Published online Feb 16, 2024. doi: 10.12998/wjcc.v12.i5.995
Peer-review started: October 13, 2023
First decision: November 28, 2023
Revised: December 1, 2023
Accepted: January 18, 2024
Article in press: January 18, 2024
Published online: February 16, 2024
Processing time: 109 Days and 15.4 Hours
A solitary fibrous tumor (SFT) is often located in the pleura, while SFT of the pancreas is extremely rare. Here, we report a case of SFT of the pancreas and discuss imaging, histopathology, and immunohistochemistry for accurate diagnosis and treatment.
A 54-year-old man presented to our hospital with pancreatic occupancy for over a month. There were no previous complaints of discomfort. His blood pressure was normal. Blood glucose, tumor markers, and enhanced computed tomography (CT) suggested a malignant tumor. Because the CT appearance of pancreatic cancer varies, we could not confirm the diagnosis; therefore, we performed endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB). Pathology and immunohistochemistry were consistent with SFT of the pancreas. The posto
Other diseases must be excluded in patients with a pancreatic mass that cannot be diagnosed. CT and pathological histology have diagnostic value for pancreatic tumors. Endoscopic puncture biopsy under ultrasound can help diagnose pancreatic masses that cannot be diagnosed preoperatively. Surgery is an effective treatment for SFT of the pancreas; however, long-term follow-up is strongly recommended because of the possibility of malignant transformation of the tumor.
Core Tip: We need to be more vigilant for indeterminate pancreatic masses, and then computed tomography and histopathology can play a very important role in clinical diagnosis. Surgery is an effective treatment for solitary fibrous tumor of the pancreas; however, long-term follow-up is strongly recommended because of the possibility of malignant transformation of the tumor.
- Citation: Wang WW, Zhou SP, Wu X, Wang LL, Ruan Y, Lu J, Li HL, Ni XL, Qiu LL, Zhou XH. Imaging, pathology, and diagnosis of solitary fibrous tumor of the pancreas: A case report and review of literature. World J Clin Cases 2024; 12(5): 995-1003
- URL: https://www.wjgnet.com/2307-8960/full/v12/i5/995.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i5.995
A solitary fibrous tumor (SFT) is histologically characterized as a mesenchymal tumor, probably fibroblastic in origin, located primarily in the pleura; however, it can be found in any other extrapleural region[1-3]. Extrapleural areas include the liver, peritoneum, kidney, and salivary glands[4-7]. SFT of the pancreas is rare, with only about 30 cases reported to date[1-3,6-35]. SFT of the pancreas is usually asymptomatic, and most are detected by physical examination, computed tomography (CT), or ultrasound as pancreatic masses[6,30,32]. The final diagnosis depends on histopathology and immunohistochemistry[7,31].
Here, we report a case of SFT of the pancreas and present the radiological and pathological differential diagnosis.
A 54-year-old man was admitted to our hospital with a pancreatic space-occupying mass of one month’s duration, identified on a physical exam.
A 54-year-old man had been one month before a medical CT finding of pancreas space-occupying lesions, with no adverse reactions, patients for further treatment at our hospital.
The patient had no other significant medical history. History of hypertension, diabetes, coronary heart disease, and other chronic disease was denied.
The patient had no significant personal or family history.
The patient had no discomfort after the physical examination.
There was no abnormal carcinoembryonic antigen [< 0.5 ng/mL (normal 0-5 ng/mL)], carbohydrate antigen 199 3.9 U/mL (average 0-7 U/mL), alpha-fetoprotein 2.4 ng/mL (normal 0-8.8 ng/mL), carbohydrate antigen 125 12.5 (average 0-30.2 U/mL). Fasting glucose was 5. 19 mmol/L (normal 3.89-6. 11 mmol/L).
