Published online Feb 16, 2024. doi: 10.12998/wjcc.v12.i5.942
Peer-review started: November 2, 2023
First decision: December 5, 2023
Revised: December 14, 2023
Accepted: January 23, 2024
Article in press: January 23, 2024
Published online: February 16, 2024
Processing time: 89 Days and 14.9 Hours
Obstructive sleep apnea-hypopnea syndrome (OSAHS) is primarily caused by airway obstruction due to narrowing and blockage in the nasal and nasopha
To investigate the clinical efficacy and mechanisms of a mid-frequency anti-snoring device in treating moderate OSAHS.
We selected 50 patients diagnosed with moderate OSAHS in our hospital between July 2022 and August 2023. They underwent a 4-wk treatment regimen involving the mid-frequency anti-snoring device during nighttime sleep. Following the treatment, we monitored and assessed the sleep apnea quality of life index and Epworth Sleepiness Scale scores. Additionally, we performed computed tomo
Compared to pretreatment measurements, patients exhibited a significant reduction in the apnea-hypopnea index, the percentage of time with oxygen saturation below 90%, snoring frequency, and the duration of the most prolonged apnea event. The lowest oxygen saturation showed a notable increase, and both sleep apnea quality of life index and Epworth Sleepiness Scale scores improved. Oropharyngeal computed tomography scans revealed that in OSAHS patients cross-sectional areas of the oropharyngeal airway in the soft palate posterior area and retrolingual area decreased during snoring compared to the awake state. Conversely, during mid-frequency anti-snoring device treatment, these areas increased compared to snoring.
The mid-frequency anti-snoring device demonstrates the potential to enhance various sleep parameters in patients with moderate OSAHS, thereby improving their quality of life and reducing daytime sleepiness. These therapeutic effects are attributed to the device’s ability to ameliorate the narrowing of the oropharynx in OSAHS patients.
Core Tip: We investigated the clinical efficacy and underlying mechanisms of a mid-frequency anti-snoring device in treating 50 moderate obstructive sleep apnea-hypopnea syndrome patients for 4 wk. Our results indicated significant improvements in the apnea-hypopnea index, the percentage of time with oxygen saturation below 90%, and the sleep apnea quality of life index and Epworth Sleepiness Scale scores. Additionally, we found compelling evidence that the mid-frequency anti-snoring device positively influenced the narrowing of the oropharynx in sleep apnea-hypopnea syndrome patients during snoring.
- Citation: Qian B, Chen ZJ, Wang YS, Hu XY, Hu XB, Zheng YH. Clinical efficacy and mechanism study of mid-frequency anti-snoring device in treating moderate obstructive sleep apnea-hypopnea syndrome. World J Clin Cases 2024; 12(5): 942-950
- URL: https://www.wjgnet.com/2307-8960/full/v12/i5/942.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i5.942
Obstructive sleep apnea-hypopnea syndrome (OSAHS) is primarily caused by airway obstruction due to narrowing and blockage in the nasal and nasopharyngeal, oropharyngeal, soft palate, and tongue base areas. Its main clinical manifestations include snoring and associated breathing pauses during nighttime sleep. Most patients experience dry mouth upon waking in the morning, and some may also have symptoms such as headaches, daytime sleepiness, fatigue, lack of concentration, etc[1].
Transcutaneous electrical stimulation of the genioglossus muscle is a non-invasive treatment method for OSAHS [2]. Recently, a mid-frequency anti-snoring device has been developed that delivers specific pulse-modulated compound waves to provide intermittent electrical stimulation to the genioglossus muscle and its sublingual nerve branches. This leads to a responsive contraction of the genioglossus muscle, an increase in upper airway tension, a reduction in the severity of oropharyngeal upper airway collapse, and the maintenance of an open upper airway, ultimately terminating snoring. This device is beneficial for improving the quality of sleep in OSAHS patients.
This study focused on patients with moderate OSAHS and investigated whether the treatment with a mid-frequency anti-snoring device can improve various sleep parameters in OSAHS patients and enhance their quality of daily life. Additionally, it explores whether the airways in the oropharyngeal region expand under the stimulation of mid-frequency waves. Currently, there is a lack of imaging evidence in this regard. Therefore, this study conducted oropharyngeal computed tomography (CT) scans in OSAHS patients in awake, snoring, and mid-frequency anti-snoring device treatment states to measure the cross-sectional area at the narrowest points in the soft palate posterior and retrolingual areas. This approach aimed to provide imaging-based confirmation of the working mechanism of this technology in treating OSAHS.
This study was conducted on 50 patients diagnosed with moderate OSAHS in our hospital from July 2022 to August 2023. The primary patient information is shown in Table 1. Upon diagnosis, patients began wearing a mid-frequency anti-snoring device during nighttime sleep. Before treatment, the study’s purpose and relevant precautions were explained to the patients. Upon the patients’ understanding and agreement, informed consent forms were signed.
| Variable | Data |
| Male/female | 35/15 |
| Age in yr | 38.5 ± 6.8 |
| Height in cm | 170.3 ± 10.8 |
| Weight in kg | 89.7 ± 15.6 |
| BMI in kg/m2 | 29.5 ± 3.4 |
| Systolic blood pressure in mmHg | 122.3 ± 11.4 |
| Diastolic blood pressure in mmHg | 71.6 ± 10.2 |
Inclusion criteria: (1) An apnea-hypopnea index (AHI) ≥ 30 events per hour during 7 h of sleep at night or an AHI ≥ 5 events per hour. The criterion for moderate OSAHS was an AHI index of 15-30 events per hour; (2) Voluntary participation and signed informed consent; (3) Complete clinical data; and (4) Good patient compliance and the ability to fully participate in the entire study.
Exclusion criteria: (1) Patients with mild or severe OSAHS; (2) Patients who have recently experienced acute illness and received related treatments; (3) Patients with severe cardiovascular or cerebrovascular diseases or multiorgan dysfunction; (4) Patients with mental illnesses or cognitive communication disorders; (5) Patients who have participated in other clinical studies in the past 3 months; and (6) Contraindications for the mid-frequency anti-snoring device, including acute purulent inflammation in the submental region, bleeding tendency, malignant tumors, severe heart diseases, severe cardiopulmonary diseases, airway obstruction due to nasal disorders, the presence of implantable devices such as pacemakers, skin allergies, and inability to express self-awareness, such as infants.
Sleep monitoring was conducted using a Sleep-Disordered Breathing Analyzer (SOMNOcheck micro, Weinmann, Germany). The first monitoring was performed before the mid-frequency anti-snoring device treatment to establish the diagnosis and assess severity. Subsequently, the mid-frequency anti-snoring device was used during nighttime sleep for 4 wk. The second sleep monitoring was conducted after the treatment and compared with the data from the first monitoring. Key sleep monitoring parameters included the AHI, lowest pulse oxygen saturation (LSPO2), and snoring percentage (the percentage of time spent snoring during sleep).
