Published online Feb 26, 2024. doi: 10.12998/wjcc.v12.i6.1050
Peer-review started: October 27, 2023
First decision: December 6, 2023
Revised: December 20, 2023
Accepted: January 19, 2024
Article in press: January 19, 2024
Published online: February 26, 2024
Processing time: 115 Days and 14.1 Hours
Immune-checkpoint inhibitor-mediated colitis (IMC) is an increasingly recognized adverse event in cancer immunotherapy, particularly associated with immune checkpoint inhibitors (ICIs) such as anti-cytotoxic T-lymphocyte antigen-4 and anti-programmed cell death protein-1 antibodies. As this revolutionary immunotherapy gains prominence in cancer treatment, understanding, diagnosing, and effectively managing IMC becomes paramount. IMC represents a unique cha
Core Tip: Diagnosing and managing immune-checkpoint inhibitor-mediated colitis (IMC) is essential for optimizing the benefits of cancer immunotherapy. This review underscores the importance of accurate diagnosis, differentiating IMC from other forms of colitis, and tailoring treatment strategies for optimal outcomes. Multidisciplinary approaches, including endoscopy, histopathology, and immune profiling, are crucial in diagnosing IMC. Treatment options range from corticosteroids to immunosuppressants, and a personalized approach is often required. Collaborative efforts between oncologists, gastroenterologists, and pathologists are critical to effectively manage this emerging immune-related adverse event.
- Citation: Velikova T, Krastev B, Gulinac M, Zashev M, Graklanov V, Peruhova M. New strategies in the diagnosis and treatment of immune-checkpoint inhibitor-mediated colitis. World J Clin Cases 2024; 12(6): 1050-1062
- URL: https://www.wjgnet.com/2307-8960/full/v12/i6/1050.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i6.1050
Over the past two decades, immune checkpoint inhibitors (ICIs) have transformed the landscape of cancer treatment, offering a glimmer of hope for patients battling a spectrum of malignancies[1]. By targeting immune checkpoint molecules like cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1), these immunotherapies harness their own defenses to combat cancer cells. The clinical success of ICIs is nothing short of remarkable, ushering in an era where some individuals previously deemed incurable experience profound and durable remissions[2].
However, this therapeutic paradigm shift has not been without its challenges. While ICIs show great promise, they also constrain immune-related adverse events (irAEs)[3]. These irAEs can affect nearly any organ or system, ranging from skin rashes to endocrine dysfunction. Gastrointestinal (GI) toxicities are the most common and clinically significant. Immune-checkpoint inhibitor-mediated colitis (IMC) stands out as a prominent and often formidable challenge[4,5].
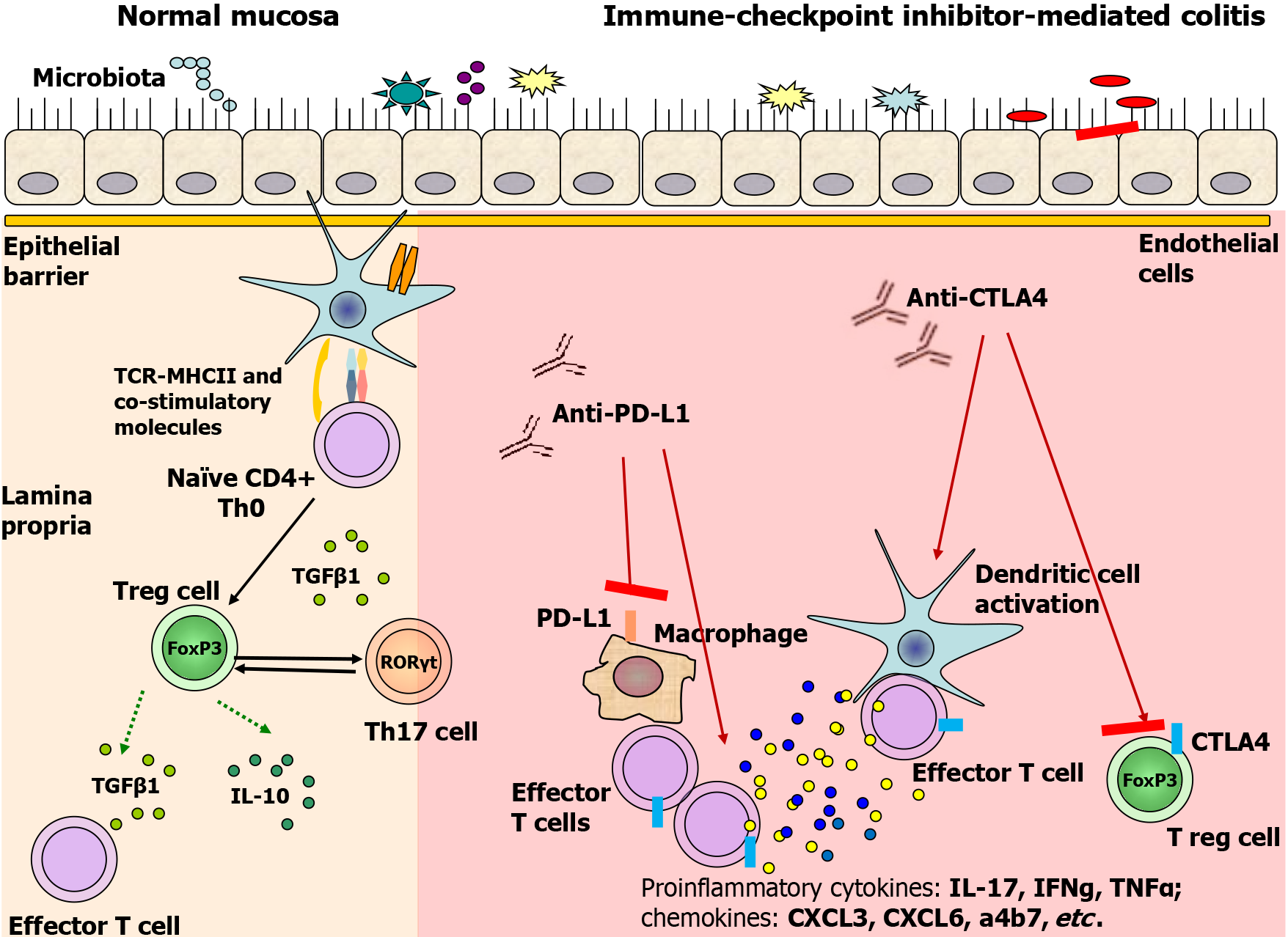
IMC is characterized by inflammation of the colonic mucosa and presents as one of the most frequent irAEs associated with ICIs[6]. This inflammatory condition arises when the delicate balance between immune activation and tolerance in the gut is disrupted, leading to dysregulated immune responses. The exact pathophysiological mechanisms of IMC are still under intense investigation, but emerging evidence indicates that gut mucosal immunity plays a pivotal role in its development[7]. Figure 1 presents some of the hypothesized immunological mechanisms of IMC development.
Precise incidence and prevalence of IMC can be challenging to ascertain, primarily due to variations in rates between different ICIs and the absence of standardized reporting mechanisms. However, some estimates have provided insights. Contemporary studies suggest that the incidence of GI irAEs, including IMC, occurs in 0.3% to 7.0% of treated patient, approximately 15%-25% of patients treated with anti-CTLA-4 agents and 5%-10% of patients treated with anti-PD-1/PD-L1 agents[8,9]. The prevalence of IMC may be underestimated, as not all cases reach clinical significance[10].