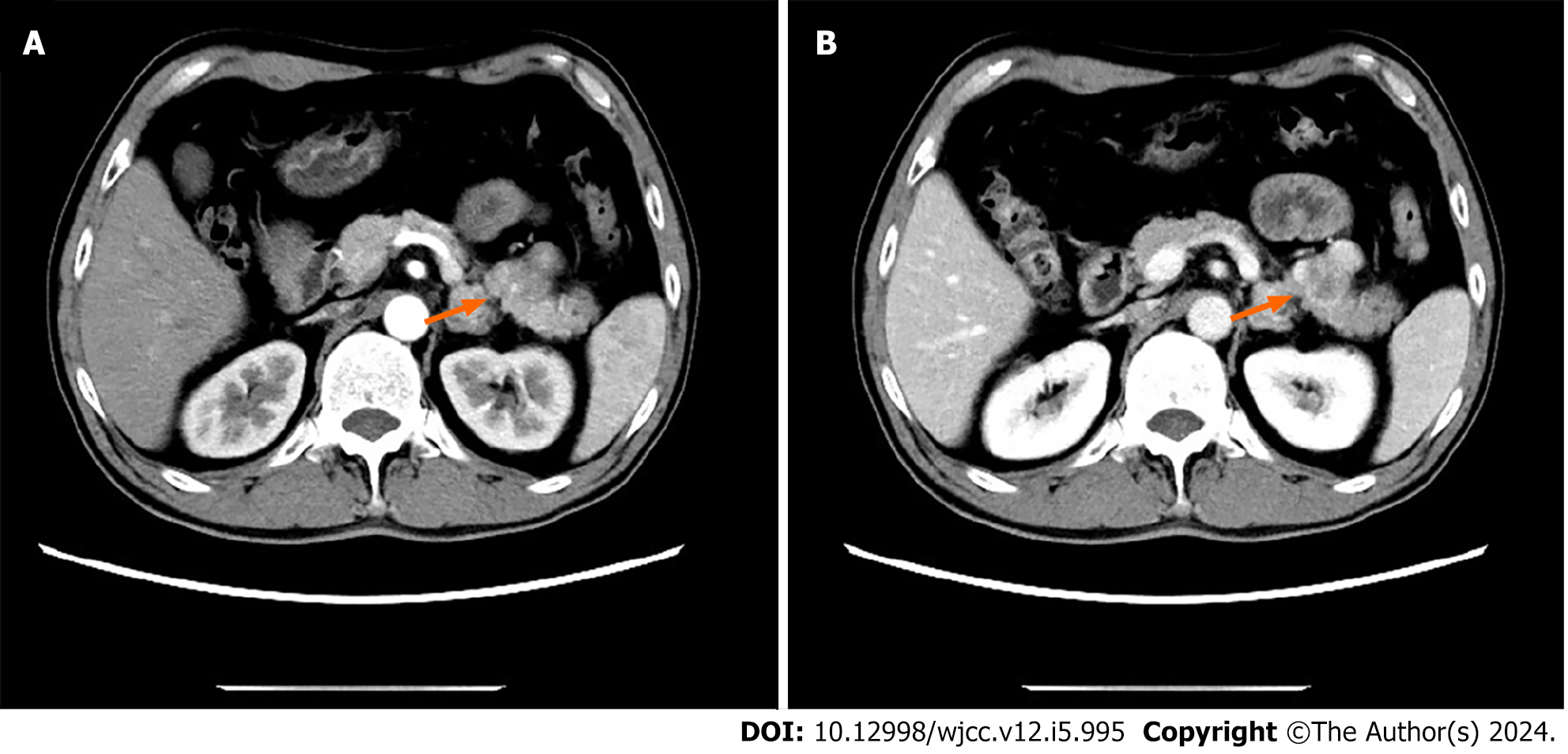
A review of an abdominal enhanced CT showed a tumor of about 3 cm × 2 cm in the tail of the pancreatic body, showing uneven enhancement after enhancement, consistent with a malignant tumor (Figure 1).
A SFT of the pancreas.
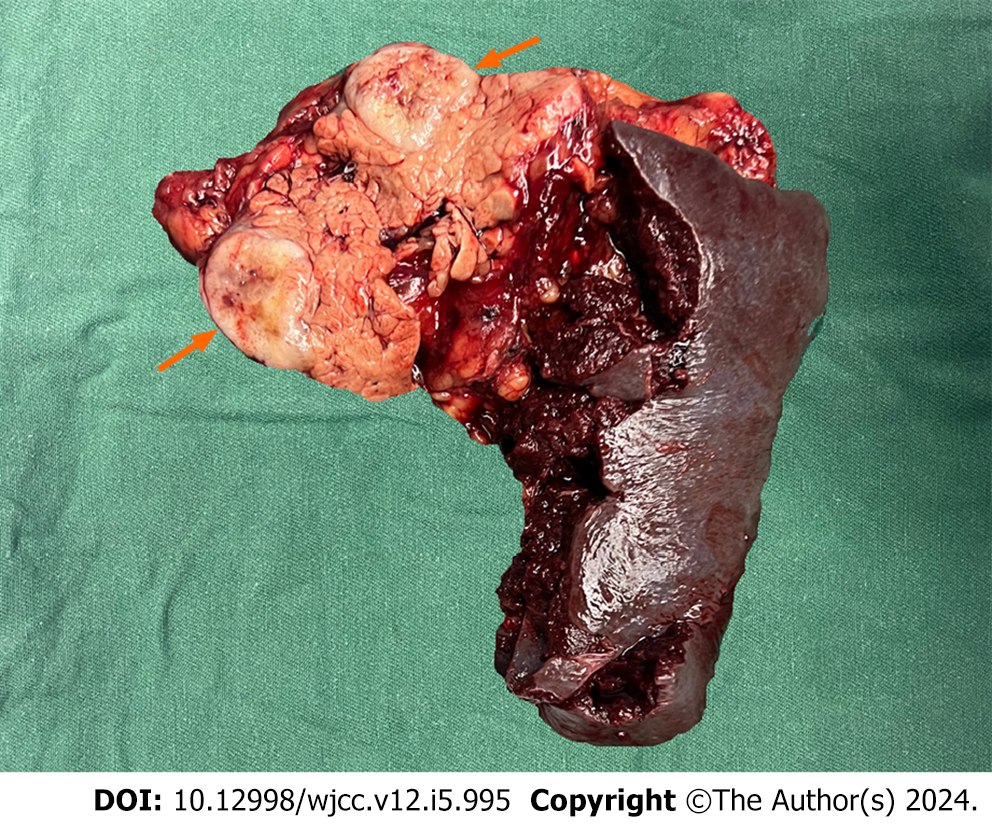
CT revealed a mass with mixed density and inadequate blood supply; these findings were inconsistent with a pancreatic tumor; therefore, we considered a pseudopapillary tumor and a non-functional pancreatic neuroendocrine tumor. We performed an ultrasound endoscopic tissue biopsy. The pathology and immunohistochemistry suggested SFT of the pancreas. After excluding contraindications to surgery and obtaining informed written consent, we performed a laparoscopic distal pancreatectomy with splenectomy. No significant adhesions were seen in the peripancreatic tissue. The pancreatic body was approximately 3 cm × 2 cm (Figure 2). Intraoperative frozen sections showed negative margins. Intraoperative blood loss was 100 mL and no blood transfusion was required.
The patient had no postoperative pancreatic fistula, abdominal infection, or bleeding. Ten days after surgery, he was discharged from the hospital after removing the drainage tube. One month after surgery, the patient returned to the hospital for examination. He did not complain of discomfort. The complete blood count, liver enzymes and renal function were normal.
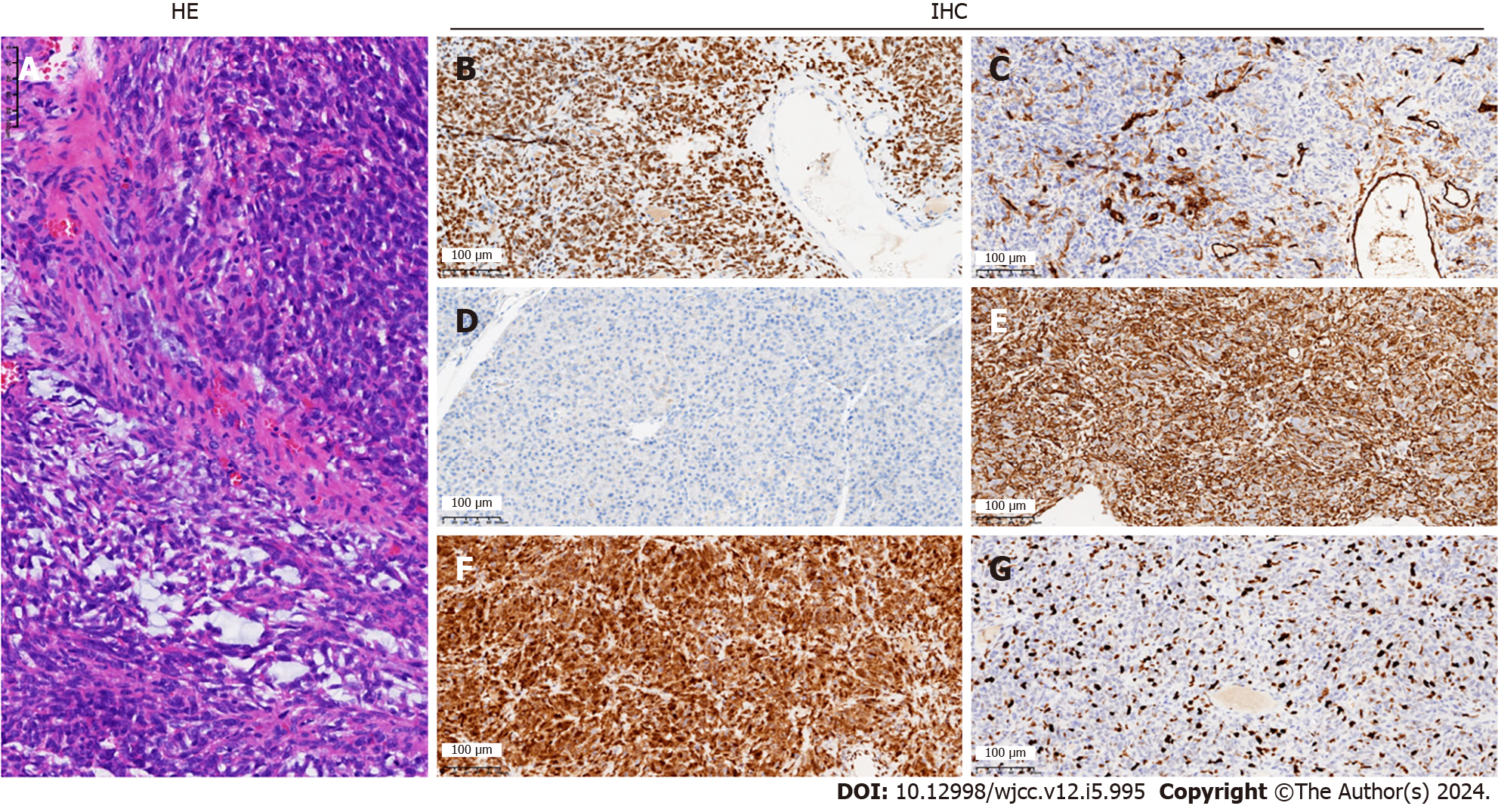
Histopathological and immunohistochemical results of the postoperative specimen suggested an SFT of the pancreas of 3.0 cm × 2.5 cm × 1.0 cm, negative margins, no tumor involvement in the surrounding lymph nodes, and no tumor involvement in the spleen. Markers were as follows: Signal transducer and activator of transcription 6 (STAT6) (+), cluster of differentiation (CD) 34 (+), B cell CLL/lymphoma-2 (Bc1-2) (+), vimentin (+), CD99 (+), CD117 (-), Ki-67 (+40%), discovered on GIST-1 (+), transducin-like enhancer protein 1 (+), S- 100 (-), cytokeratin pan (pan) (-), somatostatin receptor 2 (-) (Figure 3).
No specific treatment was given after the patient was discharged from the hospital, and he had no complaints for three months after the procedure. He returned for regular follow-up. No abnormalities were found on complete blood counts, blood glucose, tumor markers, or CT.
SFT is a mesenchymal tumor comprising less than 2% of soft tissue tumors[36]. About 65% of SFTs originate from the pleura[3]; however, they can also be found in extrapleural areas[6], with only 34 cases reported to date, including the present case (Tables 1 and 2). SFT of the pancreas is extremely rare. We searched PubMed and Google Scholar for pancreatic tumors and SFT and found 34 cases. Of these, 14 (41. 1%) were male, and 20 (58.9%) were female. The mean age was 54. 17 ± 15.4, and the median age was 54; 17 patients had lesions in the pancreatic tumor head [three (17.6%) male and 13 (76.4%) female]. Seventeen had tumors in the tail of the pancreatic body [ten (58.8%) male and seven (41.2%) female]. The mean tumor diameter was 5.2 cm ± 3.8 cm. Of the 34 patients, 12 presented with pain (12/34), 12 were discovered on physical examination (12/34), four presented with jaundice (4/34), one presented with an abdominal mass (1/34), and five were detected by other means (5/34) (Table 1).
| No | Ref. | Age | Sex | Pancreatic site | Symptoms | Size (cm) | Pancreatic surgery |
| 1 | Lüttges et al[1] | 50 | F | Body | Incidental | 55 | DP |
| 2 | Chatti et al[8] | 41 | M | Body | Abdominal pain | 13 | DP |
| 3 | Gardini et al[9] | 62 | F | Head | Abdominal pain | 3 | PD |
| 4 | Miyamoto et al[10] | 41 | F | Head | Abdominal pain | 18 × 15 | Enucleation |
| 5 | Kwon et al[11] | 54 | M | Body | Incidental | 76 × 6 | MS |
| 6 | Srinivasan et al[12] | 78 | F | Body | Back pain, weight loss | 5 | DP |
| 7 | Chetty et al[13] | 67 | F | Head | Incidental | 26 | PD |
| 8 | Ishiwatari et al[14] | 58 | F | Head | Incidental | 3 | PD |
| 9 | Sugawara et al[15] | 55 | F | Head | Incidental | 6 × 4 | PD |
| 10 | Santos et al[16] | 40 | M | Body | Incidental | 3 | Enucleation |
| 11 | Tasdemir et al[17] | 24 | F | Body | Epigastric pain | 11 | Enucleation |
| 12 | van der et al[18] | 67 | F | Head | Abdominal pain | 28 × 16 | Enucleation |
| 13 | Chen et al[19] | 49 | F | Head | Abdominal pain | 13 | PD |
| 14 | Hwang et al[20] | 53 | F | Head | Incidental | 52 × 45 × 40 | PHR |
| 15 | Baxter et al[21] | 58 | F | Head | Abdominal pain | 35 × 3 | LPD |
| 16 | Estrella et al[22] | 52 | F | Head | Jaundice | 15 × 10 × 10 | LPD |
| 17 | Han et al[23] | 77 | F | Head | Jaundice | 15 × 14 | Biopsy |
| 18 | Murakami et al[24] | 82 | M | Body | Hypokalemia hypertension, edema | 6 | DP |
| 19 | Spasevska et al[3] | 47 | M | Head | jaundice | 35 × 2 × 18 | LPD |
| 20 | Paramythiotis et al[7] | 55 | M | Body | Abdominal pain | 31 × 28 | DP |
| 21 | D'Amico FE et al[25] | 52 | M | Body | Incidental | 12 | DP |
| 22 | Oana et al[26] | 73 | M | Head | Abdominal discomfort | 65 × 55 | Enucleation |
| 23 | Sheng et al[27] | 1 | M | Head | Jaundice | 20 | DP |
| 24 | Geng et al[28] | 48 | M | Body | Hypoglycemia | 65 × 5 | DP |
| 25 | Qian et al[29] | 46 | M | Body | Hypoglycemia | 70 × 61 | DP |
| 26 | Rogers et al[30] | 37 | F | Head | Abdominal pain | 23 | PD |
| 27 | Taguchi et al[31] | 60 | M | Head | Palpable mass | 9 × 7 × 7 | PD |
| 28 | Jariwalla et al[32] | 64 | F | Body | Abdominal pain | 19 | DP |
| 29 | Marotti et al[33] | 75 | F | Body | Incidental | 13 | Enucleation |
| 30 | Addeo et al[6] | 59 | M | Body | Incidental | 4 | DP |
| 31 | Rodriguez et al[2] | 48 | F | Body | Abdominal pain | 13 × 10 × 95 | TP |
| 32 | Jones et al[34] | 61 | F | Body | NA | 27 | DP |
| 33 | Liu et al[35] | 54 | F | Head | Incidental | 31 × 23 | LDPPHRt |
| 34 | Present case | 54 | M | Body | Incidental | 3 × 2 | DP |
| No | Ref. | Immunohistochemistry | Outcome | Follow-up |
| 1 | Lüttges et al[1] | CD34, CD99, Bcl-2, vimentin | Alive | 20 months |
| 2 | Chatti et al[8] | CD34, CD99, Bcl-2, vimentin | Death | 3 d |
| 3 | Gardini et al[9] | CD34, CD99, Bcl-2, vimentin, SMA | Alive | 16 months |
| 4 | Miyamoto et al[10] | CD34, Bcl-2 | Alive | 7 months |
| 5 | Kwon et al[11] | CD34, CD99, vimentin | NA | NA |
| 6 | Srinivasan et al[12] | CD34, Bcl-2 | Alive | 7 months |
| 7 | Chetty et al[13] | CD34, CD99, Bcl-2 | 42 mo | 6 v |
| 8 | Ishiwatari et al[14] | CD34, Bcl-2 | Alive | 42 months |
| 9 | Sugawara et al[15] | CD34 | NA | NA |
| 10 | Santos et al[16] | CD34, betacatenin | NA | NA |
| 11 | Tasdemir et al[17] | CD34, Bcl-2, beta-catenin, vimentin, Ki67 < 2% | Alive | 3 months |
| 12 | van der et al[18] | CD34, CD99, Bcl-2 | NA | NA |
| 13 | Chen et al[19] | CD34, Bcl-2, vimentin, CD68, muscle-specific actin | Alive | 30 months |
| 14 | Hwang et al[20] | CD34, Bcl-2, muscle-specific actin, CD10, ER, PR | Alive | 30 months |
| 15 | Baxter et al[21] | CD34, Bcl-2 | NA | NA |
| 16 | Estrella et al[22] | CD34, Bcl-2, keratin (rare), p16, p53 | Alive | 40 months |
| 17 | Han et al[23] | CD34, CD99 | No progression | 10 months |
| 18 | Murakami et al[24] | STAT6, CD34, Bcl-2, ACTH, POMC, NSE | Death | 4 months |
| 19 | Spasevska et al[3] | CD34, vimentin, CD99, Bcl-2, nuclear betacatenin | Death | 1 wk |
| 20 | Paramythiotis et al[7] | CD34, CD99, Bcl-2 vimentin, S-100 | Alive | 40 months |
| 21 | D'Amico FE et al[25] | STAT6, CD34 | Alive | 24 months |
| 22 | Oana et al[26] | CD34, Bcl-2 | Alive | 36 months |
| 23 | Sheng et al[27] | CD34, vimentin, SMA, Ki67 < 3% | Alive | 12 months |
| 24 | Geng et al[28] | STAT6, CD34, Bcl-2, CD31, PHH-3, D2-40, Ki67 > 10% | Alive | 6 months |
| 25 | Qian et al[29] | STAT6, CD34, Bcl-2, Ki67 10% | Alive | 10 months |
| 26 | Rogers et al[30] | STAT6, CD34, Bcl-2, CD99 | Alive | 4 months |
| 27 | Taguchi et al[31] | STAT6, CD34, Bcl-2, vimentin, cytokeratin AE1/AE3 | Alive | 12 months |
| 28 | Jariwalla et al[32] | STAT6, CD34 | NA | NA |
| 29 | Marotti et al[33] | STAT6, CD34 | Alive | 6 months |
| 30 | Addeo et al[6] | STAT6, CD34, Bcl-2, Ki67 7% | NA | NA |
| 31 | Rodriguez et al[2] | STAT6 | Alive | 12 months |
| 32 | Jones et al[34] | STAT6, CD34 | Alive | 1 months |
| 33 | Liu et al[35] | CD34, STAT6, CD99 | Alive | 6 months |
| 34 | Present case | TAT6, CD34, Bc1-2, Vimentin, CD99, Ki67 40% | Alive | 3 months |
Most SFTs of the pancreas are detected by physical examination; clinical signs and symptoms include abdominal pain and jaundice. Because these are not typical symptoms, it is challenging to differentiate SFT from other pancreatic diseases. Histopathology and immunohistochemistry are the gold standards for diagnosis. We recommend ultrasound endoscopic aspiration biopsy for space-occupying pancreatic lesions that cannot be diagnosed on imaging.
Our preoperative diagnosis relied on ultrasound endoscopic puncture biopsy in the present case. The preoperative and postoperative pathological histological examination and immunohistochemistry were consistent with SFT of the pancreas with no tumor involvement in the peripheral lymph nodes, no tumor involvement in the incised margin of the pancreas, and no tumor involvement in the spleen.
The immunohistochemical differential diagnosis of SFT of the pancreas should include spindle cell tumors such as gastrointestinal stromal tumor (GIST), smooth muscle sarcoma, nerve sheath tumor, fibrous mucinous sarcoma, perivascular epithelioid cell tumor, and vascular tumors[3,16,20,37]. The immunomarkers of SFT of the pancreas include STAT6, CD34, bc1-2, vimentin, and CD99[34]. These features help to distinguish SFT from other mesenchymal tumors[34,37]. SFT expresses CD34 and vimentin in 80%-90% of cases and CD99 and bcl-2 in 70%. SFTs are usually negative for c-kit (CD117), smooth muscle actin, junctional protein, S-100 protein, and cytokeratin (markers for GIST, smooth muscle sarcoma, nerve sheath tumor, and fibrous mucinous sarcoma, respectively) are negative[3]. NAB2-STAT6 fusion is a driver mutation in SFT, where transcriptional repressors of the cytokinesis pathway are converted into transcriptional activators[31,38,39]. STAT6 has a sensitivity of 98% and a specificity of 85% for SFT and is therefore considered the most characteristic SFT marker[40,41]. In our case, the tumor was positive for STAT6, while CD34, bc1-2, vimentin, and CD99 were positive.
In this case, CT showed no enhancement in the arterial phase and heterogeneous enhancement in the venous area. We believe that it should be distinguished from neuroendocrine tumors, which show enhanced CT from the arterial phase to the portal venous phase[13,37], which makes it difficult for us to distinguish the disease, so many scholars before us also misdiagnosed it before surgery[1,10,11,13,26]. At the same time, we believe that it should also be differentiated from pancreatic cancer and solid pseudopapillary tumors of the pancreas. The imaging features of this tumor have been described in detail in our previous work on pancreatic tumors[42].
Most SFTs are benign[43], and malignant SFTs account for 10%-15%[30,39,44,45]. The histopathological features of malignant SFT: (1) Hypercellularity; (2) more than four mitotic figures per ten high-power fields; (3) nuclear pleo
Because of the non-specific clinical symptoms and radiological features of SFT of the pancreas, the diagnosis is challenging with preoperative radiological and laboratory examinations alone. A definitive diagnosis relies on histopathology and immunohistochemistry. In cases where the tumor is found in the pancreas, and the diagnosis cannot be confirmed, it is recommended to obtain histopathology with ultrasound aspiration. As this presentation is rarely reported, there is a lack of uniform treatment criteria, and surgery is effective. However, the tumor may lead to potential recurrence or metastasis; therefore, long-term follow-up is recommended.
We thank the patient's family members for providing detailed treatment information and Dr. Kevin Li for revising the language.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Triantopoulou C, Greece S-Editor: Qu XL L-Editor: A P-Editor: Yu HG
| 1. | Lüttges J, Mentzel T, Hübner G, Klöppel G. Solitary fibrous tumour of the pancreas: a new member of the small group of mesenchymal pancreatic tumours. Virchows Arch. 1999;435:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Rodríguez AH, Martino MD, Mazeyra MV, Martín-Pérez E. Solitary fibrous tumor of the pancreas. Autops Case Rep. 2021;11:e2021245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Spasevska L, Janevska V, Janevski V, Noveska B, Zhivadinovik J. Solitary Fibrous Tumor of the Pancreas: A Case Report and Review of the Literature. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2016;37:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Xie GY, Zhu HB, Jin Y, Li BZ, Yu YQ, Li JT. Solitary fibrous tumor of the liver: A case report and review of the literature. World J Clin Cases. 2022;10:7097-7104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 5. | Afzal A, Maldonado-Vital M, Khan S, Farooque U, Luo W. Solitary Fibrous Tumor of Pancreas With Unusual Features: A Case Report. Cureus. 2020;12:e10833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Addeo P, Averous G, Bachellier P. Solitary Fibrous Tumor of the Pancreas. J Gastrointest Surg. 2021;25:569-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Paramythiotis D, Kofina K, Bangeas P, Tsiompanou F, Karayannopoulou G, Basdanis G. Solitary fibrous tumor of the pancreas: Case report and review of the literature. World J Gastrointest Surg. 2016;8:461-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Chatti K, Nouira K, Ben Reguigua M, Bedioui H, Oueslati S, Laabidi B, Alaya M, Ben Abdallah N. [Solitary fibrous tumor of the pancreas. A case report]. Gastroenterol Clin Biol. 2006;30:317-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Gardini A, Dubini A, Saragoni L, Padovani F, Garcea D. [Benign solitary fibrous tumor of the pancreas: a rare location of extra-pleural fibrous tumor. Single case report and review of the literature]. Pathologica. 2007;99:15-18. [PubMed] |
| 10. | Miyamoto H, Molena DA, Schoeniger LO, Haodong Xu. Solitary fibrous tumor of the pancreas: a case report. Int J Surg Pathol. 2007;15:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Kwon HJ, Byun JH, Kang J, Park SH, Lee MG. Solitary fibrous tumor of the pancreas: imaging findings. Korean J Radiol. 2008;9 Suppl:S48-S51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Srinivasan VD, Wayne JD, Rao MS, Zynger DL. Solitary fibrous tumor of the pancreas: case report with cytologic and surgical pathology correlation and review of the literature. JOP. 2008;9:526-530. [PubMed] |
| 13. | Chetty R, Jain R, Serra S. Solitary fibrous tumor of the pancreas. Ann Diagn Pathol. 2009;13:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Ishiwatari H, Hayashi T, Yoshida M, Kuroiwa G, Sato Y, Kobune M, Takimoto R, Kimura Y, Hasegawa T, Hirata K, Kato J. [A case of solitary fibrous tumor of the pancreas]. Nihon Shokakibyo Gakkai Zasshi. 2009;106:1078-1085. [PubMed] |
| 15. | Sugawara Y, Sakai S, Aono S, Takahashi T, Inoue T, Ohta K, Tanada M, Teramoto N. Solitary fibrous tumor of the pancreas. Jpn J Radiol. 2010;28:479-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Santos LA, Santos VM, Oliveira OC, De Marco M. Solitary fibrous tumour of the pancreas: a case report. An Sist Sanit Navar. 2012;35:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Tasdemir A, Soyuer I, Yurci A, Karahanli I, Akyildiz H. A huge solitary fibrous tumor localized in the pancreas: a young women. JOP. 2012;13:304-307. [PubMed] |
| 18. | van der Vorst JR, Vahrmeijer AL, Hutteman M, Bosse T, Smit VT, van de Velde CJ, Frangioni JV, Bonsing BA. Near-infrared fluorescence imaging of a solitary fibrous tumor of the pancreas using methylene blue. World J Gastrointest Surg. 2012;4:180-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Chen JW, Lü T, Liu HB, Tong SX, Ai ZL, Suo T, Ji Y. A solitary fibrous tumor in the pancreas. Chin Med J (Engl). 2013;126:1388-1389. [PubMed] |
| 20. | Hwang JD, Kim JW, Chang JC. Imaging Findings of a Solitary Fibrous Tumor in Pancreas: A Case Report. J Korean Soc Radiol. 2014;70. [DOI] [Full Text] |
| 21. | Baxter AR, Newman E, Hajdu CH. Solitary fibrous tumor of the pancreas. J Surg Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Estrella JS, Wang H, Bhosale PR, Evans HL, Abraham SC. Malignant Solitary Fibrous Tumor of the Pancreas. Pancreas. 2015;44:988-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Han SH, Baek YH, Han SY, Lee SW, Jeong JS, Cho JH, Kwon HJ. Solitary Fibrous Tumor of the Pancreas: A Case Report and Review of the Literature. Korean J Med. 2015;88. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Murakami K, Nakamura Y, Felizola SJ, Morimoto R, Satoh F, Takanami K, Katakami H, Hirota S, Takeda Y, Meguro-Horike M, Horike S, Unno M, Sasano H. Pancreatic solitary fibrous tumor causing ectopic adrenocorticotropic hormone syndrome. Mol Cell Endocrinol. 2016;436:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | D'Amico FE, Ruffolo C, Romano M, DI Domenico M, Sbaraglia M, Dei Tos AP, Garofalo T, Giordano A, Bassi I, Massani M. Rare Neoplasm Mimicking Neuoroendocrine Pancreatic Tumor: A Case Report of Solitary Fibrous Tumor with Review of the Literature. Anticancer Res. 2017;37:3093-3097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Oana S, Matsuda N, Sibata S, Ishida K, Sugai T, Matsumoto T. A case of a "wandering" mobile solitary fibrous tumor occurring in the pancreas. Clin J Gastroenterol. 2017;10:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Sheng Q, Xu W, Liu J, Shen B, Deng X, Wu Y, Wu W, Yu S, Wang X, Lv Z. Pancreatic solitary fibrous tumor in a toddler managed by pancreaticoduodenectomy: a case report and review of the literature. Onco Targets Ther. 2017;10:1853-1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Geng H, Ye Y, Jin Y, Li BZ, Yu YQ, Feng YY, Li JT. Malignant solitary fibrous tumor of the pancreas with systemic metastasis: A case report and review of the literature. World J Clin Cases. 2020;8:343-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Qian X, Zhou D, Gao B, Wang W. Metastatic solitary fibrous tumor of the pancreas in a patient with Doege-Potter syndrome. Hepatobiliary Surg Nutr. 2020;9:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Rogers C, Samore W, Pitman MB, Chebib I. Solitary fibrous tumor involving the pancreas: report of the cytologic features and first report of a primary pancreatic solitary fibrous tumor diagnosed by fine-needle aspiration biopsy. J Am Soc Cytopathol. 2020;9:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Taguchi Y, Hara T, Tamura H, Ogiku M, Watahiki M, Takagi T, Harada T, Miyazaki S, Hayashi T, Kanai T, Mori H, Ozawa T, Nishiwaki Y. Malignant solitary fibrous tumor of the pancreas: a case report. Surg Case Rep. 2020;6:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Jariwalla NR, Park N, El Hage Chehade N, Truong A, Choi AY, Samarasena J. Solitary Fibrous Tumor of the Pancreas: Really? 2021; 116: S686. [DOI] [Full Text] |
| 33. | Marotti JD, Liu X, Jamot S, Gardner TB, Gordon SR, Kerr DA. Solitary fibrous tumor of the pancreas clinically mimicking a pancreatic neuroendocrine tumor: Cytologic pitfalls when a transgastric approach is utilized. Diagn Cytopathol. 2021;49:E405-E409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Jones VM, Wangsiricharoen S, Cornea V, Bocklage TJ, Ali SZ, Allison DB. Cytopathological characteristics of solitary fibrous tumour involving the pancreas by fine needle aspiration: Making an accurate preoperative diagnosis in an uncommon location. Cytopathology. 2022;33:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Liu W, Wu S, Cai Y, Peng B. Total laparoscopic duodenum-preserving pancreatic head resection for solitary fibrous tumor: The first case report. Asian J Surg. 2022;45:651-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 36. | Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ, Brennan MF, Coit DG. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94:1057-1068. [PubMed] |
| 37. | Yamashita H, Fujino Y, Ohara T, Kakinoki K, Sugimoto T, Kajimoto K, Tominaga M. A rare case of metastatic solitary fibrous tumor of the pancreas manifesting as a cystic neoplasm: a case report. Surg Case Rep. 2019;5:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Thway K, Ng W, Noujaim J, Jones RL, Fisher C. The Current Status of Solitary Fibrous Tumor: Diagnostic Features, Variants, and Genetics. Int J Surg Pathol. 2016;24:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 39. | Li J, Li J, Xiong Y, Xu T, Xu J, Li Q, Yang G. Atypical/malignant solitary fibrous tumor of the pancreas with spleen vein invasion: Case report and literature review. Medicine (Baltimore). 2020;99:e19783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Krsková L, Odintsov I, Fabián O, Hroudová P, Mrhalová M. Determination of biological behavior of solitary fibrous tumors: correlation of expression of Ki-67, TPX2 and TERT mRNA subunit level and NAB2-STAT6 fusion compared to morphological aspects of SFTs. Neoplasma. 2022;69:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 41. | Yoshida A, Tsuta K, Ohno M, Yoshida M, Narita Y, Kawai A, Asamura H, Kushima R. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol. 2014;38:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 42. | Wu X, Zhou S, Zhou X, Xu X, Wang L, Ruan Y, Lu J, Li H, Xu H, Ma X. Literature review of imaging, pathological diagnosis, and outcomes of metachronous lung and pancreatic metastasis of cecal cancer. World J Surg Oncol. 2022;20:341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 43. | Zambo I, Veselý K. [WHO classification of tumours of soft tissue and bone 2013: the main changes compared to the 3rd edition]. Cesk Patol. 2014;50:64-70. [PubMed] |
| 44. | Demicco EG, Wagner MJ, Maki RG, Gupta V, Iofin I, Lazar AJ, Wang WL. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol. 2017;30:1433-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 266] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 45. | Folpe AL, Devaney K, Weiss SW. Lipomatous hemangiopericytoma: a rare variant of hemangiopericytoma that may be confused with liposarcoma. Am J Surg Pathol. 1999;23:1201-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Sikri V, Chawla R. Solitary fibrous tumour of the pleura. Indian J Chest Dis Allied Sci. 2013;55:167-169. [PubMed] |
| 47. | Robinson LA. Solitary fibrous tumor of the pleura. Cancer Control. 2006;13:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 219] [Article Influence: 27.4] [Reference Citation Analysis (0)] |