Published online Feb 6, 2024. doi: 10.12998/wjcc.v12.i4.820
Peer-review started: November 6, 2023
First decision: November 16, 2023
Revised: November 22, 2023
Accepted: January 12, 2024
Article in press: January 12, 2024
Published online: February 6, 2024
Processing time: 79 Days and 23.1 Hours
Human epidermal growth factor receptor-2 (HER-2) plays a vital role in tumor cell proliferation and metastasis. However, the prognosis of HER2-positive gastric cancer is poor. Inetetamab, a novel anti-HER2 targeting drug independently developed in China, exhibits more potent antibody-dependent cell-mediated cytotoxicity than trastuzumab, which is administered as the first-line treatment for HER2-positive gastric cancer in combination with chemotherapy. In this case, the efficacy and safety of inetetamab combined with tegafur was investigated as a second-line treatment for HER2-positive gastric cancer.
A 52-year-old male patient with HER2-positive gastric cancer presented with abdominal distension, poor appetite, and fatigue two years after receiving six cycles of oxaliplatin combined with tegafur as first-line treatment after surgery, followed by tegafur monotherapy for six months. The patient was diagnosed with postoperative recurrence of gastric adenocarcinoma. He received 17 cycles of a combination of inetetamab, an innovative domestically developed anti-HER2 monoclonal antibody, and tegafur chemotherapy as the second-line treatment (inetetamab 200 mg on day 1, every 3 wk combined with tegafur twice daily on days 1–14, every 3 wk). Evaluation of the efficacy of the second-line treatment revealed that the patient achieved a stable condition and progression-free survival of 17 months. He tolerated the treatment well without exhibiting any grade 3-4 adverse events.
Inetetamab combined with chemotherapy for the treatment of metastatic HER2-positive gastric cancer demonstrates significant survival benefits and acceptable safety.
Core Tip: In this paper, we present a case involving a patient with human epidermal growth factor receptor-2 (HER2)-positive gastric cancer who was received oxaliplatin combined with tegafur as the first-line treatment post surgery. The patient was diagnosed with postoperative recurrence of gastric adenocarcinoma. He received inetetamab, an innovative domestically developed anti-HER2 monoclonal antibody, combined with tegafur chemotherapy as the second-line treatment. Evaluation of the efficacy of the second-line treatment revealed that the patient achieved a stable condition. This is significant because We provided a practical reference case for HER2-positive gastric cancer patients who was received inetetamab that is an innovative domestically developed anti-HER2 monoclonal antibody, which may help to provide survival benefits to some extent.
- Citation: Zhou JH, Yi QJ, Li MY, Xu Y, Dong Q, Wang CY, Liu HY. Inetetamab combined with tegafur as second-line treatment for human epidermal growth factor receptor-2-positive gastric cancer: A case report. World J Clin Cases 2024; 12(4): 820-827
- URL: https://www.wjgnet.com/2307-8960/full/v12/i4/820.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i4.820
Gastric cancer is a malignant tumor that poses a significant threat to human life and health. In 2020, gastric cancer ranked as the fifth most common cancer, accounting for 5.6% of all new cancer cases globally[1]. However, the 5-year survival rate for advanced gastric cancer is low, standing at 33%[1,2]. Approximately 50% of all global deaths from gastric cancer occur in China[3]. Human epidermal growth factor receptor-2 (HER-2) is a crucial member of the epidermal growth factor receptor (ERbB) family and plays a vital role in tumor cell proliferation and metastasis. HER2-positive gastric cancer is a distinct subtype. A previous study has revealed that the proportion of HER2-positive cases among gastric cancer patients in China is 8.8%[4]. The prognosis for HER2-positive gastric cancer is poor, with a 5-year survival rate of less than 20%[1]. Given the poor prognosis of HER2-positive gastric cancer and increased understanding about the HER2 gene, numerous molecular drugs targeting HER2-positive cancer have been developed. The ToGA study[5] demonstrated that the overall survival of HER2-positive gastric cancer patients improved by 2.7 months following the administration of trastuzumab combined with chemotherapy; thus, this combination was established as the first-line treatment for HER2-positive gastric cancer. However, the survival benefits of trastuzumab in HER2-positive gastric cancer patients are less than those observed in breast cancer patients. Inetetamab, which is a novel anti-HER2-targeting drug that was independently developed in China, exhibits more potent antibody-dependent cell-mediated cytotoxicity (ADCC) than trastuzumab.
In this study, we report a case of postoperative recurrence and progression of HER2-positive gastric cancer treated with inetetamab combined with chemotherapy as a second-line treatment, resulting in more than 17 months of survival. This case will provide valuable insights into the use of inetetamab in the treatment of advanced HER2-positive gastric cancer.
In May 2021, a 52-year-old man who had previously been diagnosed with HER2-positive gastric cancer presented himself to the Second Affiliated Hospital of Shandong First Medical University with symptoms of abdominal distension, poor appetite, and fatigue.
The patient experienced recurrence and metastasis more than two years after undergoing a laparoscopy-assisted D2 radical gastrectomy for gastric cancer in May 2019. Postoperative pathological analysis revealed infiltrating gastric adenocarcinoma, some of which exhibited signet-ring cell morphology.
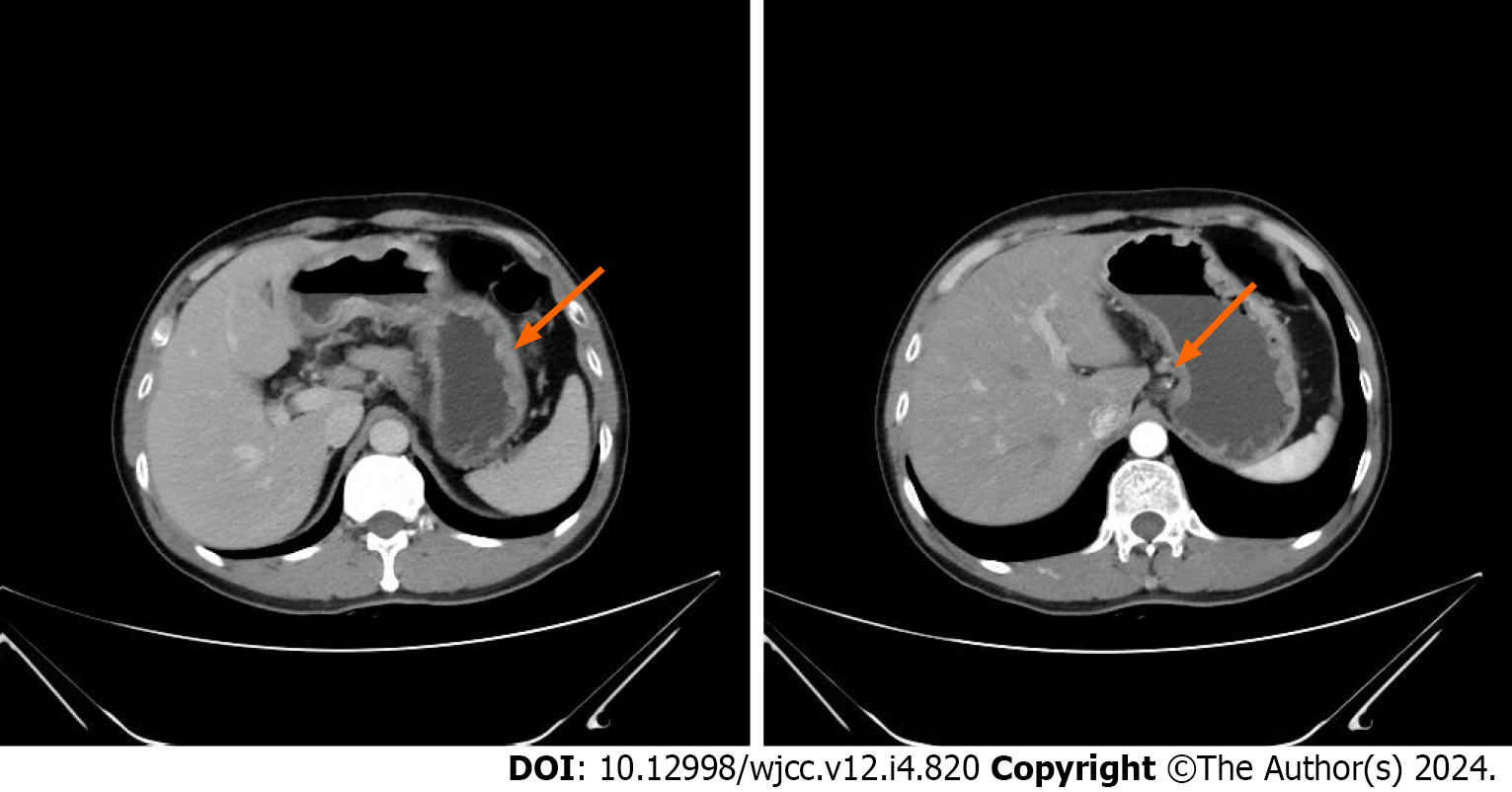
In May 2019, the patient was admitted to the Second Affiliated Hospital of Shandong First Medical University with paroxysmal dull pain in the upper abdomen. A painless gastroscopy was performed, and the pathological findings of the biopsy were consistent with poorly differentiated adenocarcinoma and signet-ring cell carcinoma. The concentration of cancer antigen (CA) 199 was 151.84 U/mL, while carcinoembryonic antigen and CA724 were within the normal range. Contrast-enhanced abdominal computed tomography (CT) revealed abnormal enhancement was observed on the greater curvature of the gastric body and antrum, with enlarged lymph nodes around it (Figure 1). In late May 2019, the patient underwent laparoscopy-assisted radical gastrectomy. The postoperative pathological report indicated a low-to-moderately differentiated invasive adenocarcinoma involving the entire stomach, with some parts displaying signet-ring cell morphology. The tumor measured 9 cm × 4.5 cm × 1.2 cm. It had penetrated the entire abdominal wall, broken through the mesothelial cell layer, and showed extensive vascular and nerve invasions. The upper incisal margin was not involved, but the lower incisal margin was affected. Cancer metastasis was observed in 8 out of 8 Lymph nodes at the greater curvature of the stomach with three cancerous nodules, 16 out of 18 Lymph nodes at the lesser curvature of the stomach as well as in the omentum and mesenteric nodules, and 2 out of 2 Lymph nodes of group 12. Immunohistochemical analysis revealed HER2 positivity (3+), PDL1 negativity (-), MSH2 positivity (+), MSH6 positivity (+), PMS2 positivity (+), and weak MLH1 positivity (+). Gastric cancer-related gene detection revealed ERBB2-IGR fusion, ERBB2 gene amplification (tissue abundance of 7.8 times), and missense mutations in exon 3 of RHOA p.L69Q (tissue abundance of 11.5%) and exon 5 of TP53p.R175H (tissue abundance of 14.9%). After surgery for newly diagnosed gastric adenocarcinoma (pT4aN3M1, stage IV, positive margin), metastases occurred in the omentum and mesentery. For economic reasons, the patient declined anti-HER2 therapy. Instead, he underwent six cycles of postoperative adjuvant chemo
The patient had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 1. He had no history of underlying diseases or family history of cancer. His cardiopulmonary examination results were normal.
The concentration of the tumor marker CA199 was 135.25 U/mL (normal range: 0-35). A hemoglobin level of 83 g/L and serum protein level of 30.2 g/L, which indicated moderate anemia and hypoproteinemia, respectively, was also observed.
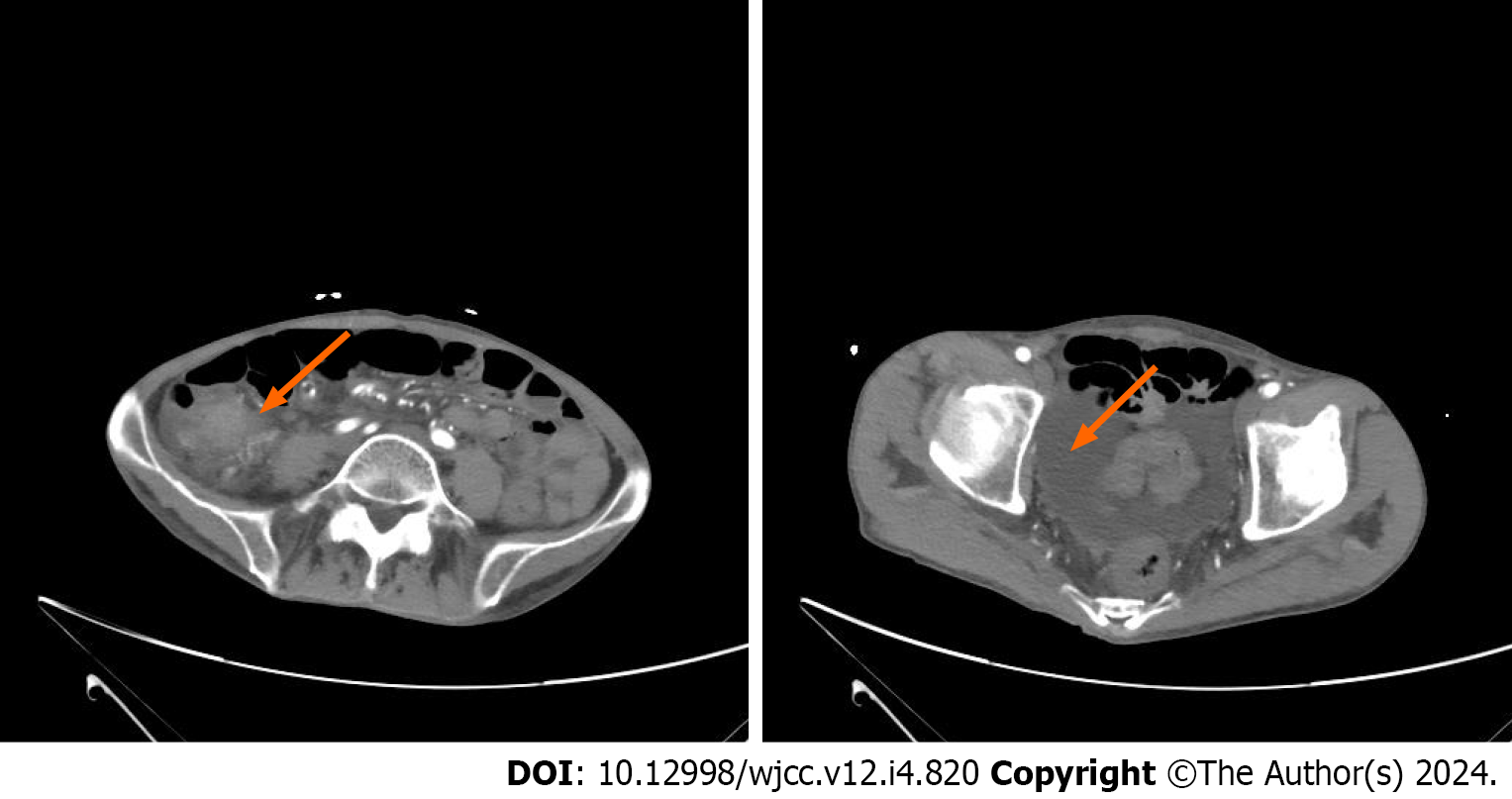
Contrast-enhanced abdominal CT revealed significant local thickening and enhancement of the ileum wall, along with abdominal and pelvic effusion and multiple enlarged lymph nodes surrounding the ileum (Figure 2).
Based on the disease progression, the patient was diagnosed with postoperative recurrence of gastric adenocarcinoma.
Second-line treatment was initiated at the end of May 2021. The treatment regimen consisted of 200 mg of inetetamab on day 1 and 40 mg of tegafur twice daily from day 1 to day 14, with a treatment cycle that repeated every three weeks. The patient underwent a total of 17 cycles of second-line treatment until September 2022.
The efficacy of second-line treatment was evaluated using contrast-enhanced CT scans conducted at cycles 2, 4, 8, 10, and 12 (Figures 3 and 4). The results of this evaluation indicated SD, and after two cycles of treatment, the expression of the tumor marker CA199 was significantly decreased (Figure 5).
In October 2022, the patient underwent re-examination, and contrast-enhanced abdominal CT revealed thickening of the urinary bladder wall and a significantly higher CA199 concentration than before (Figure 5). A cystoscopic biopsy was performed, and the postoperative pathology indicated the presence of poorly differentiated malignant tumors in the urinary bladder, some of which showed signet-like features. The results of immunohistochemical analysis showed uroplakin3 (-), villin (+), CDX-2 (-), GATA3 (-), P63 (individual+), PSA (-), TTF-1 (-), CK20 (focal +), and CK7 (+). The immunophenotypes of the tumor cells were MSH2 (+), MSH6 (+), PMS2 (+), MLH1 (+), and PDL1 (-). These results did not support a diagnosis of adenocarcinoma of lung, prostate, and intestinal origin, or squamous cell carcinoma. However, the possibility of metastatic or dedifferentiated primary cancer could not be ruled out. Following second-line treatment, the patient achieved a progression-free survival (PFS) of 17 months. As the patient’s condition worsened and disease progressed further, third-line treatment with disitamab vedotin was initiated at the end of October 2022.
During the treatment, the patient’s blood cell count, liver and kidney function, color Doppler echocardiography, and inflammatory index were regularly monitored. The patient did not report any symptoms of nausea or vomiting, and bone marrow suppression did not occur, as indicated by the absence of any decrease in leukocyte, neutrophil, or platelet levels. Additionally, no significant abnormalities were observed in liver and kidney function. Color Doppler echocardiography did not reveal any signs of cardiac toxicity. Treatment discontinuation or dose adjustment was not necessary during the course of the treatment.
Various studies[6-10] have demonstrated that the HER2 gene is overexpressed in multiple tumor types, including breast, lung, ovarian, prostate, and colorectal cancers. Around 20% of the patients with gastric cancer exhibit HER2 overexpression; these HER2-positive patients tend to have poor prognoses and are at higher risk of recurrence[5,11]. HER2 has emerged as a crucial biomarker and therapeutic target in gastric cancer treatment, leading to increased clinical interest in this gene. The results of the ToGA study revealed that the combination of the anti-HER2 drug, trastuzumab, with chemotherapy could extend the survival of patients with advanced gastric cancer. Subsequent investigations have also yielded satisfactory results[12-14]. Consequently, trastuzumab combined with chemotherapy has become the standard first-line treatment for advanced HER2-positive gastric cancer. Nevertheless, challenges such asthe high cost and development of drug resistance continue to be obstacles in clinical application.
Inetetamab is a groundbreaking anti-HER2 monoclonal antibody drug that was independently developed in China. According to Wang et al[15], both inetetamab and trastuzumab possess two identical Fab segments, each containing 214 amino acids, indicating that their binding activity and affinity for HER2 antigens are the same. However, the Fc segment of inetetamab has been modified with amino acids and subjected to optimized manufacturing, resulting in the improvement of its ADCC effect by 11.1% compared to trastuzumab, along with reduced risk of immunogenicity. The HOPES study[16,17] has further confirmed the safety and efficacy of inetetamab, demonstrating that its effectiveness is comparable to trastuzumab in treating advanced HER2-positive breast cancer. Subgroup analysis in this study showed that HER2-positive metastatic patients treated with inetetamab combined with chemotherapy achieved a median PFS of 9.2 months, while first-line chemotherapy for recurrence and metastasis resulted in a median PFS of 11.1 months. Importantly, the combination of inetetamab with chemotherapy did not lead to a significant increase in toxicity. Various case reports, such as those by Gui et al[18], Zhang et al[19], Li et al[20], and Cai et al[21], have also documented the considerable efficacy and good safety of inetetamab therapy for advanced HER2-positive breast cancer. Additionally, Deng et al[22] revealed the inhibitory effects of inetetamab combined with chemotherapy in the treatment of advanced gastric and breast cancers, thereby providing a promising therapeutic option for these two types of tumors.
The patient in this case exhibited HER2 (3+) in the immunohistochemical assay after undergoing R1 resection for stage IV gastric adenocarcinoma. The disease progressed after first-line treatment, and the patient exhibited anemia and hypoproteinemia and had an ECOG PS score of 1; therefore, intensive treatment was deemed to be intolerable and avoided. Considering these factors, the second-line treatment of choice was the combination of inetetamab, which is a domestic innovative anti-HER2 monoclonal antibody, combined with tegafur. Remarkably, after receiving 17 cycles of treatment, the patient achieved a PFS of 17 months, which significantly surpassed clinical expectations. Furthermore, the treatment was well-tolerated, safe, and led to improved quality of life. The patient’s condition remained stable throughout the treatment. In this case, the second-line treatment proved to be highly effective, with substantial survival benefits. Thus, the clinical efficacy of the ADCC-optimized monoclonal antibody inetetamab combined with chemotherapy for advanced HER2-positive gastric cancer shows great promise and merits further exploration.
The treatment of metastatic HER2-positive gastric cancer with inetetamab combined with chemotherapy demonstrates significant survival benefits and acceptable safety. As precision therapy for gastric cancer continues to evolve, researchers have focused on the molecular characteristics and immune microenvironment of this type of cancer. Immunotherapy is emerging as a new area of interest. KEYNOTE-811[23] demonstrated that the objective response rate of the immunotherapy group increased by 22% compared to that of the control group, indicating that this approach could become an effective treatment option for advanced HER2-positive gastric cancer. With further clinical research, it is anticipated that optimal treatments for HER2-positive gastric cancer will be identified and developed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lengyel CG, Italy S-Editor: Yan JP L-Editor: A P-Editor: Zhang Y
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64647] [Article Influence: 16161.8] [Reference Citation Analysis (176)] |
| 2. | Cancer.Net. Stomach cancer: statistics. August 2023. Available from: https://www.cancer.net/cancer-types/stomach-cancer/statistics. |
| 3. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11938] [Article Influence: 2984.5] [Reference Citation Analysis (4)] |
| 4. | Huang D, Li ZS, Fan XS, Wu HM, Liu JP, Sun WY, Li SS, Hou YY, Nie X, Li J, Qin R, Guo LC, Xu JH, Zhang HZ, Sun MM, Guo QN, Yang YH, Liu YH, Qin Y, Zhang LJ, Li JH, Zhang ZH, Gao P, Li YJ, Sheng WQ; Cooperative Gastric Cancer Study Group of China Anti-Cancer Association Tumor Pathology Committee. [HER2 status in gastric adenocarcinoma of Chinese: a multicenter study of 40 842 patients]. Zhonghua Bing Li Xue Za Zhi. 2018;47:822-826. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5326] [Article Influence: 355.1] [Reference Citation Analysis (3)] |
| 6. | Tarantino P, Jin Q, Tayob N, Jeselsohn RM, Schnitt SJ, Vincuilla J, Parker T, Tyekucheva S, Li T, Lin NU, Hughes ME, Weiss AC, King TA, Mittendorf EA, Curigliano G, Tolaney SM. Prognostic and Biologic Significance of ERBB2-Low Expression in Early-Stage Breast Cancer. JAMA Oncol. 2022;8:1177-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 101] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 7. | Son J, Jang J, Beyett TS, Eum Y, Haikala HM, Verano A, Lin M, Hatcher JM, Kwiatkowski NP, Eser PÖ, Poitras MJ, Wang S, Xu M, Gokhale PC, Cameron MD, Eck MJ, Gray NS, Jänne PA. A Novel HER2-Selective Kinase Inhibitor Is Effective in HER2 Mutant and Amplified Non-Small Cell Lung Cancer. Cancer Res. 2022;82:1633-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Rossini A, Giussani M, Ripamonti F, Aiello P, Regondi V, Balsari A, Triulzi T, Tagliabue E. Combined targeting of EGFR and HER2 against prostate cancer stem cells. Cancer Biol Ther. 2020;21:463-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Huang W, Chen Y, Chang W, Ren L, Tang W, Zheng P, Wu Q, Liu T, Liu Y, Wei Y, Xu J. HER2 positivity as a biomarker for poor prognosis and unresponsiveness to anti-EGFR therapy in colorectal cancer. J Cancer Res Clin Oncol. 2022;148:993-1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Ersoy E, Cao QJ, Otis CN. HER2 Protein Overexpression and Gene Amplification in Tubo-Ovarian High-grade Serous Carcinomas. Int J Gynecol Pathol. 2022;41:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, Stoss O, Hill J, Ng V, Lehle M, Thomas M, Kiermaier A, Rüschoff J. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 452] [Cited by in RCA: 428] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 12. | Rivera F, Romero C, Jimenez-Fonseca P, Izquierdo-Manuel M, Salud A, Martínez E, Jorge M, Arrazubi V, Méndez JC, García-Alfonso P, Reboredo M, Barriuso J, Muñoz-Unceta N, Jimeno R, López C. Phase II study to evaluate the efficacy of Trastuzumab in combination with Capecitabine and Oxaliplatin in first-line treatment of HER2-positive advanced gastric cancer: HERXO trial. Cancer Chemother Pharmacol. 2019;83:1175-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Rivera F, Izquierdo-Manuel M, García-Alfonso P, Martínez de Castro E, Gallego J, Limón ML, Alsina M, López L, Galán M, Falcó E, Manzano JL, González E, Muñoz-Unceta N, López C, Aranda E, Fernández E, Jorge M, Jiménez-Fonseca P. Perioperative trastuzumab, capecitabine and oxaliplatin in patients with HER2-positive resectable gastric or gastro-oesophageal junction adenocarcinoma: NEOHX phase II trial. Eur J Cancer. 2021;145:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Gong J, Liu T, Fan Q, Bai L, Bi F, Qin S, Wang J, Xu N, Cheng Y, Bai Y, Liu W, Wang L, Shen L. Optimal regimen of trastuzumab in combination with oxaliplatin/ capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): a multicenter, phase II trial. BMC Cancer. 2016;16:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Wang XW, Liu PP, Lu FH, Tan QQ. Evaluation of critical quality attributes of an anti-HER2 humanized monoclonal antibody drug. Zhongguo Yaoxue Zazhi. 2015;50:1054-1061. |
| 16. | Bian L, Xu BH, Di LJ, Wang T, Wang XJ, Jiao SC, Yang JL, Tong ZS, Liu J, Feng JF, Liu DG, Yu QT, Liu YP, Ma Y, Yu H, Jiang ZF. [Phase Ⅲ randomized controlled, multicenter, prospective study of recombinant anti-HER2 humanized monoclonal antibody (Cipterbin) combined with vinorelbine in patients with HER2 positive metastatic breast cancer: the HOPES Study]. Zhonghua Yi Xue Za Zhi. 2020;100:2351-2357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8204] [Cited by in RCA: 8139] [Article Influence: 339.1] [Reference Citation Analysis (0)] |
| 18. | Gui XJ, Zhao JL, Wang Y, Chai J, Din L, Yao H. The successful application of inetetamab combined with pyrotinib in the treatment of HER-2 positive advanced breast cancer: a case report. Lingnan Xiandai Linchuang Waike. 2021;21:675-678. [DOI] [Full Text] |
| 19. | Zhang Y, Xu ZY. Posterior line treatment with Inetetamab for HER2-positive advanced breast cancer: a case report. Zhongguo Dandai Yiyao. 2023;30:165-168. |
| 20. | Li Y, Peng W, Zhong JC. Inetetamab combining pyrotinib as second-line treatment of HER2-positive patients: A case report. Zhongguo Xinyao Zazhi. 2023;32:916-920. |
| 21. | Cai YY, Zhao JL, Wang Y, Ding LXX, Chai J, Luo SM, Yao HR. Inetetamab combined with pyrotinib in the treatment of primary drug-resistant HER2 positive advanced breast cancer: a case report. Shiyong Yaowu Yu Linchuang. 2023;26:285-288. [DOI] [Full Text] |
| 22. | Deng L, Zhao L, Liu L, Huang H. Systemic investigation of inetetamab in combination with small molecules to treat HER2-overexpressing breast and gastric cancers. Open Life Sci. 2023;18:20220535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 23. | Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, Wyrwicz LS, Xu J, Shitara K, Qin S, Van Cutsem E, Tabernero J, Li L, Shah S, Bhagia P, Chung HC. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 480] [Article Influence: 120.0] [Reference Citation Analysis (1)] |