The clinical features of IMC are broad and varied, encompassing a spectrum of presentations from mild diarrhea to severe colitis. The diversity of symptoms and the potential for rapid progression underscore the need for early detection and prompt intervention. Clinicians, oncologists, and researchers must comprehensively understand IMC to optimize patient care[7,11].
This review aims to present the mechanisms of action of ICIs and existing data on IMC risk factors, diagnosis, clinical, endoscopic, and serologic features, and treatment strategies, including medications, biologics, fecal microbiota transplantation (FMT), surgery, etc, and the emergence of novel biomarkers and treatments, leading to more effective management of this irAE and improved patient outcomes.
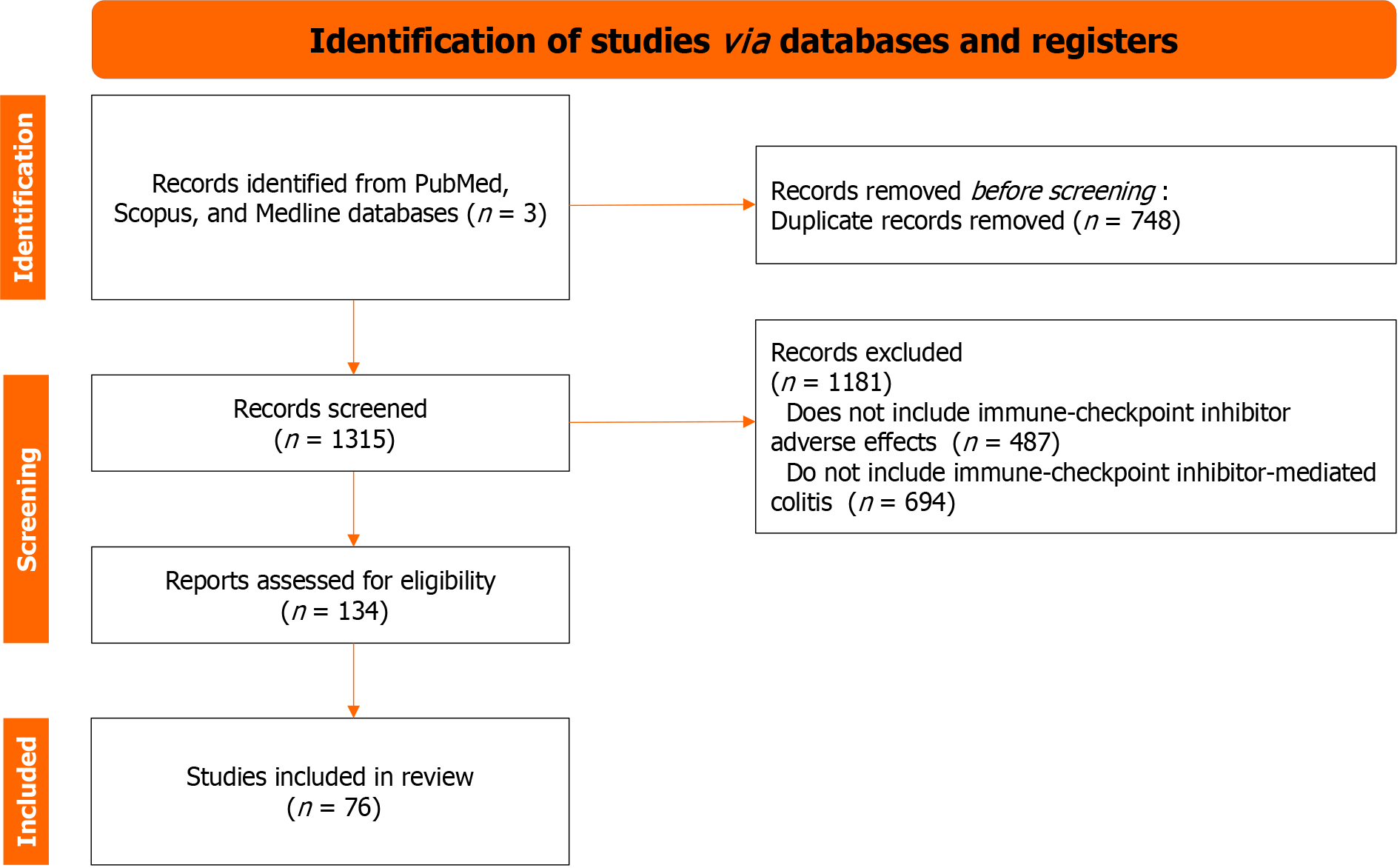
We conducted a comprehensive literature review to identify relevant studies focusing on IMC diagnosis and treatment. The search was performed in key databases, including PubMed, Scopus, and Medline. A combination of Medical Subject Headings (MESH) terms and free-text words was used to maximize the search results.
The search terms used were as follows: (“Immune-Checkpoint Inhibitor” OR “Checkpoint Inhibitor”) AND (“Colitis”); (“Immune-Checkpoint Inhibitor-Mediated Colitis” OR “Immunotherapy-Associated Colitis”) AND (“Diagnosis Strategies” OR “Diagnostic Approaches”); (“Immune-Checkpoint Inhibitor” OR “Checkpoint Inhibitor”) AND (“Gastrointestinal Adverse Effects” OR “Immune-Related Colitis”); (“Immune-Checkpoint Inhibitor-Mediated Colitis” OR “Immunotherapy-Associated Colitis”) AND (“Treatment Approaches” OR “Management”). The search strategy aimed to identify papers discussing IMC diagnosis and treatment in the context of immunotherapy and ICIs. Relevant articles were screened based on titles, abstracts, and full text to ensure their alignment with the paper’s objectives. The flow chart of identification, screening, and inclusion of retrieved papers is shown in Figure 2, following the PRISM guidelines.
The retrieved papers were further filtered to include only original research, reviews, clinical trials, and case studies that provided valuable insights into the topic. The publication date was limited to the most recent literature, primarily from the last ten years.
This systematic search strategy gathered a comprehensive selection of literature to form the basis for our review of new strategies for diagnosing and treating IMC. We wrote a modified form of a narrative review, following the recent guidelines[12].
The risk of IMC among cancer patients depends on various factors with external and intrinsic nature. One of the most significant determinants of IMC occurrence is the type of checkpoint inhibitor treatment. While both anti-CTLA4 and PD1 inhibitors have the potential to cause immune-mediated bowel inflammation and diarrhea, this toxicity is up to three times more common among patients treated with anti-CTLA4 compared to those who have received anti-PD1 or anti-PD-L1 agents[13,14]. The risk of IMC and diarrhea becomes most pronounced with checkpoint inhibitor combinations.
A meta-analysis on the incidence of immune-related diarrhea among patients treated with combinations of anti-CTLA4 and anti-PD1 or anti-CTLA1 and anti-PD-L1 reports the incidence of IMC almost three times higher in the anti-CTLA4 and anti-PD1 cohort: 40.4% for anti-CTLA4 and anti-PD-L1 vs 13.2% for the anti-CTLA4 and anti-PD1 combination[15]. Monotherapies with anti-PD1-L1 or anti-PD-1 posed the slightest risk of such GI complications, with occurrence rates of 11.0% and 9.1% for each drug subclass, respectively. Whether higher doses of ICIs are associated with more IMC is not yet clear. While it is considered more relevant to drugs like nivolumab and ipilimumab, the dose seems less significant for pembrolizumab[16].
Genetic predisposition to the occurrence of IMC has also been a subject of research and speculation. A report on the association of human leucocyte antigen (HLA) variation and immunotherapy-related adverse events revealed a significant association between HLA-DQB1*03:01 and colitis[17]. As this HLA class II variant is also found with higher prevalence among inflammatory bowel disease (IBD) patients, it further supports a potential role in the pathogenesis of immune-mediated bowel inflammation like the one observed with IMC. Clinical similarities between IMC and IBD led to the investigation of a polygenic risk score, primarily developed for ulcerative colitis, in IMC[18]. The score effectively identified subjects at risk of all grades and severe IMC in a cohort of more than 1300 non-small cell lung cancer patients receiving ICIs[18].
Most commonly, ICI-related GI irAEs manifest as colitis, characterized by watery diarrhea, abdominal pain, blood or mucus in the stool, and nocturnal bowel movements. IMC is the second most common irAE, which appears 6 wk to 8 wk after initial treatment with ICIs[19,20].
It was estimated that more frequently, GI irAEs emerged in patients treated with anti-CTLA-4 monotherapy than with PD-1/PD-L1 inhibitors. More specifically, as reported above, many studies reported that 30% to 40% of patients treated with CTLA-4 blockers developed diarrhea. Moreover, combined therapy of PD-1/PD-L1 inhibitor and CTLA-4 blockade leads to the highest rate of ICI-mediated diarrhea[14,21].
The evaluation of IMC typically involves a combination of clinical, endoscopic, histopathological, and laboratory assessments to determine the severity, extent, and underlying cause of the condition.
The clinical presentation of IMC can vary from person to person but often includes the following symptoms: diarrhea, abdominal pain, rectal bleeding, urgency tenesmus, and weight loss[7]. To rule out infectious enterocolitis, the patients should be initially tested for infections such as Salmonella, Shigella, Campylobacter, parasites, and Clostridium difficile before the diagnosis of IMC is accepted. Another important diagnostic test in patients with diarrhea includes a fecal PCR test to exclude cytomegalovirus colitis[22]. Computer tomography (CT) with oral and intravenous contrast should be performed in patients with diarrhea and abdominal pain to evaluate bowel inflammation and other intra-abdominal disorders. Long-lasting IMC could lead to severe complications such as ileus, toxic megacolon, and intestinal perforation[23].
It must be highlighted that symptom severity and duration could vary widely in patients. Clinical manifestation could present with mild, intermittent symptoms in some patients, while others could have severe symptoms that require urgent medical treatment. The exact medical evaluation and early detection of IMC are essential for better outcomes[24].
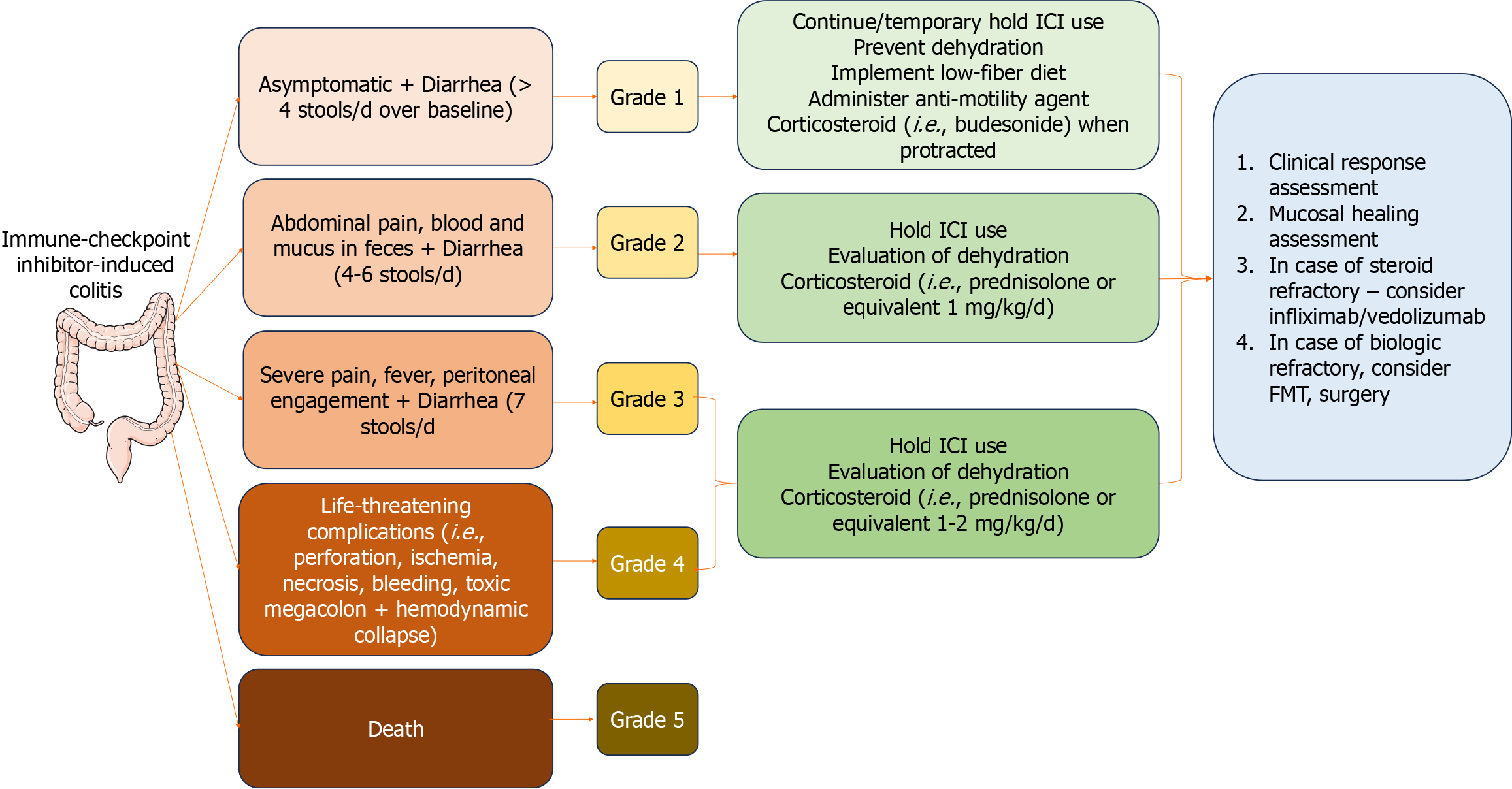
In line with this, Common Terminology Criteria for Adverse Events (CTCAE) provides a widely recognized system for evaluating the intensity of IMC[7]. IMC severity is categorized on a scale ranging from 1 (mild symptoms) to 5 (the most severe cases), taking into account symptomatology, endoscopic observations, and treatment protocols. Based on the grading, different treatment options are available (Figure 3).
It should be noted that the precise evaluation of colonic mucosa during the colonoscopy is essential for distinguishing the IMC from other GI disorders, such as IBD or infections colitis. After detailed analysis of many factors, such as clinical manifestation, endoscopic appearance, and histopathological examination of the tissue biopsies, the diagnosis of IMC could be accepted[25].
So far, there is no established grading scale for endoscopic evaluation of IMC. However, in clinical practice, the Mayo Endoscopic Score for Ulcerative Colitis and the Simple Endoscopic Score for Crohn’s disease (CD) could be used[24].
In line with this, endoscopic evaluation is essential in diagnosing and assessing the severity of IMC. The endoscopic features of IMC could be diverse, depending on the underlying cause and extent of inflammation. Some of the major endoscopic features typical for IMC include mucosal inflammation, erythema, edema, and loss of vascular pattern of the mucosa[26]. Most commonly, in clinical practice, patients on a high dose or combination of immunotherapy are diagnosed with mild colitis[27].
Many publications indicate that colonic ulcerations observed during endoscopy in patients with ipilimumab-mediated enterocolitis may be a clinical indicator of a more severe and potentially steroid-refractory disease. Extensive ulcerations suggest a more significant degree of tissue damage and inflammation. Patients with such endoscopic findings may be at higher risk of not responding well to standard steroid therapy[28].
A single-center study by Wang et al[29] demonstrated the connection between endoscopic and histologic features of ICI-related GI toxicities with long-term follow-up. The authors established that patients with more extensive and higher-grade colitis with ulcers on endoscopy and histological findings of acute inflammation have better outcomes. Another important conclusion is the correlation between endoscopic inflammation and the higher grade of colitis but not with higher-grade diarrhea.
Today, as oncology emphasizes personalized therapy, the identification of PD-L1 on tumor cells and tumor-infiltrating immune cells by histological and immunohistochemical examination is a great step forward. However, it is vital to determine whether it can be used a predictive tissue biomarker for scientific research and leverage the potential for antitumor immunotherapy in patients. Although PD-L1 expression represents a measure of the potential of the patient’s immune system to recognize the tumor and mount an effective antitumor immune response, ICIs produce various side effects[30]. According to literature data, only 4.9% to 22.0% of patients treated with ICIs report mild immune-related side effects and 4%-8% reported severe side effects that required treatment discontinuation[30]. The most commonly reported side effects are fatigue (affecting more than 36% of patients), opportunistic infections (in more than 38% of cases), disorders in the function of the thyroid gland as thyroiditis, hyper- and hypothyroidism (mainly when treated with anti-PD-1), adrenal insufficiency, hepatitis (proceeding with jaundice and asymptomatic elevation of transaminases and/or bilirubin) and diarrhea. Diarrhea is the most frequent mild side effect in patients with IMC, and perforation is rare but causes severe complications. The incidence of immune-related diarrhea is 1.3%-13.6% based on the specific agent, dose and combination of treatment for patients taking anti-PD1 and anti-PD-L1 medication[31,32]. Colitis is an immune-related adverse event of anti-PD1 (Nivolumab and Pembrolizumab) and anti-PD-L1 medications (Atezolizumab, Avelumab, and Durvalumab)[32].
Histologic assessment is a valuable tool for confirming the diagnosis of IMC and guiding treatment decisions. Depending on the severity and extent of inflammation, treatment may include corticosteroids, immunosuppressive agents, or temporary discontinuation of the ICI[20], as shown in Figure 3.
It is important to note that the histological features of IMC can overlap with those of other forms of colitis, such as IBD. Therefore, a careful evaluation of clinical history, endoscopic findings, and histopathological features is necessary to differentiate ICI-related colitis from other causes. A hallmark feature of IMC is the presence of a dense inflammatory infiltrate within the colonic mucosa[32].
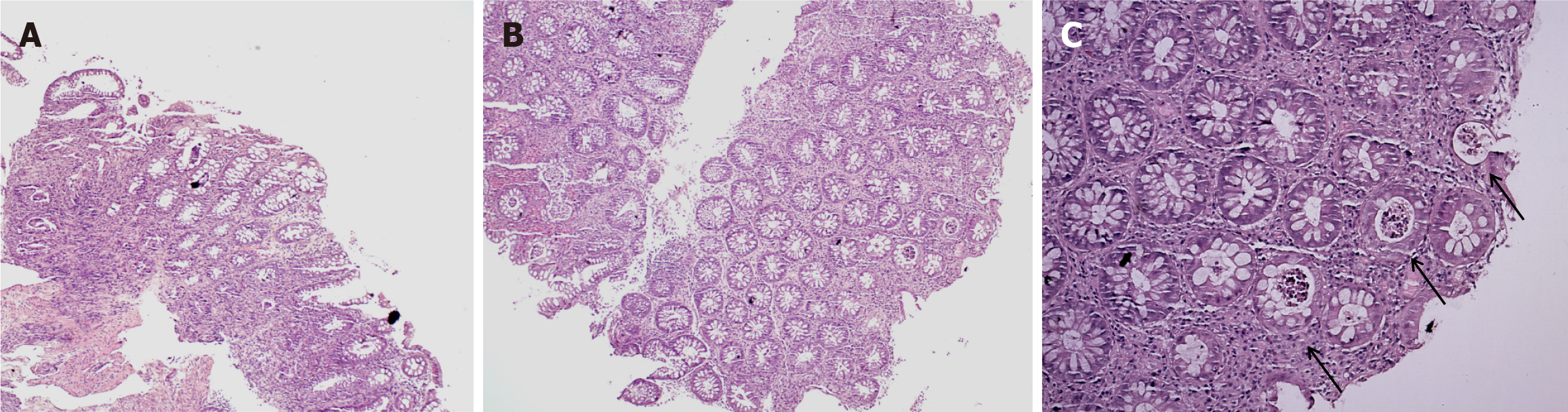
According to our experience, in just 1 mo, we had 6 patients (3/6 men and 3/6 women), aged between 27-51 years. The patients are had relapse/refractory Hodgkin’s lymphoma (4/6 with nodular sclerosis and 2/6 with mixed cellularity) and were treated with PDL-1 therapy as 4th line. All patients developed immune-related colitis after treatment with Nivolumab (4/6) and Pembrolizumab (2/6). One of the patients also had immune-related hepatitis and immune-related arthritis, and one died during therapy. The observed IMCs were grade 2 (5 of the patients) and grade 3 (1 patient). We performed fibro colonoscopy (FCS) in 1 patient with grade 3 colitis (Figure 4) and with grade 1-2 colitis (Figure 5) to evaluate mucosal involvement. We provide a histological evaluation of the biopsies (Figures 4 and 5). All patients had grade 3 diarrhea, which was treated with corticosteroids and cessation of checkpoint inhibitors.
Recent reports suggest that ICI-induced diarrhea/colitis may occur at different periods of therapy, but in most cases, occurs after an average of three infusions. However, it can occur immediately after the first infusion[33,34].
Although IMC is one of the most common side effects, there is little information on the pathological features of anti-PD-1/PD-L1 colitis. Chen et al[35] described the most common histopathologic findings in 8 patients who developed colitis while on monotherapy with ICIs. The most common injury pattern they observed (5/8 cases) was an active colitis with neutrophilic crypt microabscesses and prominent crypt epithelial cell apoptosis and crypt atrophy/dropout. The remaining cases (3/8) showed a lymphocytic colitis-like pattern characterized by increased intraepithelial lymphocytes and surface epithelial injury without crypt atrophy[35].
Interestingly, the authors reported that recurrent colitis was observed in patients several months after completion of ICI therapy[34,35]. Unfortunately, in our practice, patients with immune-related colitis are rarely biopsied, thus significantly reducing the awareness of anti-PD-1/PD-L1 colitis among pathologists and making it difficult to diagnose and treat it timely.
Nevertheless, precisely because of the non-specific morphological features of IMC and the similar changes in other colitis (i.e. cytomegalovirus-associated colitis, acute graft vs host disease, IBD; chemotherapy-induced colitis), further evaluation with endoscopy and biopsy could confirm the diagnosis.
Effective management of IMC is liable on early diagnosis, as prompt intervention can mitigate the severity of colitis. To facilitate this, there is growing interest in identifying serological and fecal biomarkers that can aid in the early detection and monitoring of IMC[36].
These markers can be obtained from circulation, target organs, etc. For CTLA-4 inhibitors, the following are discussed: from blood (i.e. T cell repertoires, T regulatory cells, eosinophils, IL-6, IL-17, serum proteins, and gene expression profiles, etc), from target organs (i.e. ectopic expression of CTLA-4, baseline gut microbiota, muscle attenuation) and from the host. On the other hand, for PD-1/PD-L1 inhibitor-related toxicity, there are some routine blood tests (i.e. complete blood count, CD4+ Th1 cells, serum autoantibodies, soluble serum proteins, HLA genotypes), etc[37-40].
Because IMC involves complex immune system dysregulation, IL-17, a pro-inflammatory cytokine implicated in several autoimmune and inflammatory conditions, has also been explored. While IL-17’s role in IMC remains an area of active investigation, its elevated levels in the serum and colon tissue may signify the immunological milieu and contribute to IMC pathogenesis. Monitoring IL-17 levels could provide insights into disease progression and therapeutic responses[41-43].
Fecal calprotectin is another biomarker whose usefulness is under investigation for IMC diagnosis and management. Elevated fecal calprotectin levels are associated with various GI disorders, including IBD. For IMC, a rise in fecal calprotectin levels may indicate an inflammatory process in the colon. However, more research is needed to establish the sensitivity and specificity of fecal calprotectin as a reliable biomarker for IMC. Its use is limited by the possibility of false-positive results in the context of irAEs and immunotherapy, making its interpretation challenging[44-46].
Despite potential biomarkers, it is essential to acknowledge that serological markers for IMC are not yet validated for routine clinical use. Current research primarily consists of small-scale studies and case reports, and there is a lack of standardized cutoff values to define IMC. Moreover, serological markers may not be exclusive to IMC, making differential diagnosis complex[37]. However, the search for reliable biomarkers for IMC continues. By identifying distinct serological profiles associated with IMC, clinicians may be better equipped to diagnose and manage these emerging irAEs. Prospective studies with larger cohorts, exploring a spectrum of potential markers, and their correlation with clinical outcomes are vital for establishing their clinical utility. Furthermore, understanding the interplay between these serological markers and the evolving immune responses during immunotherapy will provide deeper insights into the immunopathogenesis of IMC[38].
In conclusion, some established biomarkers, such as fecal calprotectin and IL-17, represent promising avenues for improving the diagnosis and management of IMC. While these markers hold potential, further research and standardization are required before they can be integrated into clinical practice. By leveraging the collective expertise of oncologists, gastroenterologists, and immunologists, the quest for biomarkers specific to IMC is a collaborative effort that underscores the need for precision medicine in the era of cancer immunotherapy.
The grade of the GI irAE is determined by the severity of the diarrhea (i.e. a rise in the number of stool movements per day relative to baseline) and the existence and seriousness of additional colitis symptoms.
Current guidelines recommend that patients with grade 1 diarrhea (< 4 bowel movements per day) without other additional symptoms of colitis be treated conservatively with hydration and loperamide or diphenoxylate/atropine[47].
In patients with more than four bowel movements per day (grade 2), treatment should start with oral corticosteroid (1-2 mg/kg) with tapering in 4-6 wk. In such cases, ICI therapy should be discontinued immediately (Figure 3). If this therapy fails, intravenous corticosteroids should be initiated. The efficacy of the treatment must be evaluated regularly every 3-5 d. In cases where the general condition of the patient deteriorates and the severity of diarrhea does not improve, a colonoscopy should be performed[48]. The results from the endoscopic assessment can give valuable information about the activity of colon inflammation and its extent. According to many retrospective clinical studies, half of the patients treated with systemic corticosteroids have improved ICI-related diarrhea[24].
However, in certain situations, corticosteroids cannot control the symptoms, and the deterioration of diarrhea may become even life-threatening without more aggressive immunosuppressant treatment. Current guidelines recommend adding infliximab or vedolizumab to corticosteroid treatment in such cases.
Initially, infliximab has been recommended in steroid-refractory IMC. Infliximab is a monoclonal antitumor necrosis factor alpha (TNF-α) antibody, which has a comprehensive implication in the treatment of various autoimmune diseases such as ulcerative colitis, CD, rheumatoid and psoriatic arthritis, and psoriasis[49]. There have been reported data concerning the implication of infliximab for treating severe steroid-refractory IMC associated with ipilimumab[50,51].
Another immunosuppressant agent used in steroid-refractory IMC cases is vedolizumab, which represents an integrin antagonist that binds to α4β7 integrin. In general, the mechanism of action of vedolizumab includes limiting the migration of T-lymphocytes into the gut mucosa, which leads to the alleviation of gut inflammation. Vedolizumab can provide a more specific immunosuppression impact over inflamed gut mucosa by remodeling antitumor immune responses[52,53].
It must be pointed out that both immunosuppressants have several weaknesses, such as drug-induced comorbidities and increased risk of infections[54,55].
In cases with severe IMC (grade 3-4), patients should be hospitalized, and specific treatment must be initiated. Intravenous corticosteroids (1-2 mg/kg/d) should be started until symptoms improve. The duration of corticosteroid treatment usually continues over 4-6 wk with tapering of corticosteroid dosage[56].
The current guidelines recommend combined therapy with steroids and infliximab or vedolizumab, preferably within 2 wk of onset, especially for patients with high-risk endoscopic features if no improvement is seen after 2 d to 3 d of intravenous methylprednisolone (1-2 mg/kg/d) therapy[57].
There is no consensus on the length of treatment with TNF-blockers (infliximab) or integrin blockers (vedolizumab). The likelihood of endoscopic/histologic remission is correlated with the treatment duration. It was estimated that the risk of recurrence is decreased by using up to three doses (at weeks 0, 2, and 6)[58].
The choice between which immunosuppressive regimen (infliximab or vedolizumab) should be administrated depends on many factors. Of great importance is to properly evaluate the risk of infections, other comorbidities, and malignancy[59].
Data related to treating colitis resistant to immunosuppressive medication with fecal transplantation has been published. Of course, the expertise of the center and the institutional availability are of great importance for the success of the procedure[60].
The impaired homeostasis of the gut microbiome plays an essential role in developing IMC. In recent decades, FMT has been used in clinical practice as a treatment method for patients with recurrent Clostridium difficile infection and IBD. FMT aims to restructure the gut microbiota to its normal composition. This medical process is characterized by transferring fecal matter obtained from a healthy donor into the GI tract of the patient[61]. The first case series concerning the implication of FMT for successfully treated patients with IMC was published by Wang et al[60]. The authors concluded that modulation of the gut microbiome could lead to improvement of the IMC.
Recently, many data represent fascinating results concerning the effect of FMT in the treatment of refractory to corticosteroids and immunosuppression therapy. A current study by Halsey et al[62] reveals 92% achieving clinical remission in patients with grade 3 or 4 ICM-related diarrhea. The authors also made a 16S rRNA sequencing of patient stool samples. They found a complete remission of the patient’s microbiota after the FMT with increases in abundance of Collinsella and Bifidobacterium.
A study by Wang et al[63] represents data on managing IMC with FMT as a first-line treatment. The results from the study demonstrate that FMT as a first-line treatment leads to quick relief of symptoms in most patients; thus, the use of steroids and immunosuppression could be avoided. Furthermore, the study revealed that first-line FMT treatment of IMC could replace the current treatment standards.
In situations where IMC fails to adequately respond to medical treatment and becomes severe, surgical intervention becomes necessary[64,65]. The surgical procedure can vary, contingent upon factors such as the severity and extent of the colitis, the patient’s overall health, and specific clinical considerations. Surgery is particularly indicated when complications arise, such as colonic perforation, toxic megacolon, or severe bleeding.
Fortunately, such complications are rare. In a phase III clinical trial comprising 511 patients who underwent ipili
The preferred surgical approach is a subtotal colectomy because the colonic lesions tend to be extensive. Opting for segmental colonic resection often leads to significant inflammation of the remaining colon during the postoperative period[68].
In non-perforative cases, particularly in critically ill patients, an alternative option is the creation of a diverting ileostomy. This can help reduce the duration of the surgery and minimize complications[69]. An ileostomy should be considered at an earlier stage, as this surgical intervention tends to be less aggressive than a colectomy. Additionally, an early ileostomy allows for the potential resumption of ICI therapy sooner[70]. Due to the possibility of relapsing with severe colitis, which in some cases can be much more severe than the initial type, the ileostomy closure period must be carefully planned[71].
Postoperative complications vary depending on the surgical procedure and patient comorbidities. At short-term follow-up (< 30 d), complications may occur in 21% of patients, often presenting as postoperative infection and intestinal obstruction. In the long term, the complication rate can vary by 39%, mainly due to fecal incontinence and small bowel obstruction[72].
Due to the lack of early warning symptoms of colitis development after ICI use, multidisciplinary team monitoring and quick action are advised[73].
As the field of immunotherapy rapidly evolves, ongoing research in diagnosing and managing IMC is essential to ensure the best possible care for patients. Several promising future directions can be explored to enhance our understanding and handling of this specific immunotherapy-related adverse event[74].
The development of these future directions relies on robust, large-scale studies, interdisciplinary collaboration, and the exchange of knowledge across the fields of oncology, immunology, and gastroenterology. A collective effort among researchers, clinicians, and pharmaceutical companies is vital to enhance our ability to diagnose and manage IMC, ultimately improving patient outcomes and the safety of cancer immunotherapy[75,76].
Precision medicine proposed tools to predict individual susceptibility to IMC, such as genomics, immunogenetics, and immune profiling, allowing for risk assessment before immunotherapy initiation.
Identifying specific serological and molecular markers for IMC is a priority. However, the search for reliable, high-sensitivity assays that can identify IMC earlier in treatment, allowing for timely intervention, is ongoing.
It is important to create comprehensive, multidisciplinary guidelines to offer oncologists, gastroenterologists, and other healthcare providers a standardized approach to the diagnosis, management, and monitoring of IMC.
Investigating tailored therapies for individual patient responses to different treatment modalities, including immunosuppressive agents, ICI discontinuation, and monoclonal antibodies, is essential for optimizing care.
More research is needed to understand the specific risk profiles for IMC and establish guidelines for managing complex cases.
These allow for regular monitoring of patients, enabling the early detection of IMC symptoms and swift intervention.
Future clinical practice should embrace shared decision-making models, allowing patients to actively participate in treatment decisions while understanding the risks and benefits of certain immunotherapy.
Novel immunomodulation strategies are needed that target and modulate immune responses and induce immune tolerance.
IMC is an emerging and complex clinical entity associated with the expanding use of cancer immunotherapies. In this comprehensive review, we have delved into the mechanisms, clinical features, diagnosis, management of IMC and promising future directions, focusing on evolving biomarker-based diagnostics.
The importance of early recognition and accurate diagnosis of IMC to ensure prompt and effective management of the condition was emphasized. Incorporating established or novel biomarkers for IMC into clinical practice holds promise for swift, accurate, and non-invasive identification of cases, enabling clinicians to intervene in a timely manner.
Nevertheless, managing IMC requires a multidisciplinary approach and tailored treatment strategies that balance effective ICI administration with the potential risk of IMC. Medical professionals from different fields must collaborate to optimize patient care while minimizing adverse effects. Overall, as immunotherapy continues to revolutionize cancer treatment, IMC remains a challenge but also represents a growing opportunity for research and innovation. The future directions we have outlined open avenues for more personalized care, early biomarker-based diagnosis, and novel treatment strategies, fostering a safer and more effective landscape for cancer immunotherapy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Tulassay Z, Hungary; Zheng L, China S-Editor: Chen YL L-Editor: Filipodia P-Editor: Zhao S
| 1. | Iranzo P, Callejo A, Assaf JD, Molina G, Lopez DE, Garcia-Illescas D, Pardo N, Navarro A, Martinez-Marti A, Cedres S, Carbonell C, Frigola J, Amat R, Felip E. Overview of Checkpoint Inhibitors Mechanism of Action: Role of Immune-Related Adverse Events and Their Treatment on Progression of Underlying Cancer. Front Med (Lausanne). 2022;9:875974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 2. | Perdyan A, Sobocki BK, Balihodzic A, Dąbrowska A, Kacperczyk J, Rutkowski J. The Effectiveness of Cancer Immune Checkpoint Inhibitor Retreatment and Rechallenge-A Systematic Review. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 3. | Esfahani K, Meti N, Miller WH Jr, Hudson M. Adverse events associated with immune checkpoint inhibitor treatment for cancer. CMAJ. 2019;191:E40-E46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, Lambotte O, Mariette X, Prat A, Suárez-Almazor ME. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 892] [Article Influence: 178.4] [Reference Citation Analysis (0)] |
| 5. | Velikova T, Krastev B, Lozenov S, Gencheva R, Peshevska-Sekulovska M, Nikolaev G, Peruhova M. Antibiotic-Related Changes in Microbiome: The Hidden Villain behind Colorectal Carcinoma Immunotherapy Failure. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Yin Q, Wu L, Han L, Zheng X, Tong R, Li L, Bai L, Bian Y. Immune-related adverse events of immune checkpoint inhibitors: a review. Front Immunol. 2023;14:1167975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 163] [Reference Citation Analysis (0)] |
| 7. | Som A, Mandaliya R, Alsaadi D, Farshidpour M, Charabaty A, Malhotra N, Mattar MC. Immune checkpoint inhibitor-induced colitis: A comprehensive review. World J Clin Cases. 2019;7:405-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 201] [Cited by in RCA: 192] [Article Influence: 32.0] [Reference Citation Analysis (6)] |
| 8. | Prieux-Klotz C, Dior M, Damotte D, Dreanic J, Brieau B, Brezault C, Abitbol V, Chaussade S, Coriat R. Immune Checkpoint Inhibitor-Induced Colitis: Diagnosis and Management. Target Oncol. 2017;12:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Weingarden AR, Rubin SJS, Gubatan J. Immune checkpoint inhibitor-mediated colitis in gastrointestinal malignancies and inflammatory bowel disease. World J Gastrointest Oncol. 2021;13:772-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 10. | Tang L, Wang J, Lin N, Zhou Y, He W, Liu J, Ma X. Immune Checkpoint Inhibitor-Associated Colitis: From Mechanism to Management. Front Immunol. 2021;12:800879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 11. | Alorfi NM, Alourfi MM. Biologic Therapy for Refractory Immune Checkpoint Inhibitor Colitis. Biologics. 2022;16:119-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011;31:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 494] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 13. | Soularue E, Lepage P, Colombel JF, Coutzac C, Faleck D, Marthey L, Collins M, Chaput N, Robert C, Carbonnel F. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut. 2018;67:2056-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 14. | Tandon P, Bourassa-Blanchette S, Bishay K, Parlow S, Laurie SA, McCurdy JD. The Risk of Diarrhea and Colitis in Patients With Advanced Melanoma Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis. J Immunother. 2018;41:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 15. | Bishay K, Tandon P, Bourassa-Blanchette S, Laurie SA, McCurdy JD. The risk of diarrhea and colitis in patients with lung cancer treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Curr Oncol. 2020;27:e486-e494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Gong Z, Wang Y. Immune Checkpoint Inhibitor-Mediated Diarrhea and Colitis: A Clinical Review. JCO Oncol Pract. 2020;16:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Hasan Ali O, Berner F, Bomze D, Fässler M, Diem S, Cozzio A, Jörger M, Früh M, Driessen C, Lenz TL, Flatz L. Human leukocyte antigen variation is associated with adverse events of checkpoint inhibitors. Eur J Cancer. 2019;107:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 18. | Middha P, Thummalapalli R, Betti MJ, Yao L, Quandt Z, Balaratnam K, Bejan CA, Cardenas E, Falcon CJ, Faleck DM; Princess Margaret Lung Group, Gubens MA, Huntsman S, Johnson DB, Kachuri L, Khan K, Li M, Lovly CM, Murray MH, Patel D, Werking K, Xu Y, Zhan LJ, Balko JM, Liu G, Aldrich MC, Schoenfeld AJ, Ziv E. Polygenic risk score for ulcerative colitis predicts immune checkpoint inhibitor-mediated colitis. medRxiv. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 19. | Weber JS, Dummer R, de Pril V, Lebbé C, Hodi FS; MDX010-20 Investigators. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119:1675-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 347] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Abu-Sbeih H, Mao E, Ali N, Qiao W, Trinh VA, Zobniw C, Johnson DH, Samdani R, Lum P, Shuttlesworth G, Blechacz B, Bresalier R, Miller E, Thirumurthi S, Richards D, Raju G, Stroehlein J, Diab A. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflamm Bowel Dis. 2018;24:1695-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 180] [Article Influence: 25.7] [Reference Citation Analysis (4)] |
| 21. | Pernot S, Ramtohul T, Taieb J. Checkpoint inhibitors and gastrointestinal immune-related adverse events. Curr Opin Oncol. 2016;28:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | McCutcheon JL, McClain CM, Puzanov I, Smith TA. Infectious Colitis Associated With Ipilimumab Therapy. Gastroenterology Res. 2014;7:28-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Shah R, Witt D, Asif T, Mir FF. Ipilimumab as a Cause of Severe Pan-Colitis and Colonic Perforation. Cureus. 2017;9:e1182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Geukes Foppen MH, Rozeman EA, van Wilpe S, Postma C, Snaebjornsson P, van Thienen JV, van Leerdam ME, van den Heuvel M, Blank CU, van Dieren J, Haanen JBAG. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3:e000278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 206] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 25. | Menon T, Afzali A. Immune-Mediated Colitis. Curr Treat Options Gastroenterol. 2019;17:506-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Verschuren EC, van den Eertwegh AJ, Wonders J, Slangen RM, van Delft F, van Bodegraven A, Neefjes-Borst A, de Boer NK. Clinical, Endoscopic, and Histologic Characteristics of Ipilimumab-Associated Colitis. Clin Gastroenterol Hepatol. 2016;14:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 27. | Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6070] [Cited by in RCA: 6205] [Article Influence: 620.5] [Reference Citation Analysis (0)] |
| 28. | Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, Lebbé C, Ferraresi V, Smylie M, Weber JS, Maio M, Bastholt L, Mortier L, Thomas L, Tahir S, Hauschild A, Hassel JC, Hodi FS, Taitt C, de Pril V, de Schaetzen G, Suciu S, Testori A. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med. 2016;375:1845-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 964] [Cited by in RCA: 1024] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 29. | Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W, Lum P, Raju G, Shuttlesworth G, Stroehlein J, Diab A. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer. 2018;6:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 30. | Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, Korenstein D. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 422] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 31. | Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, Liu N, Yan CX. Immune-Related Adverse Events Associated with Anti-PD-1/PD-L1 Treatment for Malignancies: A Meta-Analysis. Front Pharmacol. 2017;8:730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 348] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 32. | Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis. Oncoimmunology. 2017;6:e1344805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 33. | Mooradian MJ, Wang DY, Coromilas A, Lumish M, Chen T, Giobbie-Hurder A, Johnson DB, Sullivan RJ, Dougan M. Mucosal inflammation predicts response to systemic steroids in immune checkpoint inhibitor colitis. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 34. | Nishida T, Iijima H, Adachi S. Immune checkpoint inhibitor-induced diarrhea/colitis: Endoscopic and pathologic findings. World J Gastrointest Pathophysiol. 2019;10:17-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (3)] |
| 35. | Chen JH, Pezhouh MK, Lauwers GY, Masia R. Histopathologic Features of Colitis Due to Immunotherapy With Anti-PD-1 Antibodies. Am J Surg Pathol. 2017;41:643-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 36. | Xu Y, Fu Y, Zhu B, Wang J, Zhang B. Predictive Biomarkers of Immune Checkpoint Inhibitors-Related Toxicities. Front Immunol. 2020;11:2023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 37. | Li N, Hou X, Huang S, Tai R, Lei L, Li S, Abuliz A, Wang G, Yang S. Biomarkers related to immune checkpoint inhibitors therapy. Biomed Pharmacother. 2022;147:112470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Les I, Martínez M, Pérez-Francisco I, Cabero M, Teijeira L, Arrazubi V, Torrego N, Campillo-Calatayud A, Elejalde I, Kochan G, Escors D. Predictive Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Events. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 39. | Jia XH, Geng LY, Jiang PP, Xu H, Nan KJ, Yao Y, Jiang LL, Sun H, Qin TJ, Guo H. The biomarkers related to immune related adverse events caused by immune checkpoint inhibitors. J Exp Clin Cancer Res. 2020;39:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 40. | Bai R, Lv Z, Xu D, Cui J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res. 2020;8:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 314] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 41. | Callahan MK, Yang A, Tandon S, Xu Y, Subudhi SK, Roman RA, Heine AI, Pogoriler E, Kuk D, Panageas K, Yuan JD, Allison JP, Wolchok JD. Evaluation of Serum IL-17 Levels During Ipilimumab Therapy: Correlation With Colitis. J Clin Oncol. 2011;29:2505. [RCA] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 42. | Bamias G, Delladetsima I, Perdiki M, Siakavellas SI, Goukos D, Papatheodoridis GV, Daikos GL, Gogas H. Immunological Characteristics of Colitis Associated with Anti-CTLA-4 Antibody Therapy. Cancer Invest. 2017;35:443-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | Westdorp H, Sweep MWD, Gorris MAJ, Hoentjen F, Boers-Sonderen MJ, van der Post RS, van den Heuvel MM, Piet B, Boleij A, Bloemendal HJ, de Vries IJM. Mechanisms of Immune Checkpoint Inhibitor-Mediated Colitis. Front Immunol. 2021;12:768957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | Zou F, Wang X, Glitza Oliva IC, McQuade JL, Wang J, Zhang HC, Thompson JA, Thomas AS, Wang Y. Fecal calprotectin concentration to assess endoscopic and histologic remission in patients with cancer with immune-mediated diarrhea and colitis. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 45. | Abu-Sbeih H, Ali FS, Alsaadi D, Jennings J, Luo W, Gong Z, Richards DM, Charabaty A, Wang Y. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: a multi-center study. J Immunother Cancer. 2018;6:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 46. | Kennedy LC, Grivas P. Immunotherapy-Related Colitis: An Emerging Challenge and a Quest for Prospective Data. JCO Oncol Pract. 2020;16:464-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M, Dunnington D, Ernstoff MS, Frigault M, Kaffenberger BH, Lunning M, McGettigan S, McPherson J, Mohindra NA, Naidoo J, Olszanski AJ, Oluwole O, Patel SP, Pennell N, Reddy S, Ryder M, Santomasso B, Shofer S, Sosman JA, Wang Y, Weight RM, Johnson-Chilla A, Zuccarino-Catania G, Engh A. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J Natl Compr Canc Netw. 2020;18:230-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 312] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 48. | Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2245] [Cited by in RCA: 2586] [Article Influence: 369.4] [Reference Citation Analysis (0)] |
| 49. | Sfikakis PP. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun. 2010;11:180-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 50. | Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 3151] [Article Influence: 450.1] [Reference Citation Analysis (0)] |
| 51. | Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2:1346-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 613] [Article Influence: 76.6] [Reference Citation Analysis (1)] |
| 52. | Bergqvist V, Hertervig E, Gedeon P, Kopljar M, Griph H, Kinhult S, Carneiro A, Marsal J. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother. 2017;66:581-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 53. | Hsieh AH, Ferman M, Brown MP, Andrews JM. Vedolizumab: a novel treatment for ipilimumab-induced colitis. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Youssef J, Novosad SA, Winthrop KL. Infection Risk and Safety of Corticosteroid Use. Rheum Dis Clin North Am. 2016;42:157-176, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 305] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 55. | Siegel CA, Hur C, Korzenik JR, Gazelle GS, Sands BE. Risks and benefits of infliximab for the treatment of Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:1017-24; quiz 976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 56. | Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M, Dunnington D, Ernstoff MS, Frigault M, Hoffner B, Hoimes CJ, Lacouture M, Locke F, Lunning M, Mohindra NA, Naidoo J, Olszanski AJ, Oluwole O, Patel SP, Reddy S, Ryder M, Santomasso B, Shofer S, Sosman JA, Wahidi M, Wang Y, Johnson-Chilla A, Scavone JL. Management of Immunotherapy-Related Toxicities, Version 1.2019. J Natl Compr Canc Netw. 2019;17:255-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 381] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 57. | Abu-Sbeih H, Ali FS, Luo W, Qiao W, Raju GS, Wang Y. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2018;6:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 58. | Abu-Sbeih H, Ali FS, Wang X, Mallepally N, Chen E, Altan M, Bresalier RS, Charabaty A, Dadu R, Jazaeri A, Lashner B, Wang Y. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2019;7:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (1)] |
| 59. | Evangelatos G, Bamias G, Kitas GD, Kollias G, Sfikakis PP. The second decade of anti-TNF-a therapy in clinical practice: new lessons and future directions in the COVID-19 era. Rheumatol Int. 2022;42:1493-1511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 60. | Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, Jiang ZD, Abu-Sbeih H, Sanchez CA, Chang CC, Parra ER, Francisco-Cruz A, Raju GS, Stroehlein JR, Campbell MT, Gao J, Subudhi SK, Maru DM, Blando JM, Lazar AJ, Allison JP, Sharma P, Tetzlaff MT, Wargo JA, Jenq RR. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24:1804-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 550] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 61. | Abu-Sbeih H, Wang Y. Gut Microbiome and Immune Checkpoint Inhibitor-Induced Enterocolitis. Dig Dis Sci. 2020;65:797-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Halsey TM, Thomas AS, Hayase T, Ma W, Abu-Sbeih H, Sun B, Parra ER, Jiang ZD, DuPont HL, Sanchez C, El-Himri R, Brown A, Flores I, McDaniel L, Ortega Turrubiates M, Hensel M, Pham D, Watowich SS, Hayase E, Chang CC, Jenq RR, Wang Y. Microbiome alteration via fecal microbiota transplantation is effective for refractory immune checkpoint inhibitor-induced colitis. Sci Transl Med. 2023;15:eabq4006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 63. | Wang YH, Varatharajalu K, Shatila M, Campbell MT, Msaouel P, Kovitz CA. First-line treatment of fecal microbiota transplantation for immune-mediated colitis. J Clini Onco. 2023;41 Suppl 16:2510. [DOI] [Full Text] |
| 64. | Samaan MA, Pavlidis P, Papa S, Powell N, Irving PM. Gastrointestinal toxicity of immune checkpoint inhibitors: from mechanisms to management. Nat Rev Gastroenterol Hepatol. 2018;15:222-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 65. | Mitchell KA, Kluger H, Sznol M, Hartman DJ. Ipilimumab-induced perforating colitis. J Clin Gastroenterol. 2013;47:781-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 66. | Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11779] [Article Influence: 785.3] [Reference Citation Analysis (0)] |
| 67. | Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, Quezado M, Lowy I, Yellin M, Rosenberg SA, Yang JC. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283-2289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 700] [Cited by in RCA: 647] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 68. | Marthey L, Mateus C, Mussini C, Nachury M, Nancey S, Grange F, Zallot C, Peyrin-Biroulet L, Rahier JF, Bourdier de Beauregard M, Mortier L, Coutzac C, Soularue E, Lanoy E, Kapel N, Planchard D, Chaput N, Robert C, Carbonnel F. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 267] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 69. | Le KDR, Choy KT, Roth S, Heriot AG, Kong JCH. Immune mediated colitis: a surgical perspective. ANZ J Surg. 2023;93:1495-1502. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 70. | Portenkirchner C, Kienle P, Horisberger K. Checkpoint Inhibitor-Induced Colitis-A Clinical Overview of Incidence, Prognostic Implications and Extension of Current Treatment Options. Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Horisberger K, Portenkirchner C, Rickenbacher A, Biedermann L, Gubler C, Turina M. Complete Recovery of Immune Checkpoint Inhibitor-induced Colitis by Diverting Loop Ileostomy. J Immunother. 2020;43:145-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 72. | Peyrin-Biroulet L, Germain A, Patel AS, Lindsay JO. Systematic review: outcomes and post-operative complications following colectomy for ulcerative colitis. Aliment Pharmacol Ther. 2016;44:807-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 73. | Nakamura Y. Biomarkers for Immune Checkpoint Inhibitor-Mediated Tumor Response and Adverse Events. Front Med (Lausanne). 2019;6:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 74. | Terrin M, Migliorisi G, Dal Buono A, Gabbiadini R, Mastrorocco E, Quadarella A, Repici A, Santoro A, Armuzzi A. Checkpoint Inhibitor-Induced Colitis: From Pathogenesis to Management. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 75. | Cai X, Zhan H, Ye Y, Yang J, Zhang M, Li J, Zhuang Y. Current Progress and Future Perspectives of Immune Checkpoint in Cancer and Infectious Diseases. Front Genet. 2021;12:785153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 76. | Del Gaudio A, Di Vincenzo F, Petito V, Giustiniani MC, Gasbarrini A, Scaldaferri F, Lopetuso LR. Focus on Immune Checkpoint Inhibitors-related Intestinal Inflammation: From Pathogenesis to Therapeutical Approach. Inflamm Bowel Dis. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |