Published online Feb 26, 2024. doi: 10.12998/wjcc.v12.i6.1045
Peer-review started: November 13, 2023
First decision: January 9, 2024
Revised: January 10, 2024
Accepted: January 31, 2024
Article in press: January 31, 2024
Published online: February 26, 2024
Processing time: 99 Days and 4.1 Hours
Tumor deposits (TDs) are defined as discrete, irregular clusters of tumor cells lying in the soft tissue adjacent to but separate from the primary tumor, and are usually found in the lymphatic drainage area of the primary tumor. By definition, no residual lymph node structure should be identified in these tumor masses. At present, TDs are mainly reported in colorectal cancer, with a few reports in gastric cancer. There are very few reports on breast cancer (BC). For TDs, current domi
Core Tip: In this editorial, we comment on a case report by Li et al published in the recent issue of the World Journal of Clinical Cases. According to the authors of this article, the objective of presenting this case was to bring to attention the detection and reporting of tumor deposits (TDs) in breast cancer. TDs are being increasingly detected and reported in many other types of surgically resected cancers, but in this editorial article, we will focus specifically on the significance of TDs in primary breast carcinoma.
- Citation: Mubarak M, Rashid R, Shakeel S. Tumor deposits in axillary adipose tissue in patients with breast cancer: Do they matter? World J Clin Cases 2024; 12(6): 1045-1049
- URL: https://www.wjgnet.com/2307-8960/full/v12/i6/1045.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i6.1045
In this editorial, we comment on a case report by Li et al[1] published in the recent issue of the World Journal of Clinical Cases. According to the authors of this article, the objective of presenting this case was to bring to attention the detection and reporting of tumor deposits (TDs) in breast cancer (BC). TDs are being increasingly detected and reported in many other types of surgically resected cancers, but in this editorial article, we will focus specifically on the significance of TDs in primary BC.
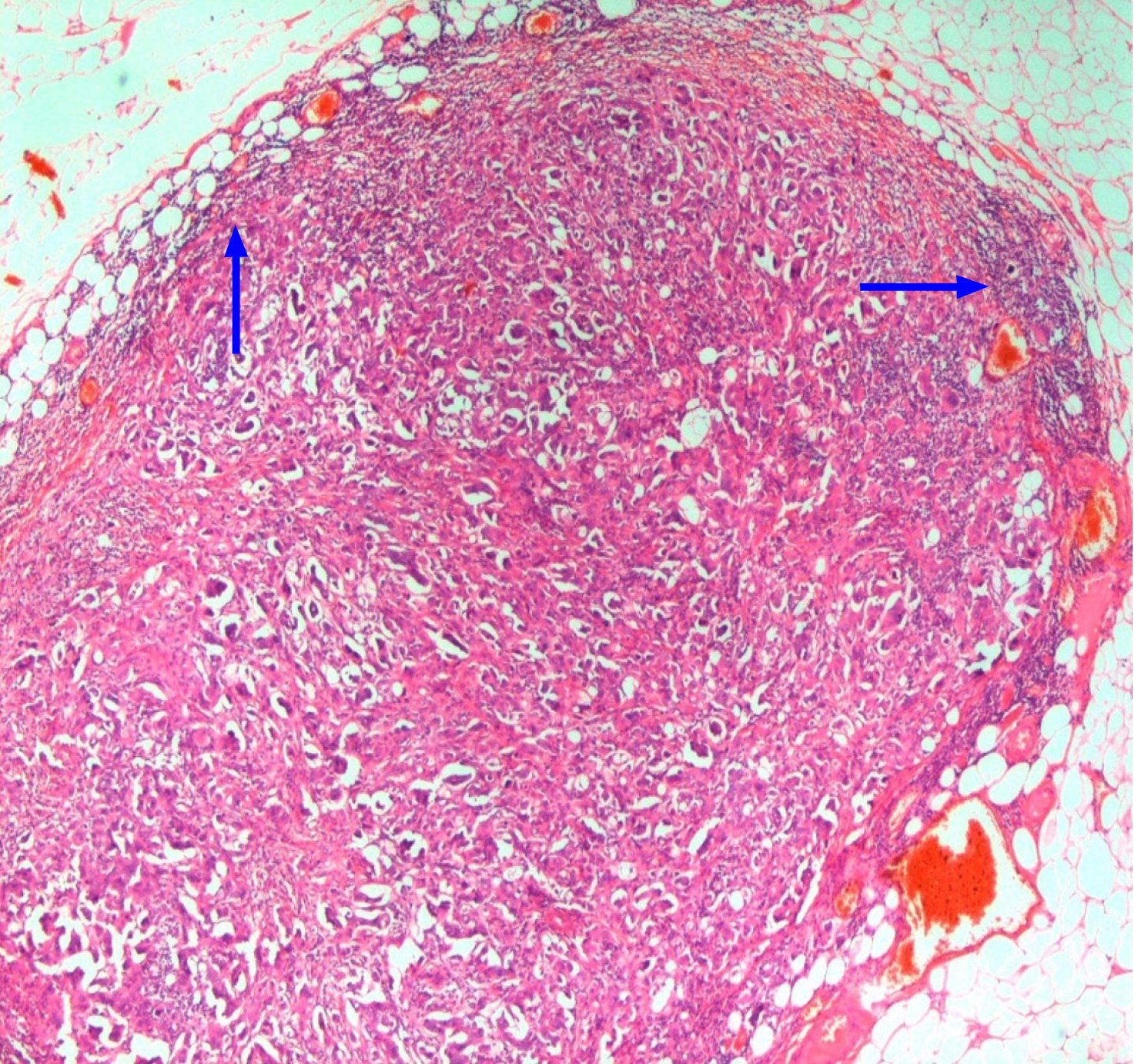
TDs are defined as discrete, irregular clusters of tumor cells lying in the soft tissue adjacent to but separate from the primary tumor (PT), and are usually found in the draining lymphatic area of the PT. By convention, no remaining lymph node (LN) structure should be discernible in these TDs. These can only be diagnosed on histopathological examination (Figures 1-3). These are an important prognostic feature in a variety of malignant tumors. Their origin, classification, and significance are still not completely understood. Moreover, their definitions have also changed, particularly in colorectal cancer (CRC). They are often thought to be derived from metastasis by the lymphatic route, but origins from venous and perineural pathways of tumor spread have also been suggested. Some pathologists even classify a TD as two LN meta
TDs were first described by Gabriel et al[2] in 1935 in CRC. However, these were not given due importance for staging or prognostic purposes for CRC or other tumor types for a considerable time. The interest in TDs was revived around the late 1990s and early 2000s when several studies were conducted on the significance of TDs in CRCs and gastric cancer (GC) reporting a significant adverse effect on prognosis[3-6]. TDs have also been detected in many other malignant tu
In GC and CRC, TDs in the LN drainage area have been identified as independent prognostic factors. In CRC, TDs have been incorporated into the TNM staging system[12]. However, the categorization, and incorporation in staging, of TDs have changed considerably in each TNM Edition since their original description in TNM 5, and the terminology remains controversial. Currently, these are assigned N1c in the absence of associated LNMs[13]. Currently, TDs are not included in the clinical or pathological staging of any other cancer including BC.
BC is the most common malignant tumor in women throughout the world[14]. BC staging is performed using the TNM staging system and is a major determinant of prognosis. According to the most recent TNM staging updates, invasive tumor masses within axillary fat distinct from identifiable LN structure, are designated as regional LNMs (pN)[15]. How
Li et al[1] have done a commendable job in reporting this case of isolated TD in the axillary area in a 70-year-old female patient with primary BC. However, given the clinical course and follow-up of this particular patient, TD does not fulfill the role of a prognostic marker in this case as the follow-up is short and no adverse outcome occurred till the last follow-up in this patient. However, they have succeeded in provoking thoughts and consideration of this lesion in BC through their case study and literature review.
In an interesting study on TDs in BC, Durak et al[16] retrospectively reviewed 145 cases of BC, detected and managed between 2001 and 2006 at a single center for determining the frequency of TDs. TDs were found in 42 (29%) of cases. After exclusion of TDs from the number of metastatic LNs, the pN stage of nine patients changed. On multivariate exploration, the presence of TDs was independently and significantly associated with distant metastases. The probability of distant metastases was 3.3-fold higher in patients with TDs. TDs were also associated with shortened patient survival time as compared to those patients without TDs, although this was not statistically significant. The results from the study by Du
In another large single-center study, Mamtani et al[17] studied the clinical significance of extranodal TDs (ETDs) in 1114 consecutive patients with T1T2cN0 invasive BCs. Overall, 113 (10.1%) patients had ETDs in this study. It was found that among T1-T2cN0 patients with sentinel LNMs, ETDs in axillary fat were strongly associated with ≥ 4 positive non-sentinel lymph nodes at axillary LN dissection (ALND). They concluded that even among the patients who may other
More similar studies are warranted by other investigators on patients with BC to delineate the long-term prognosis of TDs in this type of malignancy. At the same time, a modified Delphi process can also be initiated to streamline the diag
In summary, in this era of precision diagnostics and personalized medicine, the interest has been rekindled in TDs in many types of malignant tumors including BC. There is a need to report these and perform large, multicenter, prospective studies to detect their clinical significance in improving patient care.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gu GL, China; Jiang L, China S-Editor: Liu JH L-Editor: A P-Editor: Xu ZH
| 1. | Li T, Zhang WH, Liu J, Mao YL, Liu S. Isolated axillary tumor deposit consistent with primary breast carcinoma: A case report. World J Clin Cases. 2023;11:7718-7723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Gabriel WB, Dukes C, Bussey HJR. Lymphatic spread in cancer of the rectum. Br J Surg. 1935;1:395-413. [RCA] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 265] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Nagtegaal ID, Knijn N, Hugen N, Marshall HC, Sugihara K, Tot T, Ueno H, Quirke P. Tumor Deposits in Colorectal Cancer: Improving the Value of Modern Staging-A Systematic Review and Meta-Analysis. J Clin Oncol. 2017;35:1119-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (1)] |
| 4. | Kobayashi T, Ishida M, Miki H, Hatta M, Hamada M, Hirose Y, Sekimoto M. Significance of desmoplastic reactions on tumor deposits in patients with colorectal cancer. Oncol Lett. 2023;25:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 5. | Pu H, Pang X, Fu J, Zheng R, Chen Y, Zhang D, Fang X. Significance of tumor deposits combined with lymph node metastasis in stage III colorectal cancer patients: a retrospective multi-center cohort study from China. Int J Colorectal Dis. 2022;37:1411-1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Zheng P, Lai C, Yang W, Chen Z. Prognostic Significance of Tumor Deposits in Combination with Lymph Node Metastasis in Stage III Colon Cancer: A Propensity Score Matching Study. Am Surg. 2020;86:164-170. [PubMed] |
| 7. | Mirkin KA, Kulaylat AS, Hollenbeak CS, Messaris E. Prognostic Significance of Tumor Deposits in Stage III Colon Cancer. Ann Surg Oncol. 2018;25:3179-3184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 8. | Liang Y, Wu L, Liu L, Ding X, Wang X, Liu H, Meng J, Xu R, He D, Liang H. Impact of extranodal tumor deposits on prognosis and N stage in gastric cancer. Surgery. 2019;166:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Liang Y, Chang S, Guo H, Man Q, Zang F, Gao S. Presence of tumor deposits is an indicator of poor prognosis in patients with pancreatic ductal adenocarcinoma. Am J Cancer Res. 2023;13:1970-1984. [PubMed] |
| 10. | Zhou M, Yang W, Zou W, Yang J, Zhou C, Zhang Z, Wang Y, Zhang J, Li G, Xia F. Prognostic significance of tumor deposits in radically resected gastric cancer: a retrospective study of a cohort of 1915 Chinese individuals. World J Surg Oncol. 2022;20:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | González-Vallejo L, Blanco-Sainzdelamaza J, Querejeta-Ayerra A, Chiesa-Estomba C. Extracapsular nodal extension and tumor deposits in head and neck squamous cell carcinoma. Cancer Rep (Hoboken). 2023;6:e1897. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Chen P, Zuo ZL, Feng LB, Chen XL, Hu XY, Liu Q, Xia D. Questioning the staging of tumor deposits of colorectal cancer in the eighth edition of the TNM classification: validation by prognosis. Int J Clin Exp Pathol. 2019;12:4309-4318. [PubMed] |
| 13. | Delattre JF, Selcen Oguz Erdogan A, Cohen R, Shi Q, Emile JF, Taieb J, Tabernero J, André T, Meyerhardt JA, Nagtegaal ID, Svrcek M. A comprehensive overview of tumour deposits in colorectal cancer: Towards a next TNM classification. Cancer Treat Rev. 2022;103:102325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 14. | Sapna F, Athwal PSS, Kumar M, Randhawa S, Kahlon S. Therapeutic Strategies for Human Epidermal Receptor-2 Positive Metastatic Breast Cancer: A Literature Review. Cureus. 2020;12:e9522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Teichgraeber DC, Guirguis MS, Whitman GJ. Breast Cancer Staging: Updates in the AJCC Cancer Staging Manual, 8th Edition, and Current Challenges for Radiologists, From the AJR Special Series on Cancer Staging. AJR Am J Roentgenol. 2021;217:278-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Durak MG, Canda T, Yilmaz B, Seker NS, Kokkoz SE, Alicikus ZA, Akturk N, Gorken IB, Ellidokuz H, Sevinc AI, Saydam S, Sarioglu S. Prognostic Importance of Tumor Deposits in the Ipsilateral Axillary Region of Breast Cancer Patients. Pathol Oncol Res. 2019;25:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 17. | Mamtani A, Barrio AV, Goldman DA, Wen HY, Vincent A, Morrow M. Extranodal Tumor Deposits in the Axillary Fat Indicate the Need for Axillary Dissection Among T1-T2cN0 Patients with Positive Sentinel Nodes. Ann Surg Oncol. 2020;27:3585-3592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Lord A, Brown G, Abulafi M, Bateman A, Frankel W, Goldin R, Gopal P, Kirsch R, Loughrey MB, Märkl B, Moran B, Puppa G, Rasheed S, Shimada Y, Snaebjornsson P, Svrcek M, Washington K, West N, Wong N, Nagtegaal I. Histopathological diagnosis of tumour deposits in colorectal cancer: a Delphi consensus study. Histopathology. 2021;79:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |