Published online Feb 6, 2024. doi: 10.12998/wjcc.v12.i4.835
Peer-review started: October 23, 2023
First decision: November 22, 2023
Revised: December 4, 2023
Accepted: January 5, 2024
Article in press: January 5, 2024
Published online: February 6, 2024
Processing time: 94 Days and 5.6 Hours
Presently, there is no established standard anti-blood clot therapy for patients facing acute myocardial infarction (AMI) complicated by left ventricular thrombus (LVT). While vitamin K antagonists are the preferred choice for oral blood thinning, determining the best course of blood-thinning medication remains challenging. It is unclear if non-vitamin K antagonist oral blood thinners have different effectiveness in treating LVT. This study significantly contributes to the medical community.
The blood-thinning treatment of a patient with AMI and LVT was analyzed. Triple blood-thinning therapy included daily enteric-coated aspirin tablets at 0.1 g, daily clopidogrel hydrogen sulfate at 75 mg, and dabigatran etexilate at 110 mg twice daily. After 15 d, the patient’s LVT did not decrease but instead increased. Clinical pharmacists comprehensively analyzed the cases from the perspective of the patient’s disease status and drug interaction. The drug regimen was reformulated for the patient, replacing dabigatran etexilate with warfarin, and was administered for six months. The clinical pharmacist provided the patient with professional and standardized pharmaceutical services. The patient’s condition was discharged after meeting the international normalized ratio value (2-3) criteria. The patient fully complied with the follow-up, and the time in the therapeutic range was 78.57%, with no serious adverse effects during pharmaceutical monitoring.
Warfarin proves to be an effective drug for patients with AMI complicated by LVT, and its blood-thinning course lasts for six months.
Core Tip: Current guidelines predominantly offer low-grade recommendations favoring warfarin combined with DAPT for treating left ventricular thrombus (LVT) in acute myocardial infarction, unless contraindicated. However, the ideal duration of this triple therapy remains uncertain. Despite vitamin K antagonists being the preferred oral anticoagulant, it is unclear whether non-vitamin K antagonist oral anticoagulants exhibit distinct effectiveness in LVT treatment. This case report emphasizes that not all patients have sufficient evidence supporting the use of direct-acting oral anticoagulants in LVT management, and the recommended duration of triple therapy is six months.
- Citation: Song Y, Li H, Zhang X, Wang L, Xu HY, Lu ZC, Wang XG, Liu B. Individualized anti-thrombotic therapy for acute myocardial infarction complicated with left ventricular thrombus: A case report. World J Clin Cases 2024; 12(4): 835-841
- URL: https://www.wjgnet.com/2307-8960/full/v12/i4/835.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i4.835
Acute myocardial infarction (AMI) is an abrupt thrombus obstruction caused by the rupture of coronary artery plaques, leading to sustained myocardial ischemia and cell death. Left ventricular thrombus (LVT) commonly occurs as a complication after AMI and is linked to systemic thromboembolism[1]. Despite advancements in coronary intervention reducing AMI mortality and LVT incidence, LVT occurs in 2.7%-12.3% of AMI cases and increases to 15% in ST-segment elevation myocardial infarction (STEMI) patients, rising to 25% in anterior wall STEMI cases[2]. While warfarin, the traditional LVT treatment, has drawbacks like a narrow treatment window, numerous drug-food interactions, and frequent monitoring, its use persists. The effectiveness of non-vitamin K antagonist oral anticoagulants (NOACs) in LVT treatment and their comparative advantages remain unclear. This article analyzes anti-thrombotic therapy for AMI complicated by LVT, explores reasons for LVT enlargement despite triple anti-thrombotic drugs, outlines criteria for determining anti-thrombotic therapy, discusses anticoagulation courses, and highlights key pharmaceutical monitoring points. The aim is to offer guidance for treating AMI complicated by LVT.
A 52-year-old male was admitted to the hospital on December 7, 2019, primarily reporting “chest pain persisting for over one month, with a recent worsening over the last two hours”.
On November 7, 2019, the patient experienced persistent dull precordial pain without radiating pain in the shoulder and back, chest tightness, or breath-holding, alleviated by rest. The patient, in good physical health with no history of heart disease, hypertension, diabetes, cerebrovascular disease, or mental illness, initially self-medicated with painkillers and aspirin, providing slight relief. On November 14, 2019, precordial crushing pain ensued, persisting with radiating pain in the shoulder and back, accompanied by general fatigue. Seeking medical attention, an electrocardiogram at a local hospital revealed a sinus heart rate and low limb conduction voltage, indicative of acute inferior, extensive anterior, and posterior myocardial infarction patterns. Urgently transferred to our hospital, emergency coronary angiography diagnosed coronary atherosclerotic heart disease with a single branch lesion (involving the anterior descending branch), necessitating the placement of two stents in the anterior descending branch.
Post-surgery, the patient received triple anti-thrombotic drugs (daily 0.1 g aspirin enteric-coated tablets, daily 75 mg clopidogrel hydrogen sulfate, and twice-daily 60 mg enoxaparin injections), along with daily 47.5 mg metoprolol succinate tablets, daily 20 mg atorvastatin calcium tablets, twice-daily 50 mg sacubitril valsartan sodium tablets, daily 10 mg rabeprazole enteric-coated tablets, and tongxinluo capsules four capsules thrice daily. On November 18, the cardiac ultrasound revealed a left ventricular ejection fraction (LVEF) of 0.49, normal heart chamber diameters, left ventricular wall segmental dyskinesis, slightly decreased left ventricular function, and a thrombus in the apical wall of the left ventricle (approximately 21.0 mm × 15.8 mm × 10.7 mm), along with small pericardial effusion. Discharged on November 25 with an AMI diagnosis, the patient's compliance led to replacing enoxaparin injections with dabigatran etexilate capsules at 110 mg bid, while other discharge medications remained unchanged. One week post-discharge, the patient exhibited 100% medication compliance but presented at our hospital due to chest pain two hours prior, located in the precordial area, without sweating or discernible severity, prompting further treatment.
In October 2019, the patient underwent minimally invasive meniscal surgery for both knees at a local hospital.
The personal and family history did not reveal any notable features.
Temperature: 36.5 °C, pulse rate: 77 beats/min, respiratory rate: 18 beats/min, blood pressure: 109/74 mmHg.
N-terminal brain natriuretic peptide precursor: 1051 pg/mL (0-125 pg/mL); troponin: 0.01 ng/mL. Five markers of coagulation were as follows: D-dimer 1.49 mg/L (0-0.55 mg/L), fibrinogen 4.51 g/L (2-4 g/L), thrombin time 32.9s (14-21s); blood biochemistry: aspartate aminotransferase 52.9 U/L (15-40 U/L), alanine aminotransferase 146.6 U/L (9-50 U/L).
Cardiac ultrasound revealed coronary artery stent implantation, normal heart chamber diameters, segmental dyskinesis of the left ventricular wall, slightly decreased left ventricular function, a LVEF of 0.49, and a band-shaped slightly higher echo of about 45.3 mm × 42.7 mm × 17.4 mm in the apex of the left ventricle. Admission diagnosis was as follows: (1) AMI; (2) Coronary atherosclerotic heart disease; (3) Post-coronary stent implantation; and (4) Ventricular thrombus.
Final diagnosis are as follows: (1) AMI; (2) Coronary atherosclerotic heart disease; (3) Post-coronary stent implantation; (4) Ventricular thrombus; (5) Cerebral infarction; and (6) Hepatic insufficiency.
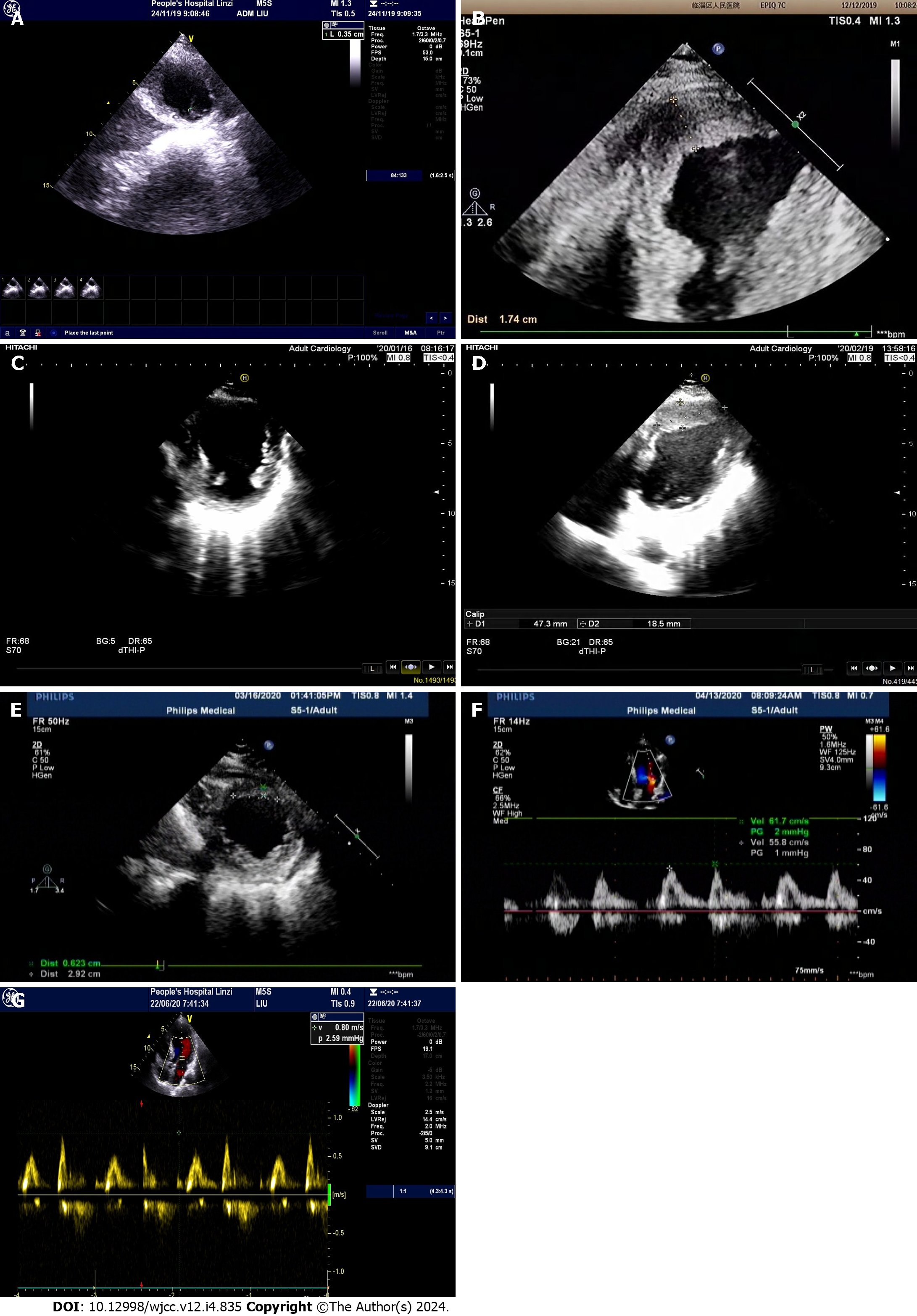
The patient initiated triple anti-thrombotic medication (daily 0.1 g aspirin enteric-coated tablets, daily 75 mg clopidogrel hydrogen sulfate, and twice-daily 110 mg dabigatran ester capsules) on November 25. In comparison with the color ultrasound results on November 24 (LVEF of 0.48 and a left ventricular apex thrombus measuring 17.6 mm in height, 3.5 mm in width, and 4.8 mm at the base), the cardiac ultrasound at admission (December 7) revealed a 112.83 times enlargement of the thrombus (Figures 1A and B). On December 14, 2019, four anti-thrombotic tests, antinuclear antibody spectrum, five vasculitis tests, two phospholipid antibodies, immunoglobulin (Ig) A, M, G, C3, C4 were normal, and IgE was 1841.1. Clinical pharmacists, after reviewing relevant information and case reports, noted that complex disease states post-AMI might exacerbate coagulation falls. While warfarin and NOACs are employed to treat AMI patients with LVT, large-scale randomized controlled trials are lacking. Warfarin, a multi-target drug, acts on the exogenous coagulation pathway (factor VII), endogenous coagulation pathway (factor IX), and common pathway (factor II and X) to block the coagulation network at multiple sites. In contrast, dabigatran, a single-target drug, inhibits only thrombin IIa, and increasing its dose or frequency may elevate the risk of bleeding. Consequently, the clinical pharmacist recommended discontinuing dabigatran etexilate on December 12 and replacing it with warfarin 3 mg orally once daily on December 13 [enoxaparin injection 60 mg intravenously every 12 h was not discontinued until the international normalized ratio (INR) value reached 2-3], a recommendation adopted by the physician. At 3:40 on December 16, 2019, the patient experienced a sudden headache, pronounced dizziness, right-side pain, visual rotation, nausea, vomiting, sweating, shortness of breath, and impaired speech. Plain cranial scanning revealed cerebral infarction (right cerebellum) and cerebral malacia (left basal ganglia to radial crown). The monitored INR value of 0.97 (target value: 2-3) did not meet the standard, but prothrombin time, activated partial thromboplastin time, and thrombin time were normal. Given the thrombus’s instability and the potential for thrombus shedding leading to cerebral artery thromboembolism, the warfarin dose was continually adjusted. Until December 27, the four coagulation tests indicated PT of 31s (normal range: 10.5-14), INR of 2.86 (normal range: 0.8-1.5), fibrinogen of 4.82 g/L (normal range: 2-4), and APTT of 43.3s (normal range: 23-35). The patient remained asymptomatic, leading to discharge once the INR value reached the target range of 2-3. Post-discharge, regular follow-ups were conducted.
The patient remained asymptomatic, leading to discharge once the INR value reached the target range of 2-3. Post-discharge, regular follow-ups were conducted. An outpatient review on January 16, 2020, confirmed a 100% resolution rate of LVT by color ultrasound (Figure 1C). The patient discontinued warfarin and continued dual antiplatelet therapy. On February 19, 2020, a subsequent color ultrasound revealed an enlarged left atrium, a LVEF of 0.45, near disappearance of left ventricular apical motion, paradoxical movement in the thin left ventricular apical part, and an attached wall thrombus measuring approximately 47.3 mm in length, 44.7 mm in width, and 18.5 mm in thickness (Figure 1D). The clinical pharmacist, upon consulting the literature, identified insufficient anticoagulation time in the patient. Accordingly, it was recommended that the patient resume warfarin treatment. The clinical pharmacist referred the patient to our hospital’s anticoagulation clinic for drug education and regular monitoring of INR values. The patient exhibited 100% compliance during the four-month follow-up, color ultrasound on March 16, 2020, showing an LVEF of 0.46 and an attached wall thrombus measuring about 32.8 mm × 29.2 mm × 6.2 mm (Figure 1E), repeat color ultrasound on April 13, 2020 and on June 20, 2020, indicated thrombus resolution (Figures 1F and G). The patient continued warfarin therapy for eight months and dual antiplatelet therapy for at least 12 mo. Throughout the one-year follow-up, the patient reported no discomfort.
The patient developed LVT within four days after AMI. Consequently, triple anti-thrombotic drugs (daily 0.1g aspirin enteric-coated tablets, daily 75 mg clopidogrel hydrogen sulfate, and twice-daily 110 mg dabigatran etexilate) were administered. However, after 15 d, the LVT did not decrease but instead increased. The clinical pharmacist conducted an analysis considering the patient’s disease state, drug interactions, and other factors, ultimately devising an individualized drug therapy for the patient.
The mechanism behind LVT formation in AMI patients remains unclear, with studies indicating the involvement of multiple factors acting on different pathways. Key risk factors are believed to be associated with Virchow’s triad, encompassing altered blood flow due to decreased left ventricular contractility, cardiomyocyte damage, and increased blood coagulability. Myocardial necrosis resulting from AMI can lead to local movement disorders or even reverse movement, causing blood flow stasis that promotes thrombosis[3,4]. Extensive myocardial injury stimulates the agglutination and adherence of numerous fibrin, red blood cells, and platelets to exposed collagen, triggering coagulation cascades and contributing to LVT formation. Myocardial infarction-induced damage to the endocardium, inflammation, and collagen exposure lead to platelet accumulation and coagulation activation[5]. In this case, the optimal period for vascular opening was missed, resulting in an increased infarct area. AMI in the anterior wall (LAD) area, as seen in this patient, is more prone to causing LVT. The extensive transmural myocardial infarction and early revascularization performed in this study may have led to the formation of microchannels in the myocardium, releasing blood and causing a small effusion in the pericardium, indicating the possibility of hemorrhagic transformation. This transformation can activate platelets and further enlarge the thrombus.
Dabigatran is a substrate of P-glycoprotein (P-gp), a type of efflux transporter responsible for the reverse transport of various structurally diverse compounds out of cells. Atorvastatin acts as both a moderate inhibitor and substrate of P-gp, in addition to inhibiting CYP3A4, which can impact P-gp activity. The combination of these two drugs may result in an 18% reduction in dabigatran etexilate exposure under homeostasis (area under the plasma concentration-time curve of the drug under homeostasis)[6]. In a substantial retrospective cohort study involving 91330 patients with non-valvular atrial fibrillation in Taiwan, the incidence of major bleeding significantly decreased in patients receiving dabigatran (49.65% of participants) with atorvastatin compared to those on single NOACs[7]. Clinical pharmacists posit that this drug combination may reduce the anticoagulant activity of dabigatran etexilate. However, the guidelines do not currently clarify the interaction between these two drugs, and additional clinical evidence is required to confirm this result.
Guidelines recommend dual antiplatelet plus anticoagulation for AMI complicated with LVT. However, there is a lack of substantial randomized controlled trials to guide the selection of anticoagulant therapy[8,9]. Considering medication compliance, this patient received triple anti-thrombotic therapy, including aspirin enteric-coated tablets, clopidogrel tablets, and dabigatran etexilate. The primary reason for the unsatisfactory efficacy was that dabigatran etexilate, being a single-target drug with gene polymorphisms, may have limitations. In contrast, warfarin, a multi-target drug, can inhibit the synthesis of various coagulation factors and modify precursor proteins of anticoagulant proteins C and S simultaneously, disrupting the coagulation process without causing thrombus shedding. Consequently, the anti-thrombotic therapy was modified to include aspirin enteric-coated tablets, clopidogrel, and warfarin, yielding significant clinical effectiveness. The ventricular thrombus resolved by January 16, 2020, as confirmed by cardiac ultrasound. Adar et al[10] reported suboptimal efficacy and safety of NOACs in treating LVT.
After the resolution of the thrombus, the patient ceased warfarin. However, a repeat color ultrasound on February 19, 2020, revealed a 100% thrombus enlargement rate within one month, presenting a challenge in defining the course of anticoagulant drugs. Clinical pharmacists conducted a thorough review of relevant information, referring to the US ACCF/AHA STEMI guideline from 2013, which suggests the addition of warfarin anticoagulation therapy to dual antiplatelet therapy for three months in STEMI patients with asymptomatic LVT. The recommended target INR is 2.0-2.5 (evidence level: class IIb and level C)[8]. The ESC STEMI guideline from 2017 proposes that STEMI patients with LVT should be considered for oral anticoagulation for six months, guided by regular echocardiography, and taking into account bleeding risk and platelet factors[9]. Current guidelines regarding oral anticoagulant recommendations for LVT primarily rely on clinical expert consensus or observational findings. Due to the scarcity of evidence from randomized controlled trials, the recommendation level for this therapy remains low. In summary, clinical pharmacists advised that anticoagulation for AMI complicated with LVT should be administered for a minimum of six months, followed by dual antiplatelet therapy for at least 12 mo. Physicians agreed to reintroduce warfarin for anticoagulation. Subsequent follow-ups and repeat color ultrasounds on April 13, 2020, indicated thrombus elimination. Warfarin was not discontinued until August 2020, and the patient remained thrombus-free three months after warfarin cessation.
During the hospitalization period, the patient received detailed information about the use of warfarin, along with precautions regarding the impact of food and other medications on warfarin’s anticoagulant effect. Continuous monitoring of coagulation indicators was recommended to maintain the INR value within the target range of 2-3, allowing for the detection of bleeding or other related adverse effects. Patients were advised to promptly seek medical attention if any discomfort arose. Upon discharge, patients were enrolled in the anticoagulation group of the chronic disease system, ensuring uniform management by clinical pharmacists during follow-up. During follow-up hospital visits, the clinical pharmacist assessed signs and symptoms of bleeding and thrombus, current medical issues, dietary habits, and patient compliance. If necessary, the patient was referred to a physician for further diagnosis and treatment. In cases where consultation with a physician was not required, the clinical pharmacist updated or adjusted the warfarin dose, scheduled the next follow-up, and maintained up-to-date medical records. Throughout the follow-up period, the patient demonstrated 100% medication compliance, and no bleeding-related adverse effects were reported. Current domestic and foreign guidelines advocate using the therapeutic range (TTR) compliance rate to evaluate the stability of warfarin anticoagulation[11]. The TTR for this case reached 78.57%, surpassing the stability compliance standard of 65%, as calculated using the follow-up days compliance calculation method[12]. Clinical pharmacists delivered professional, streamlined, and standardized pharmacy services throughout the process, significantly enhancing patient compliance and the overall compliance rate of anticoagulation therapy.
The existing guidelines suffer from a dearth of large-scale clinical randomized controlled studies focusing on anti-thrombotic therapy and treatment courses for patients experiencing AMI complicated by LVT. Consequently, the recommendation level in these guidelines is relatively low. The conventional approach revolves around anticoagulant therapy primarily using warfarin. Acknowledging the limitations associated with warfarin, clinical pharmacists take charge of delivering comprehensive anticoagulant pharmacy services. This approach not only enhances compliance rates during follow-up but also diminishes the occurrence of bleeding, embolism, and other complications. Additionally, it aids physicians in managing drug interactions, conducting drug monitoring, and performing other related tasks. This collaborative effort serves to alleviate the burden on physicians, affording them more energy and time to attend to a greater number of patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Palomino ZJ, Brazil S-Editor: Wang JJ L-Editor: A P-Editor: Chen YX
| 1. | Zhang Q, Wang CM, Shi ST, Chen H, Zhou YJ. Relationship of left ventricular thrombus formation and adverse outcomes in acute anterior myocardial infarction in patients treated with primary percutaneous coronary intervention. Clin Cardiol. 2019;42:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Xiao TH, Wang SW, Chen YM, Chen Q, Zhang XY, Xu B. [Analysis of related factors for development of left ventricular thrombosis after ventricular aneurysm following myocardial infarction]. Clinical Focus. 2007;22:464-466. [DOI] [Full Text] |
| 3. | Gianstefani S, Douiri A, Delithanasis I, Rogers T, Sen A, Kalra S, Charangwa L, Reiken J, Monaghan M, MacCarthy P. Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol. 2014;113:1111-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med. 1984;100:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 197] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Shacham Y, Leshem-Rubinow E, Ben Assa E, Rogowski O, Topilsky Y, Roth A, Steinvil A. Frequency and correlates of early left ventricular thrombus formation following anterior wall acute myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. 2013;111:667-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Wessler JD, Grip LT, Mendell J, Giugliano RP. The P-glycoprotein transport system and cardiovascular drugs. J Am Coll Cardiol. 2013;61:2495-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 7. | Chang SH, Chou IJ, Yeh YH, Chiou MJ, Wen MS, Kuo CT, See LC, Kuo CF. Association Between Use of Non-Vitamin K Oral Anticoagulants With and Without Concurrent Medications and Risk of Major Bleeding in Nonvalvular Atrial Fibrillation. JAMA. 2017;318:1250-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 313] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 8. | O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Stevenson WG, Yancy CW; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362-e425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 1136] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 9. | Ibánez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol (Engl Ed). 2017;70:1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 10. | Adar A, Onalan O, Cakan F. Newly developed left ventricular apical thrombus under dabigatran treatment. Blood Coagul Fibrinolysis. 2018;29:126-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Huang D, Wong CL, Cheng KW, Chan PH, Yue WS, Wong CK, Ho CW, Wong ICK, Chan EW, Siu CW. Impact of provision of time in therapeutic range value on anticoagulation management in atrial fibrillation patients on warfarin. Postgrad Med J. 2018;94:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Esteve-Pastor MA, Rivera-Caravaca JM, Roldán-Rabadán I, Roldán V, Muñiz J, Raña-Míguez P, Ruiz-Ortiz M, Cequier Á, Bertomeu-Martínez V, Badimón L, Anguita M, Lip GYH, Marín F; FANTASIIA Investigators. Quality of oral anticoagulation with vitamin K antagonists in 'real-world' patients with atrial fibrillation: a report from the prospective multicentre FANTASIIA registry. Europace. 2018;20:1435-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |