Published online Feb 16, 2024. doi: 10.12998/wjcc.v12.i5.913
Peer-review started: October 18, 2023
First decision: November 22, 2023
Revised: December 6, 2023
Accepted: January 15, 2024
Article in press: January 15, 2024
Published online: February 16, 2024
Processing time: 104 Days and 22.8 Hours
Intrahepatic duct (IHD) stones are among the most important risk factors for cholangiocarcinoma (CCC). Approximately 10% of patients with IHD stones develop CCC; however, there are limited studies regarding the effect of IHD stone removal on CCC development.
To investigate the association between IHD stone removal and CCC development.
We retrospectively analyzed 397 patients with IHD stones at a tertiary referral center between January 2011 and December 2020.
CCC occurred in 36 of the 397 enrolled patients. In univariate analysis, chronic hepatitis B infection (11.1% vs 3.0%, P = 0.03), carbohydrate antigen 19-9 (CA19-9, 176.00 vs 11.96 II/mL, P = 0.010), stone located in left or both lobes (86.1% vs 70.1%, P = 0.042), focal atrophy (52.8% vs 26.9%, P = 0.001), duct stricture (47.2% vs 24.9%, P = 0.004), and removal status of IHD stone (33.3% vs 63.2%, P < 0.001) were significantly different between IHD stone patients with and without CCC. In the multivariate analysis, CA19-9 > upper normal limit, carcinoembryonic antigen > upper normal limit, stones located in the left or both lobes, focal atrophy, and complete removal of IHD stones without recurrence were independent factors influencing CCC development. However, the type of removal method was not associated with CCC risk.
Complete removal of IHD stones without recurrence could reduce CCC risk.
Core Tip: It is well known that intrahepatic duct (IHD) stones are the most important risk factors for cholangiocarcinoma (CCC), but there are limited studies regarding the effect of IHD stone removal on CCC development. It has been reported that remnant stones after percutaneous transhepatic cholangioscopy could be a risk factor for CCC, but the effect of recurrence after complete removal of stones on CCC is unclear. Based on this, we investigated the association of IHD stone removal and CCC development.
- Citation: Kim TI, Han SY, Lee J, Kim DU. Removal of intrahepatic bile duct stone could reduce the risk of cholangiocarcinoma: A single-center retrospective study in South Korea. World J Clin Cases 2024; 12(5): 913-921
- URL: https://www.wjgnet.com/2307-8960/full/v12/i5/913.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i5.913
Cholangiocarcinoma (CCC) has a poor prognosis, and its incidence is increasing worldwide, especially in East Asia[1]. Surgical resection is the optimal method for curing cancer, but only about 10%-40% of patients that are diagnosed are considered suitable for operation at the time of diagnosis[2]. Research on the use of immune checkpoint inhibitors to treat local advanced or metastatic CCC is rapidly progressing[2]. A recent study found that adding durvalumab to gemcitabine and cisplatin, the standard first-line treatment for advanced CCC, can extend patients’ overall survival[3]. However, the 5-year survival rate for advanced CCC that is not amenable to surgery has not exceeded 5% until now. Therefore, one of the main approaches to increasing CCC survival rate of is to identify and eliminate its risk factors.
While the most common risk factor in Western countries is primary biliary cirrhosis, parasitic infections, intrahepatic duct (IHD) stones, and viral hepatitis are more common causes of CCC in Eastern countries[4]. IHD stones cause repetitive inflammation of the liver parenchyma and structural changes, and 10% of these result in CCC[5]. Therefore, it is important to consistently manage IHD stones. Hence, most patients undergo operative or endoscopic removal of stones. While operative treatment is known to be more effective in managing IHD stones, the endoscopic method can be an option in cases of bilateral IHD stones without structural change, while considering severe comorbidity.
Depending on the method, the removal rate in surgical treatment is 95%-100%, with a recurrence rate of 5.7%-13.9%. The non-surgical treatments such as percutaneous transhepatic cholangioscopy (PTCS) and endoscopic retrograde cholangiopancreatography (ERCP) have a removal rate of approximately 80% and a recurrence rate of 35%-63.2%[6,7]. It has been reported that remnant stones after non-surgical treatment could be a risk factor for CCC[8]; however, research on the risk of CCC development after IHD stone removal is still lacking. Therefore, this study aimed to investigate the relationship between IHD stone removal and the risk of CCC development.
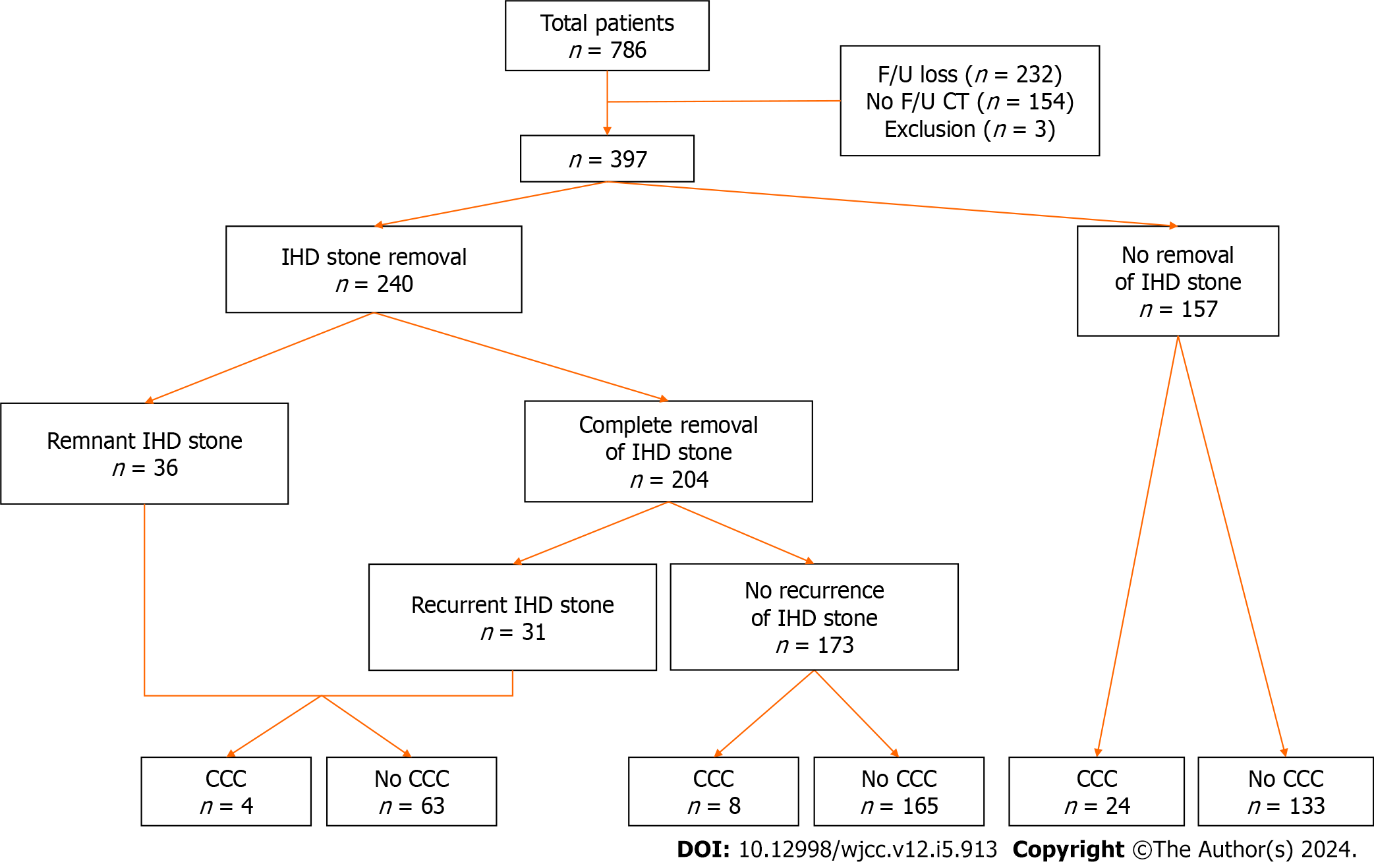
From January 2011 to December 2020, we retrospectively analyzed the medical records of patients diagnosed with IHD stones who underwent imaging tests such as computed tomography (CT) or magnetic resonance (MR) and had a follow-up record of more than 6 mo. We excluded patients who had a possibility of malignancy on diagnosis of IHD stones. We reviewed age, sex, past medical history, medication, laboratory findings, imaging tests, and pathologic results. A total of 397 patients with IHD stones were enrolled and 36 were diagnosed with CCC. This study was performed in accordance with the ethical principles of the Declaration of Helsinki (2013) and was approved by the institutional review board of Pusan National University Hospital (IRB No. 2103-010-101).
We checked for remnants and recurrence after IHD stone removal. A remnant stone was defined as a stone confirmed by CT or MR within 6 mo after IHD stone removal, while a recurrent stone was confirmed more than 6 mo after treatment. We then investigated the occurrence rate of CCC in accordance with the presence of remnant stones from pathological data.
We analyzed sex, age, height, body weight, and body mass index (BMI) and the presence of liver cirrhosis, viral hepatitis, diabetes mellitus, and hypertension for past medical history. As serologic markers, carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) were analyzed for their minimal value in consecutive tests during the follow-up period. For medication history, aspirin, ursodeoxycholic acid (UDCA), and statin use for 6 mo or more were considered. In imaging tests, we analyzed the location, size, number of stones, and the presence of stricture of the bile duct or atrophy of the liver parenchyma and distinguished stones larger than the duct by comparing the size of the stone with the duct diameter. Regardless of treatment, we defined recurrent cholangitis as taking antibiotics in outpatient or hospital administration two times or more (Figure 1).
We used SPSS statistical software (version 26.0, IBM, Armonk, NY) for statistical analysis. Qualitative data, including differences between the two groups, were summarized and expressed as frequencies and percentile using the χ2 test. For continuous variables, we analyzed the difference between the two groups and expressed it as the median with or without standard deviation using an independent two-sample t-test. The effect of independent variables on response variable was analyzed using the multivariate logistic regression, and the statistically significant variables were included in the univariate logistic regression with 0.05 alpha level.
The data of the 397 patients enrolled in this study are summarized in Table 1. CCC occurred in 36 patients. The two groups’ mean follow-up period was approximately 7 years (96.3 vs 83.9 mo). In the two groups, 33.3% and 39.6% were male, and the median age at the diagnosis of IHD stone was 60.75-years-old and 61.31-years-old, respectively. There was no significant difference in BMI and underlying viral hepatitis between the two groups. The number of patients with diabetes mellitus and hypertension was similar between the group (13.9% and 14.7%, 13.9% and 18.4%, respectively). In serologic tests, the minimum value of CA19-9 showed statistically significant differences [CA19-9 (176.00 vs 11.96, P = 0.010)] and the number of patients who had results exceeding the reference showed significant differences in CA19-9 (19.4% vs 1.9%, P < 0.001) and CEA (13.9% vs 1.9%, P = 0.002). On imaging, there was no statistical difference in the occurrence of multiple stones or stones bigger than the duct diameter. IHD stones on the left or both sides of the liver showed a higher rate of accompanying CCC than stones only on the right side (86.1% vs 70.1%, P = 0.042). Furthermore, the coexistence of atrophy of the liver parenchyma (52.8% vs 26.9%, P = 0.001) and bile duct stricture (47.2% vs 24.9%, P = 0.004) showed a statistically significant difference in the rate of CCC.
| Variable | IHD stone with CCC, n = 36 | IHD stone without CCC, n = 361 | P value |
| Male sex | 12 (33.3) | 143 (39.6) | 0.4613 |
| Age | 60.75 ± 10.20 | 61.31 ± 10.58 | 0.6872 |
| BMI | 23.27 ± 3.23 | 22.93 ± 3.27 | 0.5511 |
| Hepatitis | |||
| None | 31 (86.1) | 342 (94.7) | 0.0494 |
| HBV | 4 (11.1) | 11 (3.0) | |
| HCV | 1 (2.8) | 8 (2.2) | |
| DM | 5 (13.9) | 53 (14.7) | 0.8933 |
| HT | 5 (13.9) | 66 (18.4) | 0.5033 |
| CA19-9, minimum | 176.00 ± 463.47 | 11.96 ± 15.65 | 0.0102 |
| CA19-9 > UNL | 7 (19.4) | 7 (1.9) | < 0.0014 |
| CEA, minimum | 8.23 ± 29.45 | 1.96 ± 1.32 | 0.3322 |
| CEA > UNL | 5 (13.9) | 7 (1.9) | 0.0024 |
| Location | |||
| Lt & both | 31 (86.1) | 253 (70.1) | 0.0423 |
| Rt | 5 (13.9) | 108 (29.9) | |
| Multiple stone | 30 (83.3) | 254 (70.4) | 0.1003 |
| Stone size > duct diameter | 19 (52.8) | 173 (47.9) | 0.5783 |
| Focal atrophy | 19 (52.8) | 97 (26.9) | 0.0013 |
| Duct stricture | 17 (47.2) | 90 (24.9) | 0.0043 |
| IHD stone removal | 12 (33.3) | 228 (63.2) | < 0.0013 |
| Complete removal & no recurrence | 8 (22.2) | 165 (45.7) | 0.0073 |
| Complete removal & recurrence | 2 (5.6) | 29 (8.0) | 10.0004 |
| Incomplete removal | 2 (5.6) | 34 (9.4) | 0.7594 |
| Removal method | |||
| Non-surgery, PTCS, ERCP | 6 (50.0) | 128 (56.1) | 0.6763 |
| Surgery | 6 (50.0) | 100 (43.9) | |
| Medication | |||
| Aspirin | 0 (0.0) | 14 (3.9) | 0.6264 |
| UDCA | 28 (77.8) | 265 (73.4) | 0.5703 |
| Metformin | 3 (8.3) | 19 (5.3) | 0.4374 |
| Statin | 2 (5.6) | 23 (6.4) | 1.0004 |
| Recurrent cholangitis | 20 (55.6) | 185 (51.2) | 0.6223 |
| F/U period, mean ± SD | 96.33 ± 59.06 | 83.93 ± 60.38 | 0.1642 |
Although the number of patients who underwent IHD stone removal was lower in those with CCC (33.3% vs 63.2%, P < 0.001), there was no statistical difference in the method of stone removal, recurrence rate (5.6% vs 8.0%) or the incomplete stone removal rate (5.6% vs 9.4%). Medication history also showed no difference in the use of aspirin (0% vs 3.9%), UDCA (77.8% vs 73.4%), metformin (8.3% vs 5.3%), or statins (5.6% vs 6.4%). There was no difference in the occurrence of recurrent cholangitis (55.6% vs 51.2%) (Table 1).
We calculated the odds ratio (OR) of each factor and conducted multivariate regression analysis of seven factors that showed statistical importance (Table 2): the number of patients who exceeded the reference for CA19-9 (P < 0.001) and CEA (P = 0.001), IHD stone on the left side (P = 0.049), atrophy of the liver parenchyma (P = 0.002), bile duct stricture (P = 0.005), complete removal without recurrence (P = 0.009), and non-surgical removal method (P = 0.004) (Table 2). Of these, the frequency of bile duct stricture and removal method of non-surgery showed a P value of 0.061 and 0.141, respectively, indicating that they were not significant risk factors for CCC. We verified the ORs of CA19-9 (P < 0.001), CEA (0.005), left-sided and both IHD stone (0.013). In the case of complete removal without recurrence, the OR of CCC showed a statistically significant decrease to 0.21 (P < 0.001) (Table 2).
| Variable | Univariate analysis | Multivariate analysis | ||||
| OR | 95%CI | P value | OR | 95%CI | P value1 | |
| Male sex | 0.76 | 0.37-1.57 | 0.463 | |||
| Age | 0.99 | 0.96-1.03 | 0.761 | |||
| BMI | 1.03 | 0.93-1.15 | 0.550 | |||
| Hepatitis | ||||||
| None | Reference | |||||
| HBV | 4.01 | 1.21-13.35 | 0.023 | |||
| HCV | 1.38 | 0.17-11.39 | 0.765 | |||
| DM | 0.93 | 0.35-2.51 | 0.893 | |||
| HT | 0.72 | 0.27-1.91 | 0.505 | |||
| CA19-9, minimum | 1.01 | 1.00-1.02 | 0.013 | |||
| CA19-9 > UNL | 12.21 | 4.01-37.19 | 0.000 | 15.85 | 3.79-66.31 | 0.000 |
| CEA, minimum | 1.28 | 1.03-1.59 | 0.026 | |||
| CEA > UNL | 8.16 | 2.44-27.21 | 0.001 | 8.12 | 1.87-35.35 | 0.005 |
| Location, Lt and both | 2.65 | 1.00-6.99 | 0.049 | 4.37 | 1.37-13.94 | 0.013 |
| Multiple stone | 2.11 | 0.85-5.21 | 0.107 | |||
| Stone size > duct diameter | 1.21 | 0.61-2.41 | 0.579 | |||
| Focal atrophy | 3.04 | 1.52-6.09 | 0.002 | 2.59 | 1.13-5.94 | 0.025 |
| Duct stricture | 2.69 | 1.34-5.41 | 0.005 | 2.24 | 0.96-5.23 | 0.061 |
| IHD stone removal | ||||||
| Complete removal and no recurrence | 0.34 | 0.15-0.76 | 0.009 | 0.21 | 0.09-0.50 | 0.000 |
| Complete removal and recurrence | 0.67 | 0.15-2.95 | 0.600 | |||
| Incomplete removal | 0.57 | 0.13-2.46 | 0.447 | |||
| Removal method | ||||||
| None | Reference | |||||
| Non-surgery, PTCS, ERCP | 0.26 | 0.10-0.66 | 0.004 | 0.33 | 0.07-1.45 | 0.141 |
| Surgery | 0.33 | 0.13-0.84 | 0.021 | 0.37 | 0.08-1.70 | 0.200 |
| Medication | ||||||
| Aspirin | 0.00 | 0.00-0.00 | 0.999 | |||
| UDCA | 1.27 | 0.56-2.88 | 0.570 | |||
| Metformin | 1.64 | 0.46-5.82 | 0.447 | |||
| Statin | 0.86 | 0.20-3.83 | 0.848 | |||
Table 1 also shows the CCC occurrence rate according to the state of IHD stones. Of 157 patients who did not undergo IHD stone removal, 24 were diagnosed with CCC, resulting in an occurrence rate of 15.3%. Conversely, 12 of the 240 patients who underwent IHD stone removal treatment were diagnosed with CCC, with an occurrence rate of 5.0%. Among the 173 patients with complete removal of IHD stone without recurrence, eight were diagnosed with CCC, showing an occurrence rate of 4.6% and a statistically significant difference (P = 0.007). Complete removal with recurrent (6.4%) and incomplete removal with remnant (5.6%) stone groups showed a tendency toward decreased CCC risk (OR = 0.57-0.67) in univariate analysis, however, the difference was not statistically significant (Table 2).
Regardless of removal methods, surgical (ERCP and PTCS) or non-surgical (hepatectomy), all patients who underwent IHD stone removal showed a decreased risk of CCC, and there was no difference between the two groups in CCC development (P = 0.676) (Table 3). However, the rates of remnant or recurrent stones and recurrent cholangitis were higher in non-surgically treated patients (Table 3).
| Removal method | Surgery, n = 106 | Non-surgery, n = 134 | P value |
| Cholangiocarcinoma | 6 (5.7) | 6 (4.5) | 0.676 |
| Remnant stone | 9 (8.5) | 27 (20.1) | 0.012 |
| Recurrent stone | 12 (11.3) | 52 (38.8) | < 0.001 |
| Recurrent cholangitis | 37 (34.9) | 96 (71.6) | < 0.001 |
In the current study, we found that the CCC occurrence rate was lower in patients who underwent IHD stone removal than in those who did not. The CCC occurrence rate (9%) was similar to that of another study (1.3%-13%)[5,9]. In multivariate analysis, the factors affecting CCC were increased CA19-9 (OR = 15.85, P < 0.001) and CEA (OR = 8.12, P = 0.005) above the reference value, left-sided or bilateral IHD stones (OR = 4.37, P = 0.013), and atrophy of the liver parenchyma (OR = 2.59, P = 0.025). Complete removal of IHD stones without recurrence was identified as a factor decreasing CCC risk to 79% (OR = 0.21, P = 0.001). Although not in CCC development, there was a difference in the rate of remnant and recurrence between the surgically and non-surgically treated groups, including the rate of recurrent cholangitis. These results could suggest that surgical treatment can be better for younger patients or those with higher risk of CCC like Clonorchis sinensis or viral hepatitis infection and primary sclerosing cholangitis, etc.
The stone removal rate was 91.5% and 79.9% in surgically treated groups and the others, respectively. This was similar to another study. In the case of recurrence rate, there was no significant difference with other studies (5.7%-13.9%, 35%-63.2%) compared to 11.5% in operation and 45.5% in the PTCS group[6,7]. Although the number of patients who underwent ERCP was too small to directly compare with other groups, the removal rate was high, and the recurrence rate was low in this study because it was applied to patients with relatively small stones in the hilum.
It is well known that structural changes are risk factors for CCC, including atrophy of the parenchyma and bile duct stricture. Therefore, hepatectomy, including resection, could lower the risk of CCC. In multivariate analysis, atrophy of the liver parenchyma was identified as a risk factor; however, focal bile duct stricture did not affect CCC. This may be due to the relief of congestion by balloon dilatation during PTCS, which could influence cancer development. In this study, we expect that PTCS can be preferentially applied to patients with IHD stones since PTCS does not increase CCC risk if there are no structural changes, such as atrophy or stricture.
The risk factors for CCC were elevated tumor markers, atrophy of the liver parenchyma, and left-sided IHD stones. In one cohort study in Japan, it was reported that bile duct stricture and age above 65 years were risk factors for CCC[9], and in Korea, there was a study that remnant stone increased the risk[10]. These factors were all related to chronic inflammation, which was reported as a major factor of CCC. This was thought to be related to the increased risk in the remnant stone group from another study and the group with remnant or recurrent stone in this study[11]. In our study, remnant or recurrent stone showed a tendency of decreased CCC risk, though it did not reach statistical significance. This result emphasized the importance of reducing stones to relieve the obstruction and the complete removal and long-term surveillance after treatment. Additionally, there is a possibility that the effect of remnant or recurrent stones on the risk of CCC could not be fully evaluated because of the short follow-up period. In one study about IHD stones in Korea, the average period of occurrence of CCC was approximately 10 years; therefore, the 7-year follow-up period in this study could be too short to verify the effect of remnant stones on CCC[9,10,12].
Recently, there have been reports that drugs such as aspirin, metformin, and statins reduce cancer risk[13-15]. Some studies suggested that aspirin may help prevent cancer, and there are ongoing studies to address how metabolic factors like diabetes, hypertension, and obesity affect malignancy[16]. In a study of 2395 CCC patients and 4769 controls in 2016, Choi et al[17] reported that aspirin reduced the risk of bile duct cancer to 65%-71%, specifically 65% in intrahepatic CCC. However, they simultaneously reported that patients with bile duct disease had a 12.1-fold increase in CCC risk; thus, we expect that the higher risk of CCC could offset aspirin use in patients with IHD stones. Although there were a small number of aspirin users in this study, none were diagnosed with CCC. Tseng[18] reported that the risk of CCC was lower in metformin users than in non-users by approximately 50%-60%. However, in this study, metformin users had a higher proportion of CCC without statistical significance. Liu et al[19] revealed that statins could reduce the risk of CCC by approximately 12% in a big-data study of 3118 CCC patients and 15519 controls. These studies about drugs decreasing cancer risk were big data research generally conducted on a large number of people, so our small study on approximately 400 patients could not prove drug efficacy. In the future, large-scale studies should be conducted to investigate the effects of drugs or metabolic factors on CCC occurrence. In the long term, we need to actively implement education and campaigns targeting the general population to identify and address these risk factors.
Our study had some limitations. First, this was a single-center study with a small number of patients, approximately 400. Because IHD stones are more common among East Asian countries than in the Western world[20], there might be some limitation to generalize the conclusion of a retrospective study conducted at a single center study in South Korea. Second, some patients’ medications could not be precisely investigated due to dropout. Third, this was a retrospective study, so it requires attention for interpretation because of defective data. This study has no information about Clonorchis sinensis infection as an important risk factor for CCC. Finally, this study’s relatively short average follow-up period of 7 years could have affected the risk evaluation for CCC.
In conclusion, patients who underwent removal of IHD stones showed a decreased risk of CCC regardless of the methods, especially in the absence of remnant stone after treatment. Therefore, it is important to remove IHD stones as much as possible. Medications such as statins, metformin, and aspirin[13-15] are not expected to affect CCC occurrence. Further studies are warranted to verify these results.
Cholangiocarcinoma (CCC) is a type of gastrointestinal malignancy that has a poor prognosis and is difficult to treat. It has a low possibility of operative resection for cure at the time of diagnosis, so research for systemic chemotherapy is underway, including the use of immune check point inhibitors. In East Asia, the incidence of CCC is increasing, but there are few methods for early diagnosis. Therefore, it is very important to recognize and estimate CCC risk factors.
In East Asia, intrahepatic duct (IHD) stones have been recognized as a risk factor for developing CCC. They block the normal outflow of bile, resulting in repetitive inflammation of liver parenchyma. Chronic inflammation of biliary tract and liver parenchyma are known to contribute to malignant change, so it is important to relieve the obstruction. There have been several studies about IHD stones and CCC, but most of them had a small number of subjects and few studies identified the correlation between removal of IHD stones and CCC development.
We wanted to perform a large cohort study about the effect of IHD stone removal on CCC development, including the optimal method for removal. We also analyzed the effect of medication for metabolic disease like diabetes mellitus, dyslipidemia, and hypertension.
We retrospectively analyzed patients who were diagnosed with IHD stone with imaging tests and underwent removal in Pusan National University Hospital from January 2011 to December 2020. Based on medical records, we investigated the occurrence of CCC and factors affecting CCC development.
CCC occurred in 36 of the 397 enrolled patients. In multivariate analysis, carbohydrate antigen 19-9 > upper normal limit, carcinoembryonic antigen > upper normal limit, stones located in the left or both lobes, focal atrophy, and complete removal of IHD stones without recurrence were independent factors influencing CCC development. However, the type of removal method or medication for metabolic disease did not seem to affect CCC development.
Regardless of methods, the complete removal of IHD stones without recurrence could reduce CCC development. Therefore, it is important to choose the optimal method for removal depending on the patient and follow up. Repetitive tests or procedures may be necessary.
In the future, optimal method for removal of IHD stone regarding patient’s age, sex, social or economic factors and underlying disease should be studied. In addition, systemic treatment for CCC including cytotoxic or immune-targeted chemotherapy specific to CCC should be developed.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Rizzo A, Italy S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Zhao S
| 1. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Rizvi S, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1539] [Article Influence: 307.8] [Reference Citation Analysis (0)] |
| 2. | Rizzo A, Brandi G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat Res Commun. 2021;27:100354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 3. | Oh DY, He AR, Qin S, Chen LT, Okusaka T, Vogel A, Kim JW, Suksombooncharoen T, Lee MA, Kitano M, Burris H, Bouattour M, Tanasanvimon S, McNamara MG, Zaucha R, Avallone A, Tan B, Cundom J, Lee C, Takahashi H, Ikeda M, Chen JS, Wang J, Makowsky M, Rokutanda N, He P, Kurland JF, Cohen G, Valle JW. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022;1. [RCA] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 534] [Article Influence: 178.0] [Reference Citation Analysis (1)] |
| 4. | Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 722] [Cited by in RCA: 689] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 5. | Kim HJ, Kim JS, Joo MK, Lee BJ, Kim JH, Yeon JE, Park JJ, Byun KS, Bak YT. Hepatolithiasis and intrahepatic cholangiocarcinoma: A review. World J Gastroenterol. 2015;21:13418-13431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (3)] |
| 6. | Cha SW. [Management of Intrahepatic Duct Stone]. Korean J Gastroenterol. 2018;71:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Park JS, Han HS, Hwang DW, Yoon YS, Cho JY, Koh YS, Kwon CH, Kim KS, Kim SB, Kim YH, Kim HC, Chu CW, Lee DS, Kim HJ, Park SJ, Han SS, Song TJ, Ahn YJ, Yoo YK, Yu HC, Yoon DS, Lee MK, Lee HK, Min SK, Jeong CY, Hong SC, Choi IS, Hur KY. Current status of laparoscopic liver resection in Korea. J Korean Med Sci. 2012;27:767-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Huang MH, Chen CH, Yang JC, Yang CC, Yeh YH, Chou DA, Mo LR, Yueh SK, Nien CK. Long-term outcome of percutaneous transhepatic cholangioscopic lithotomy for hepatolithiasis. Am J Gastroenterol. 2003;98:2655-2662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Suzuki Y, Mori T, Yokoyama M, Nakazato T, Abe N, Nakanuma Y, Tsubouchi H, Sugiyama M. Hepatolithiasis: analysis of Japanese nationwide surveys over a period of 40 years. J Hepatobiliary Pancreat Sci. 2014;21:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 10. | Cheon YK, Cho YD, Moon JH, Lee JS, Shim CS. Evaluation of long-term results and recurrent factors after operative and nonoperative treatment for hepatolithiasis. Surgery. 2009;146:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (3)] |
| 11. | Roy S, Glaser S, Chakraborty S. Inflammation and Progression of Cholangiocarcinoma: Role of Angiogenic and Lymphangiogenic Mechanisms. Front Med (Lausanne). 2019;6:293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Chang JS, Tsai CR, Chen LT. Medical risk factors associated with cholangiocarcinoma in Taiwan: a population-based case-control study. PLoS One. 2013;8:e69981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Brower V. Of cancer and cholesterol: studies elucidate anticancer mechanisms of statins. J Natl Cancer Inst. 2003;95:844-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Patrignani P, Patrono C. Aspirin and Cancer. J Am Coll Cardiol. 2016;68:967-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 208] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 15. | Saraei P, Asadi I, Kakar MA, Moradi-Kor N. The beneficial effects of metformin on cancer prevention and therapy: a comprehensive review of recent advances. Cancer Manag Res. 2019;11:3295-3313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 249] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 16. | Rose PW, Watson EK, Jenkins LS. Aspirin for prevention of cancer and cardiovascular disease. Br J Gen Pract. 2011;61:412-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Choi J, Ghoz HM, Peeraphatdit T, Baichoo E, Addissie BD, Harmsen WS, Therneau TM, Olson JE, Chaiteerakij R, Roberts LR. Aspirin use and the risk of cholangiocarcinoma. Hepatology. 2016;64:785-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Tseng CH. Metformin and Biliary Tract Cancer in Patients With Type 2 Diabetes. Front Oncol. 2020;10:587666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Liu Z, Alsaggaf R, McGlynn KA, Anderson LA, Tsai HT, Zhu B, Zhu Y, Mbulaiteye SM, Gadalla SM, Koshiol J. Statin use and reduced risk of biliary tract cancers in the UK Clinical Practice Research Datalink. Gut. 2019;68:1458-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Pausawasdi A, Watanapa P. Hepatolithiasis: epidemiology and classification. Hepatogastroenterology. 1997;44:314-316. [PubMed] [DOI] [Full Text] |