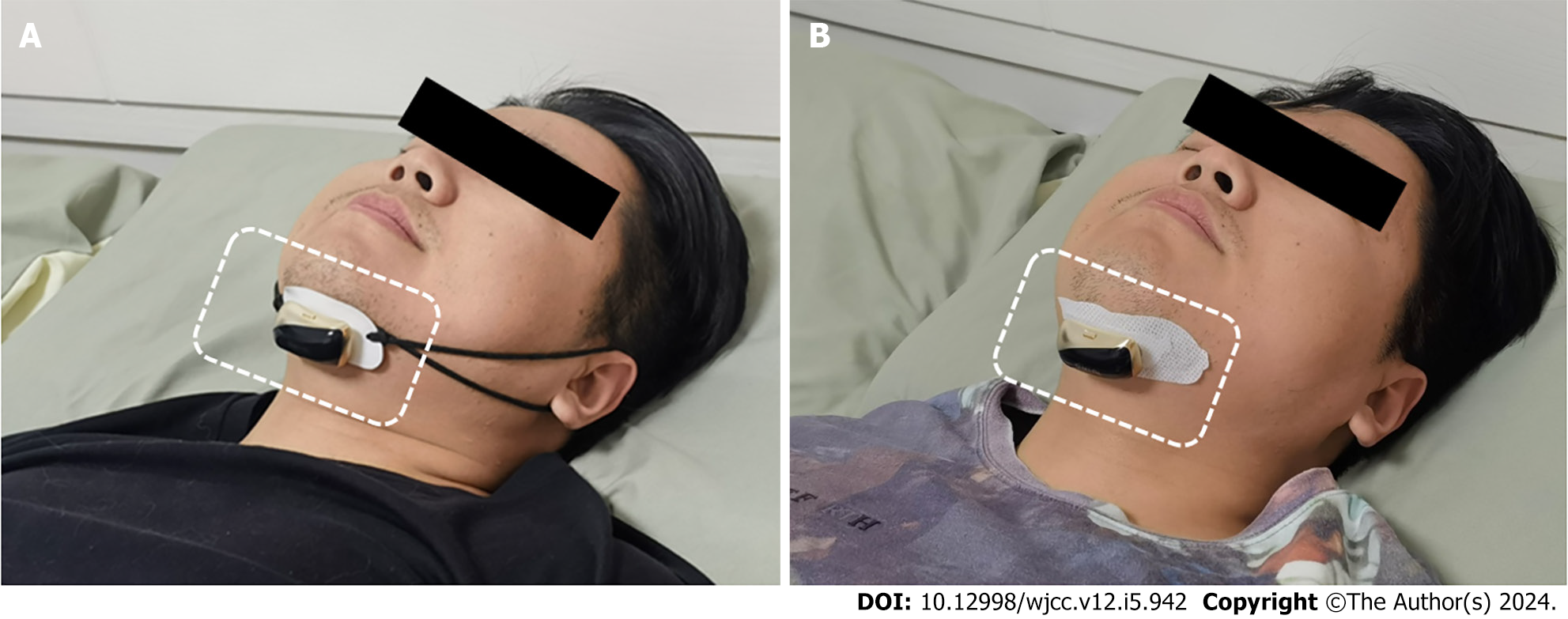
Treatment was performed using a mid-frequency anti-snoring device (JLY-Y3 or JLY-Z3, Taizhou Jinliyou Medical Technology Co. Ltd.). The device parameters included a rated voltage of D.C 3.7 V, a power of 0.1 W, a modulation waveform of bidirectional symmetric trapezoidal waves, and a frequency of 20 KHz. The treatment process involved fixing the anti-snoring device to the lower jaw using either the ear-worn (Figure 1A) or patch (Figure 1B) method. After wearing the device, it was powered on by pressing and holding the device’s switch for approximately 3 s. The power indicator would blink to confirm a successful startup. Patients would lie flat for sleep, and the anti-snoring device would automatically operate. If snoring or breathing pauses were detected, it would provide mid-frequency electrical pulses for intervention.
To better detect upper airway obstruction or narrowing in patients, overnight upper airway CT scans were conducted while they were sleeping. The United Imaging uCT760-64 slice spiral CT scanner and its matching workstation were used. Patients were placed supine, with their eyes closed, and proper positioning was ensured. The scanning range extended from the top of the maxillary sinus to 5 cm below the hyoid bone. The scanning parameters were set at 125 KV, 200 mA, a scanning pitch of 1.5, a slice thickness of 5.0 mm, and an interlayer spacing of 5.0 mm. Reconstructed images included coronal and sagittal multiplanar reformation images and three-dimensional maximum intensity projection images. The narrowest cross-sectional area of the upper airway (soft palate and retrolingual airway) was measured three times using multi-slice spiral CT 3D software. Measurements were taken with a fixed window width and window level. Upper airway CT scans were conducted 1 wk after mid-frequency anti-snoring device treatment. Patients wore the device and laid flat on the CT examination bed. The first upper airway CT scan was performed while the patient was awake. The second scan was performed when the patient started snoring, with the device switched off, and the third scan was completed when snoring ceased with the device switched on.
The study protocol was approved by the medical ethics committee of Shanghai Jinshan Tinglin Hospital for research ethics. Each patient was informed of the nature of the study and signed an informed consent form.
SPSS 22.0 software was used for analysis. Quantitative data were represented as (mean ± SEM). Self-matching paired t-tests were employed, with P < 0.05 considered statistically significant.
After 4 wk of treatment with the mid-frequency anti-snoring device, the patient’s sleep monitoring parameters, including AHI, snoring percentage, the most prolonged apnea duration(s), and the duration of SPO2 < 90%, decreased compared to before treatment (P < 0.05). LSPO2 increased compared to before treatment (P < 0.05). Various indicators of the autonomous arousal index (AAI resp) improved, with AAI resp decreased compared to before treatment (P < 0.05). AAI non-resp also decreased compared to before treatment (P < 0.05), as did respiratory effort-related arousals (P < 0.05), as shown in Table 2.
| Parameter | Before treatment | After treatment | t value | P value |
| AHI, % | 24.38 ± 9.16 | 12.63 ± 8.27 | 7.47 | < 0.05 |
| LSPO2, % | 72.62 ± 9.53 | 84.84 ± 8.71 | 4.32 | < 0.05 |
| SPO2 < 90%, % | 14.65 ± 7.26 | 10.33 ± 5.32 | 3.15 | < 0.05 |
| Snoring, % | 44.54 ± 26.13 | 15.07 ± 9.25 | 3.37 | < 0.05 |
| Most prolonged apnea duration in s | 56.08 ± 20.11 | 25.58 ± 11.27 | 4.21 | < 0.05 |
| AAI resp as events/h | 19.89 ± 11.61 | 11.74 ± 6.39 | 5.01 | < 0.05 |
| AAI non resp as events/h | 15.71 ± 7.18 | 9.37 ± 4.27 | 6.11 | < 0.05 |
| RERA as events/h | 5.91 ± 3.99 | 2.61 ± 2.65 | 5.69 | < 0.05 |
After 4 wk of treatment with the mid-frequency anti-snoring device, patients showed improvements in sleep apnea quality of life index scale scores, including social activity, emotional state, and symptom scores, all of which increased compared to before treatment. Epworth Sleepiness Scale scores decreased, and these differences were statistically significant (P < 0.05), as shown in Table 3.
| Index | Before treatment | After treatment | t value | P value |
| Daily life activity rating | 3.8 ± 0.9 | 5.4 ± 1.3 | 5.67 | < 0.05 |
| Social activity rating | 3.7 ± 1.0 | 4.8 ± 0.7 | 5.92 | < 0.05 |
| Emotional state score | 4.6 ± 1.1 | 5.3 ± 1.2 | 3.15 | < 0.05 |
| Symptom score | 4.4 ± 1.2 | 5.2 ± 0.9 | 3.37 | < 0.05 |
| SAQLI scale score | 4.2 ± 0.5 | 4.8 ± 0.4 | 8.94 | < 0.05 |
| ESS scale score | 14.8 ± 5.7 | 10.2 ± 4.3 | 3.31 | < 0.05 |
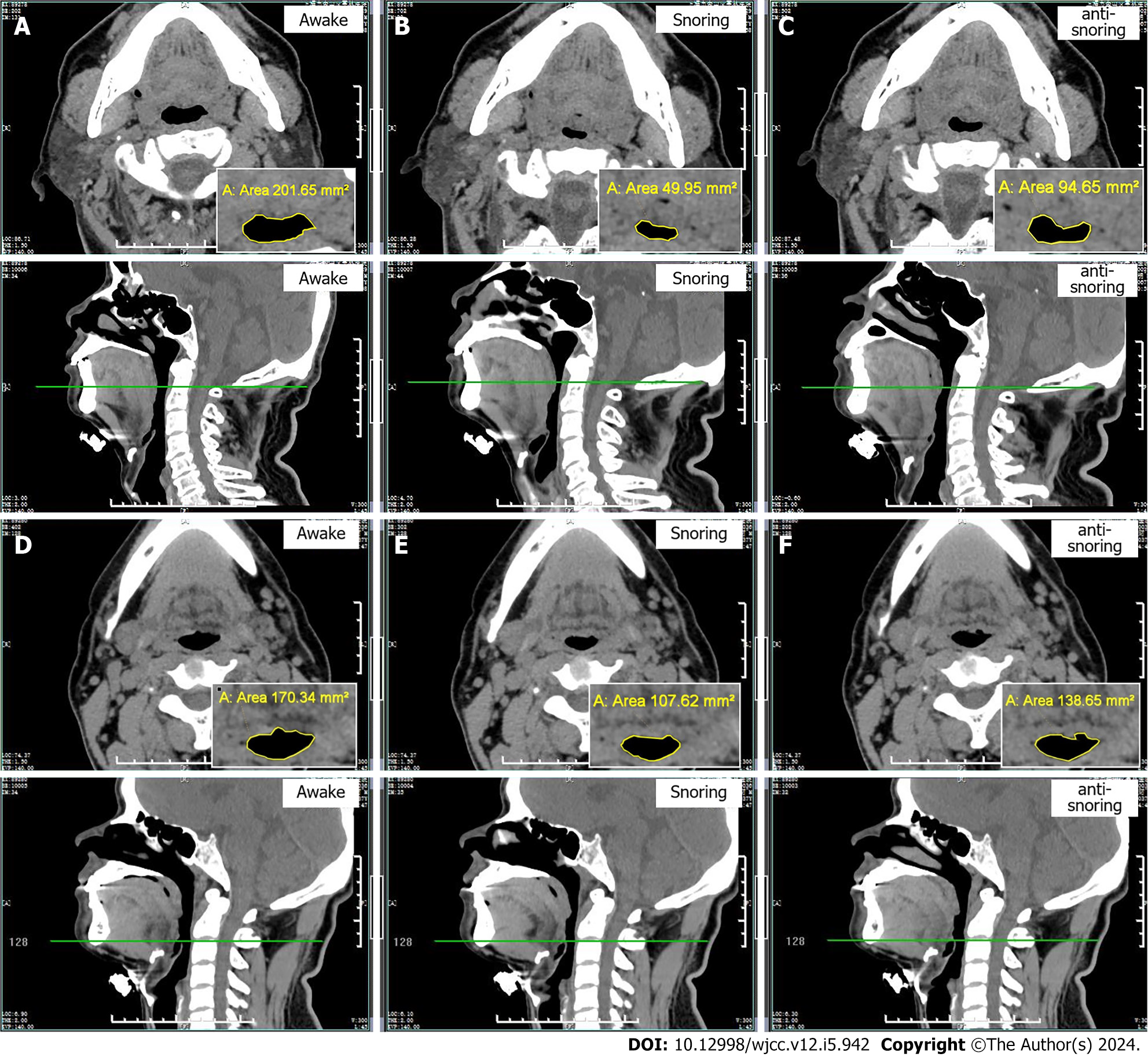
In this study, we selected the narrowest points of the oropharyngeal airway in the soft palate posterior area and retrolingual area of OSAHS patients as the measurement points. These two areas were relatively stable during CT scanning and less susceptible to external interference. In the awake, snoring, and mid-frequency anti-snoring device treatment states, the cross-sectional areas of the narrowest part of the soft palate airway in OSAHS patients were 80.47 ± 18.56 mm², 22.05 ± 16.47 mm², and 71.61 ± 17.38 mm², respectively.
Compared to the awake state, OSAHS patients had a reduction in the cross-sectional areas of the soft palate posterior area and retrolingual area during snoring (P < 0.05), as shown in Table 4 and Figure 2A-C. The cross-sectional areas of the narrowest part of the retrolingual airway in OSAHS patients were 155.36 ± 13.29 mm², 120.45 ± 12.23 mm², and 150.61 ± 12.35 mm² in the awake, snoring, and mid-frequency anti-snoring device treatment states, respectively. Compared to snoring, OSAHS patients experienced an increase in the cross-sectional areas of the soft palate and retrolingual airways during treatment with the mid-frequency anti-snoring device (P < 0.05), as shown in Table 4 and Figure 2D-F.
With the advancement of medical diagnostic technology and increased awareness of personal health, the incidence of OSAHS in the population has increased. For example, a recent study found that out of 38000 Russian citizens (aged 30-70 years), 48.9% suffered from an AHI ≥ 5, 18.1% from an AHI ≥ 15, and 4.5% from an AHI ≥ 30[3]. OSAHS significantly impacts people’s safety, quality of life, social interactions, and relationships with family members. As such, it requires active intervention and treatment[4]. The pathological mechanism of OSAHS lies in the obstruction and collapse of the upper airway during sleep. The obstruction can occur in the nasopharynx, oropharynx, or hypopharynx, with over 80% of patients experiencing combined oropharyngeal obstruction[5]. Therefore, the core of OSAHS treatment is to relieve narrowing in the oropharyngeal region and keep the airway open.
Currently, OSAHS treatment includes general therapy, medication, devices, surgery, and rehabilitation[6]. Each treatment method has its advantages and limitations, and the treatment choice should be based on the patient’s specific condition. Hypoglossal nerve stimulation is one effective method for treating OSAHS, where a nerve stimulator is implanted under the patient’s skin in the neck and is connected to a sublingual nerve[7]. It stimulates the sublingual nerve to generate nerve signals synchronized with inhalation, helping the muscles tighten, pulling the tongue forward, expanding the pharynx, and relieving airway narrowing caused by the posterior displacement of the tongue root. However, this method is only effective in the short term, with limited long-term efficacy, and the equipment is expensive, restricting its clinical use[8].
In recent years, the mid-frequency anti-snoring device has been developed based on hypoglossal nerve stimulation, and it is a new technology used to treat OSAHS. It delivers specific frequency pulse-modulated composite waves and provides intermittent electrical stimulation to the genioglossus muscle and its sublingual nerve branches. This stimulates the genioglossus muscle to contract responsively, increases the tension of the upper airway, reduces airway collapse, and maintains airway patency, thus terminating snoring and apnea and improving sleep quality. While this method is theoretically feasible, it lacks clinical practice and research support. Therefore, this study aimed to explore this issue.
In the preliminary experiments of this study, it was found that mild OSAHS patients had low treatment willingness, poor compliance, and could not complete the entire study due to their mild symptoms. Therefore, they were not included in the study. Severe OSAHS patients, on the other hand, had more severe symptoms and showed unclear effects of the mid-frequency anti-snoring device. Clinically, they were mainly treated with non-invasive positive pressure ventilation. Therefore, they were not included either. Nevertheless, moderate OSAHS patients had a strong willingness to be treated, good compliance, and could complete the study. Therefore, they were chosen as the subjects of this study. The results of this study indicated that after 4 wk of treatment with the mid-frequency anti-snoring device, the AHI of moderate OSAHS patients decreased, the time of snoring during nighttime sleep decreased, the duration of the most prolonged apnea decreased, the duration of SPO2 < 90% decreased, and the LSPO2 increased, confirming a significant improvement in the patients’ nighttime sleep quality. The study results also indicated that the sleep apnea quality of life index scores of patients increased after treatment compared to before treatment, including social activity scores, emotional state scores, and symptom scores. The Epworth Sleepiness Scale scores decreased, confirming that this treatment improved the patient’s clinical symptoms and quality of life.
The study results also showed that after using the mid-frequency anti-snoring device, the number of AAI resp occurrences decreased, AAI non-resp decreased, and respiratory effort-related arousals decreased. OSAHS patients experience repeated awakenings during sleep, which hurts sleep quality. During the initial treatment phase, this study found that mid-frequency anti-snoring treatment increased patient awakenings, affecting sleep quality. The main reasons were that the initial stimulation intensity was too high, and patients had a low tolerance for stimulation. The study continuously adjusted stimulation intensity based on patients’ actual experiences. After a period, patients fully adapted, and the number of awakenings significantly decreased.
This study conducted oropharyngeal CT examinations on OSAHS patients in awake, snoring, and mid-frequency anti-snoring treatment states to confirm that the mid-frequency anti-snoring device worked by expanding the narrow oropharyngeal airway. The narrowest points of the airway in the soft palate posterior area and retrolingual area were chosen as measurement points, and the cross-sectional areas of the airways were measured. The results showed that in the awake state the cross-sectional area of the narrowest part of the soft palate posterior airway was 80.47 ± 18.56 mm² and the narrowest part of the retrolingual airway was 155.36 ± 13.29 mm². When patients snored during sleep, there was evidence of tongue root or uvula collapse, leading to oropharyngeal airway narrowing or obstruction. The cross-sectional area of the narrowest part of the soft palate posterior airway decreased to 22.05 ± 16.47 mm², and the cross-sectional area of the retrolingual airway decreased to 120.45 ± 12.23 mm² showing significant reductions. During mid-frequency anti-snoring treatment, the cross-sectional area of the narrowest part of the soft palate posterior airway was 71.61 ± 17.38 mm², and the cross-sectional area of the retrolingual airway was 150.61 ± 12.35 mm², showing significant improvement compared to the snoring state. The study results confirmed that during treatment with the anti-snoring device, the relaxation of the tongue root and uvula was alleviated, and the degree of oropharyngeal airway narrowing was significantly improved, providing strong evidence from an imaging perspective for the working mechanism of the mid-frequency anti-snoring device.
Several objective issues were encountered throughout this study, resulting in some limitations in the examination results. First, this study employed a natural sleep approach and did not use sedative-hypnotic drugs to induce sleep. The examination process was time-consuming and challenging. In addition, patients were influenced by unfamiliar environments, such as the low temperature and high noise in the CT examination room, making it difficult for OSAHS patients to fall asleep naturally. Consequently, CT examinations were prone to interruptions and were not reflective of the patient’s actual home sleep state, potentially affecting the accuracy of the examination results. Second, the number of cases included in this study was relatively small, which may lead to statistical bias. Third, differences in patient height, weight, and disease severity, as well as differences in upper airway anatomy, could impact CT examination results. Fourth, during treatment with the anti-snoring device, high pulse stimulation intensity could cause patient awakenings, affecting the accuracy of CT examinations. Resolving the above issues requires further increasing the number of patients, expanding the sample size, improving the environment in the CT examination room, optimizing the CT examination process, and using sedative-hypnotic drugs to induce sleep if necessary.
In conclusion, this study confirmed that the mid-frequency anti-snoring device can expand the oropharyngeal airway in patients with moderate OSAHS, thereby improving their clinical symptoms and sleep quality. Our study provided a new method for the clinical treatment of OSAHS.
The mid-frequency anti-snoring device is a new technology based on sublingual nerve stimulation. Its principle is to improve the degree of oropharyngeal airway stenosis in obstructive sleep apnea-hypopnea syndrome (OSAHS) patients under mid-frequency wave stimulation.
There is a lack of clinical application and imaging evidence for the use of mid-frequency anti-snoring devices in the treatment of moderate OSAHS.
To provide imaging-based confirmation of the working mechanism of mid-frequency anti-snoring devices in treating OSAHS. This study also aimed to observe the clinical efficacy of medium-frequency anti-snoring devices in treating moderate obstructive OSAHS.
Fifty patients diagnosed with moderate OSAHS underwent a 4-wk treatment regimen involving the mid-frequency anti-snoring device during nighttime sleep. Following the treatment, we monitored and assessed the sleep apnea quality of life index and Epworth Sleepiness Scale scores. Additionally, we performed computed tomography scans of the oropharynx in the awake state, during snoring, and while using the mid-frequency anti-snoring device. Cross-sectional area measurements in different states were taken at the narrowest airway point in the soft palate posterior and retrolingual areas.
Compared to pretreatment measurements, patients exhibited a significant reduction in the apnea-hypopnea index, the percentage of time with oxygen saturation below 90%, snoring frequency, and the duration of the most prolonged apnea event. The lowest oxygen saturation (%) showed a notable increase, and both sleep apnea quality of life index and Epworth Sleepiness Scale scores improved. Oropharyngeal computed tomography scans revealed that in OSAHS patients the cross-sectional areas of the oropharyngeal airway in the soft palate posterior area and retrolingual area decreased during snoring compared to the awake state. Conversely, during mid-frequency anti-snoring device treatment, these areas increased compared to snoring.
This study confirmed that the mid-frequency anti-snoring device can expand the oropharyngeal airway in patients with moderate OSAHS, thereby improving their clinical symptoms and sleep quality.
The sample size of this study was limited, and there may be statistical bias. Further efforts are needed to increase the number of patients, expand the sample size, and conduct in-depth research on some scientific issues, such as the therapeutic effect of mid-frequency anti-snoring devices on patients with only snoring, the patient dependency and efficacy of long-term use, the impact on the anatomical structure of the upper airway of the oropharynx, and the impact of long-term use on abnormal lipid metabolism in patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nooripour R, Iran S-Editor: Chen YL L-Editor: Filipodia P-Editor: Yu HG
| 1. | Gottlieb DJ, Punjabi NM. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA. 2020;323:1389-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 761] [Article Influence: 152.2] [Reference Citation Analysis (0)] |
| 2. | Olson MD, Junna MR. Hypoglossal Nerve Stimulation Therapy for the Treatment of Obstructive Sleep Apnea. Neurotherapeutics. 2021;18:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Khokhrina A, Andreeva E, Degryse JM. The prevalence of sleep-disordered breathing in Northwest Russia: The ARKHsleep study. Chron Respir Dis. 2020;17:1479973120928103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Lee JJ, Sundar KM. Evaluation and Management of Adults with Obstructive Sleep Apnea Syndrome. Lung. 2021;199:87-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 5. | Lv R, Liu X, Zhang Y, Dong N, Wang X, He Y, Yue H, Yin Q. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduct Target Ther. 2023;8:218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 155] [Reference Citation Analysis (0)] |
| 6. | Gambino F, Zammuto MM, Virzì A, Conti G, Bonsignore MR. Treatment options in obstructive sleep apnea. Intern Emerg Med. 2022;17:971-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Mashaqi S, Patel SI, Combs D, Estep L, Helmick S, Machamer J, Parthasarathy S. The Hypoglossal Nerve Stimulation as a Novel Therapy for Treating Obstructive Sleep Apnea-A Literature Review. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Zhu Z, Hofauer B, Wirth M, Heiser C. Long-term changes of stimulation intensities in hypoglossal nerve stimulation. J Clin Sleep Med. 2020;16:1775-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |