Published online Feb 16, 2024. doi: 10.12998/wjcc.v12.i5.1018
Peer-review started: December 6, 2023
First decision: December 21, 2023
Revised: January 4, 2024
Accepted: January 24, 2024
Article in press: January 24, 2024
Published online: February 16, 2024
Processing time: 56 Days and 1.6 Hours
Ankylosing spondylitis (AS) is a chronic immune-mediated inflammatory disease. The prevailing theory links AS onset to infections in susceptible individuals. Furthermore, infections may impair the immune responses. Numerous studies have investigated links between AS and various infections-bacterial, viral, fungal, and other microorganism infections. However, limited attention has been given to the association between AS and Clonorchis sinensis (C. sinensis) infection.
A 27-year-old male with a 10-yr history of AS presented to our hospital with inflammatory lower back pain as the primary manifestation. Ten years ago, the patient had achieved a stable condition after treatment with biological agents. However, he experienced a recurrence of lumbosacral pain with an unexplained cause 10 d before hospital admission. A lumbosacral magnetic resonance imaging (MRI) scan revealed bone marrow edema in the left sacroiliac joint, and laboratory indicators were elevated. Moreover, the presence of C. sinensis eggs was detected in the stool. The patient was prescribed praziquantel, resulting in the disap
C. sinensis infections could potentially trigger the exacerbation of AS. Clinicians should pay attention to investigating the presence of infections.
Core Tip: This study explored the link between Clonorchis sinensis (C. sinensis) infection and ankylosing spondylitis (AS). While previous research extensively explored the association between AS and various infections, the association with C. sinensis received limited attention. The findings highlight the potential role of parasitic infections, particularly C. sinensis, in affecting AS disease activity. This study provides valuable insights into the less-explored aspects of AS etiology, emphasizing the need for further investigation into parasitic infections to comprehend and manage AS.
- Citation: Yi TX, Liu W, Leng WF, Wang XC, Luo L. Ankylosing spondylitis coexisting with Clonorchis sinensis infection: A case report. World J Clin Cases 2024; 12(5): 1018-1024
- URL: https://www.wjgnet.com/2307-8960/full/v12/i5/1018.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i5.1018
Ankylosing spondylitis (AS) is a chronic inflammatory and rheumatic disease resulting from an imbalance between innate and acquired immune responses[1]. While it can affect any part of the spine, its primary symptoms are persistent back pain and stiffness in the lower back and pelvis. The prevalence of AS per 10000 individuals is 23.8 in Europe, 31.9 in North America, 16.7 in Asia, 10.2 in Latin America, and 7.4 in Africa[2]. Infections commonly occur in the first 3 months and may act as potential triggers for the first symptoms of AS, often manifesting as gastrointestinal, urinary tract, and respiratory infections of microbiological origin[3,4].
Clonorchis sinensis (C. sinensis) infection is a severe parasitic disease affecting millions globally, especially prevalent in China, South Korea, the Far East of Russia, and Vietnam, with an estimated 15 million cases[5]. Transmission occurs through the consumption of undercooked freshwater fish containing metacercariae. Adult C. sinensis parasites then establish themselves within the human hepatobiliary system[6]. C. sinensis infection triggers the activation of sphingosine 1-phosphate receptor 2, leading to the injury and fibrosis of the hepatobiliary[7]. Recent research in a rat model found that C. sinensis infection increases the risk of hepatocellular carcinoma by stimulating hepatic progenitor cell proliferation[8]. Complications of C. sinensis infection include cholestasis, cholangitis, biliary system fibrosis, and in severe cases, the development of cholangiocarcinoma[9]. Consequently, the primary preventive measure is to abstain from consuming raw or undercooked freshwater fish. Praziquantel is the recommended and effective treatment for this infection[10].
While there is existing literature on the coexistence of AS and parasitic infections, there is limited research specifically addressing the simultaneous presence of AS and C. sinensis infection. This case report details a rare scenario of AS coexisting with C. sinensis infection, underscoring the potential impact of C. sinensis infection on AS disease activity.
A 27-year-old male patient with persistent lumbosacral pain for more than 10 yr had a recurrence 10 d before a hospital visit.
The patient, in his late twenties, reported experiencing lumbosacral pain for more than 10 years. The pain intensified while sitting or at rest but improved in the mornings. Throughout this period, there were no signs of heel pain, eye inflammation, symmetrical swelling, pain in small joints, psoriasis, sausage fingers/toes, facial erythema, photosensitivity, oral ulcer, alopecia, frequent urination, or urgency. Consequently, he was admitted to the Rheumatology Department at the Traditional Chinese Medicine Hospital of Dianjiang, Chongqing. Examinations conducted indicated a positive response to human leukocyte antigen B27, and magnetic resonance imaging (MRI) scans revealed localized edema around the bilateral sacroiliac joint bone marrow. Upon confirmation of AS, rheumatologists recommended a tailored treatment regimen. Subsequently, the patient managed his condition with subcutaneous administration of recombinant human tumor necrosis factor (TNF)-α receptor II: Immunoglobulin G Fc fusion protein, leading to a reduction in lumbosacral pain. Lumbosacral pain recurred 10 d before the patient's hospital admission without a clear cause. Despite orally taking aceclofenac at a dosage of 100 mg twice daily, the pain persisted and was not alleviate.
He had no notable past medical history.
No significant medical findings were identified in the patient's personal history or family background.
The physical examination confirmed tenderness and percussive pain in the left sacroiliac joint. The Schober test recorded a value of 5 cm, with a finger-to-ground distance of 30 cm, a measured pillow wall distance of 0 cm, and a chest expansion of 4 cm. The physiological curvature of the spine was within the normal range, and no tenderness was detected within the cervical, thoracic, and lumbar spinous processes, vertebral bodies, and paravertebral bodies.
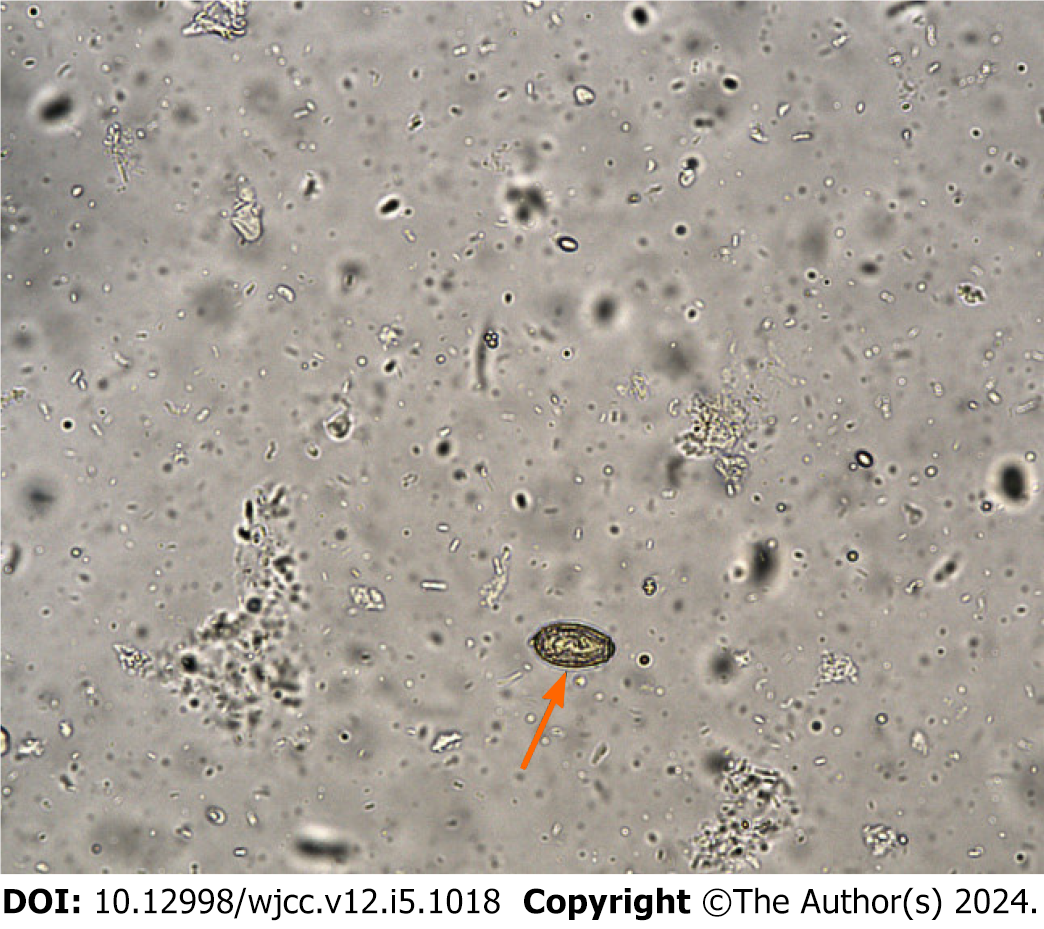
Elevated levels of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) indicated inflammation, while blood routine tests, liver and kidney functions, blood coagulation functions, and a routine urinalysis showed values within the normal range. Subsequent T-SPOT.TB and tumor marker tests were conducted to detect tuberculosis infection and prepare for the use of biological agents. The T-SPOT.TB result was negative, but alpha-fetoprotein levels were slightly elevated. However, routine stool examination revealed the presence of C. sinensis eggs (Figure 1).
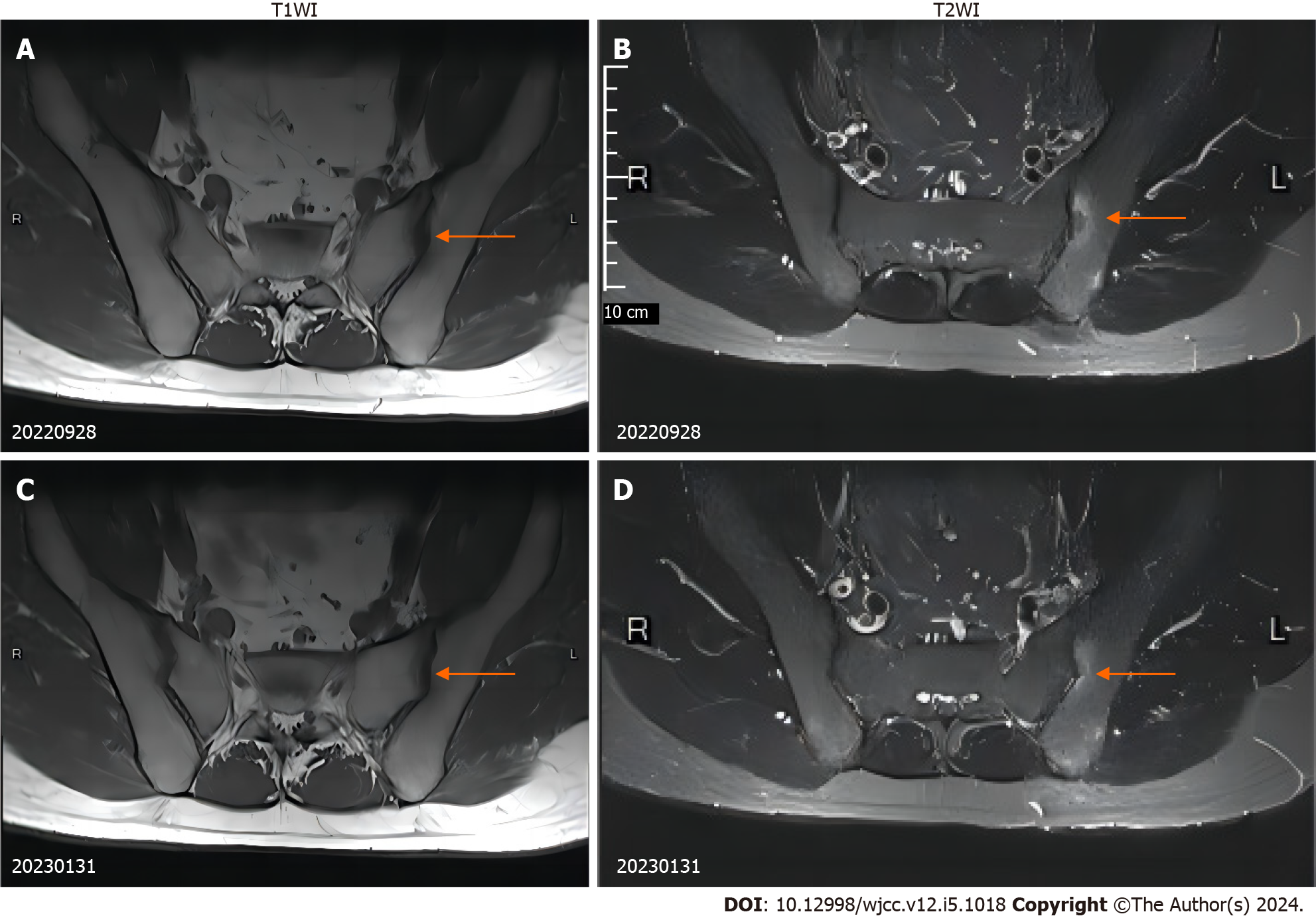
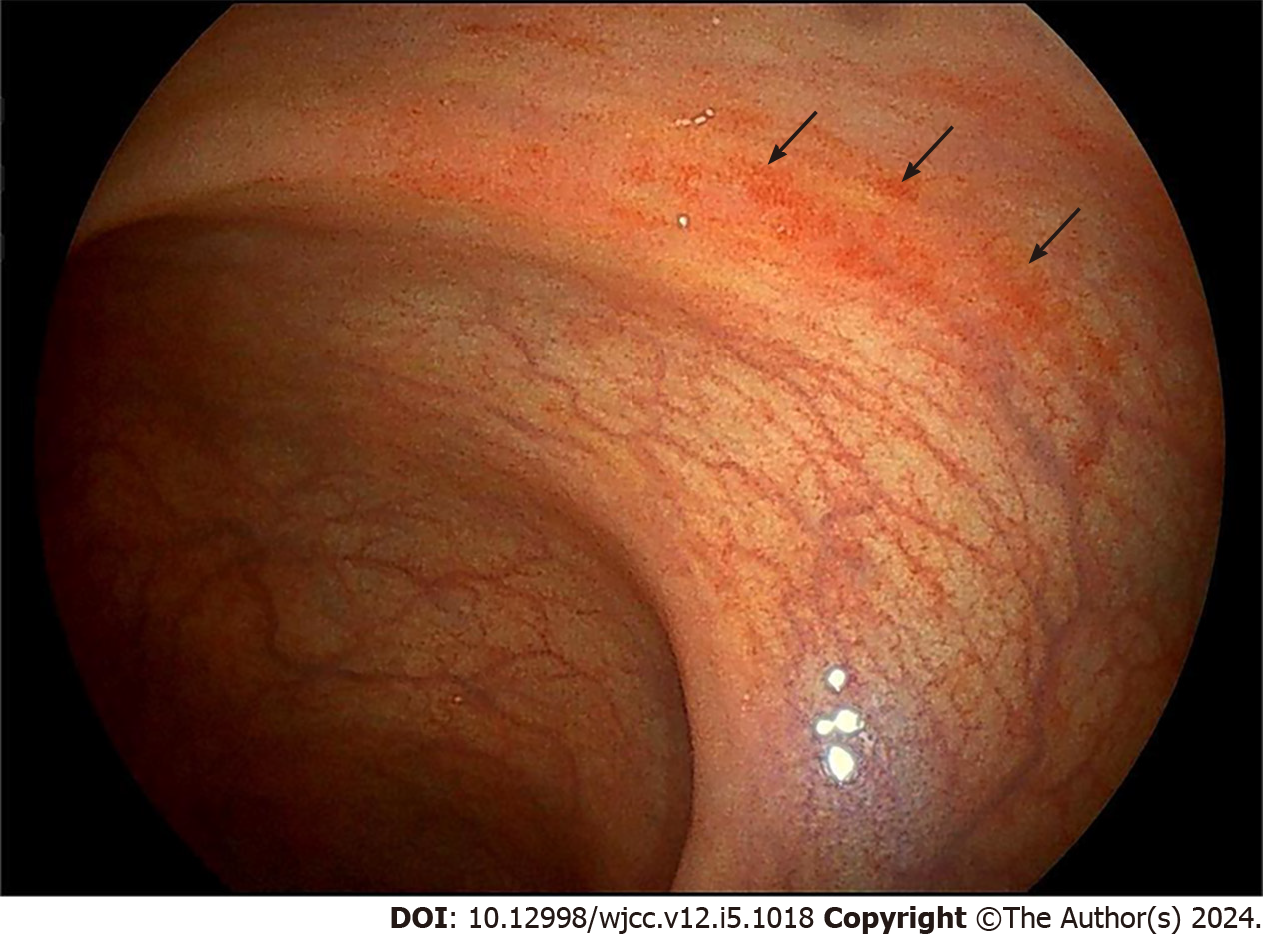
The MRI scan of the sacroiliac joint displayed bone marrow edema in the left sacroiliac joint (Figure 2A and B). A subsequent colonoscopy indicated congestion in the descending colon, sigmoid colon, and rectum (Figure 3). No liver abnormalities were observed in the upper abdominal computed tomography.
Considering the patient's symptoms, physical examination, laboratory tests, and imaging results, the final diagnosis was AS coexisting with C. sinensis infection.
Based on the patient's medical history, he had consumed uncooked freshwater fish the month before hospital admission. Therefore, praziquantel was administered orally at a dosage of 210 mg/kg/d, three times daily for 3 d.
One week later, a post-treatment fecal examination showed no C. sinensis eggs, and both ESR and CRP levels were normal. Notably, the lumbosacral pain had also subsided, and no C. sinensis eggs were detected in the three subsequent follow-up visits. The MRI indicated a reduction in bone marrow edema around the left sacroiliac joint after 4 months (Figure 2C and D).
The prevalence of AS in China is currently 0.29%, showing an upward trend. Sex-based differences in prevalence have been observed, with males exhibiting a prevalence rate 2.8 times higher than that of females[11]. The patient was diagnosed with AS 10 years ago and achieved a stable health condition following treatment with biologicals. Before the onset of backache, the patient ate uncooked freshwater fish, and C. sinensis eggs were detected. Both laboratory and imaging tests revealed an active AS disease. Concurrently, lumbosacral pain symptoms notably improved after insecticidal treatment, and relevant laboratory findings demonstrated improvement. Thus, there is speculation about the potential association between disease activity and C. sinensis infection.
Recent reviews have suggested that AS might coexist with various parasitic infections. Some researchers have reported the occurrence of AS alongside mucosal leishmaniasis, Strongyloides stercoralis, and Toxocara canis[12-14]. A study emphasized the potential risk of latent infection reactivation in individuals undergoing immunosuppressive anti-TNF treatment[12]. Additionally, another study supported a notable link between C. sinensis infection and immune suppression, influenced by gender dynamics[15]. Notably, AS patients with parasitic infection may undergo treatment using non-steroidal anti-inflammatory agents. However, administering corticosteroids is contraindicated until insecticidal treatment has been initiated[13].
Microbial infections have shown a close correlation with autoimmune diseases, supported by evidence from clinical studies and animal experiments[16]. Earlier research indicated that C. sinensis might worsen arthritis progression, with this adverse effect potentially linked to changes in immune cell profiles and associated cytokine levels[17]. However, another study suggested that C. sinensis-induced protein could suppress new bone formation in a mouse model in vivo[18]. Notably, the role of C. sinensis infections in modulating arthritis symptoms is a multifaceted and complex phenomenon. Presently, it remains unclear whether there are differences in the pathogenic mechanisms of C. sinensis between humans and other animals. Hence, further research is crucial to explore this correlation.
This case report provided significant insights, an imbalance in the Th1/Th2 immune response might compromise the body's defense mechanisms against specific viral, bacterial, and parasitic pathogens, potentially heightening the risk of opportunistic infections[19]. Patients with rheumatic diseases are frequently treated with biologicals, such as anti-TNF, which increases susceptibility to opportunistic infections[20]. Therefore, rheumatologists must evaluate the patient's condition and manage the duration of biological treatment appropriately. Various microbial infections should be ruled out before initiating biologicals, particularly parasitic infections that might be overlooked due to improving environmental conditions. Early detection and treatment of these parasitic infections can significantly improve patient outcomes.
During the diagnosis of this case, some limitations were encountered. For instance, although colonoscopy suggested intestinal mucosal congestion, further pathological biopsies were not conducted. Given these limitations, advocating for continued monitoring of this patient through scheduled follow-up colonoscopy examinations is essential. If any signs of colonic abnormalities emerge during these assessments, then it is prudent to consider subsequent pathological biopsies for a more comprehensive evaluation.
Our findings suggest that C. sinensis infections could potentially trigger the exacerbation of AS. Clinicians should be mindful of the occurrence of C. sinensis infections, particularly in endemic areas when evaluating patients with rheumatic diseases. Despite providing significant insights, further studies are necessary to elucidate the mechanisms underlying the association between AS and C. sinensis infections.
We thank the colleagues in the laboratory at Traditional Chinese Medicine Hospital Dianjiang Chongqing for providing pictures of C. sinensis eggs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA, India S-Editor: Gao CC L-Editor: A P-Editor: Zhao S
| 1. | Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1266] [Cited by in RCA: 1377] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 2. | Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford). 2014;53:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 447] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 3. | Zochling J, Bohl-Bühler MH, Baraliakos X, Feldtkeller E, Braun J. Infection and work stress are potential triggers of ankylosing spondylitis. Clin Rheumatol. 2006;25:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Zhang X, Sun Z, Zhou A, Tao L, Chen Y, Shi X, Yin J, Ding G. Association Between Infections and Risk of Ankylosing Spondylitis: A Systematic Review and Meta-Analysis. Front Immunol. 2021;12:768741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Qian MB, Zhou XN. Clonorchis sinensis. Trends Parasitol. 2021;37:1014-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Qian MB, Patel C, Palmeirim MS, Wang X, Schindler C, Utzinger J, Zhou XN, Keiser J. Efficacy of drugs against clonorchiasis and opisthorchiasis: a systematic review and network meta-analysis. Lancet Microbe. 2022;3:e616-e624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Liu JX, Liu M, Yu GZ, Zhao QQ, Wang JL, Sun YH, Koda S, Zhang B, Yu Q, Yan C, Tang RX, Jiang ZH, Zheng KY. Clonorchis sinensis infection induces hepatobiliary injury via disturbing sphingolipid metabolism and activating sphingosine 1-phosphate receptor 2. Front Cell Infect Microbiol. 2022;12:1011378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Qi Y, Hu J, Liang J, Hu X, Ma N, Xiang B. Clonorchis sinensis infection contributes to hepatocellular carcinoma progression in rat. Parasitol Res. 2022;121:3403-3415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Harrington D, Lamberton PHL, McGregor A. Human liver flukes. Lancet Gastroenterol Hepatol. 2017;2:680-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Singh S, El-Sakkary N, Skinner DE, Sharma PP, Ottilie S, Antonova-Koch Y, Kumar P, Winzeler E, Poonam, Caffrey CR, Rathi B. Synthesis and Bioactivity of Phthalimide Analogs as Potential Drugs to Treat Schistosomiasis, a Neglected Disease of Poverty. Pharmaceuticals (Basel). 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Zhao J, Huang C, Huang H, Pan JK, Zeng LF, Luo MH, Liang GH, Yang WY, Liu J. Prevalence of ankylosing spondylitis in a Chinese population: a systematic review and meta-analysis. Rheumatol Int. 2020;40:859-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Nicodemo AC, de Andrade HF, Muñoz Torres P, Amato VS. Secondary Prophylaxis with Liposomal Amphotericin B in a Patient with Mucosal Leishmaniasis Undergoing Immunobiological Therapy for Active Ankylosing Spondylitis. Am J Trop Med Hyg. 2019;101:402-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Richter J, Müller-Stöver I, Strothmeyer H, Göbels K, Schmitt M, Häussinger D. Arthritis associated with Strongyloides stercoralis infection in HLA B-27-positive African. Parasitol Res. 2006;99:706-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Jiménez-Balderas FJ, García-Jaimes J, Ríos R, Zonana-Nacach A, Tapia-Romero R, Villanueva N, Méndez-Samperio P, de-la-Rosa-Arana JL. Isolation of IgG antibodies to Toxocara in ankylosing spondylitis patients with acute anterior uveitis. Korean J Ophthalmol. 2014;28:207-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Kan S, Li Q, Li HM, Yao YH, Du XY, Wu CY, Chen GJ, Guo XK, Qian MB, Wang ZJ. Clonorchis sinensis infection modulates key cytokines for essential immune response impacted by sex. PLoS Negl Trop Dis. 2022;16:e0010726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Verma A, Sharda S, Rathi B, Somvanshi P, Pandey BD. Elucidating potential molecular signatures through host-microbe interactions for reactive arthritis and inflammatory bowel disease using combinatorial approach. Sci Rep. 2020;10:15131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Li X, Yang Y, Qin S, Kong F, Yan C, Cheng W, Pan W, Yu Q, Hua H, Zheng K, Tang R. The impact of Clonorchis sinensis infection on immune response in mice with type II collagen-induced arthritis. BMC Immunol. 2020;21:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Lee YJ, Kim MJ, Jo S, Jin SH, Park PR, Park K, Song HC, Kim J, Kim JY, Shim SC, Kim TH, Hong SJ, Kang H, Kim TJ, Won EJ. Clonorchis sinensis-Derived Protein Attenuates Inflammation and New Bone Formation in Ankylosing Spondylitis. Front Immunol. 2021;12:615369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Rostevanov IS, Betesh-Abay B, Nassar A, Rubin E, Uzzan S, Kaplanski J, Biton L, Azab AN. Montelukast induces beneficial behavioral outcomes and reduces inflammation in male and female rats. Front Immunol. 2022;13:981440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Byun JM, Lee CK, Rhee SY, Kim HJ, Kim JW, Shim JJ, Jang JY. The risk of tuberculosis in Korean patients with inflammatory bowel disease receiving tumor necrosis factor-α blockers. J Korean Med Sci. 2015;30:173-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |