Published online Feb 6, 2024. doi: 10.12998/wjcc.v12.i4.806
Peer-review started: October 11, 2023
First decision: November 20, 2023
Revised: December 8, 2023
Accepted: January 8, 2024
Article in press: January 8, 2024
Published online: February 6, 2024
Processing time: 105 Days and 14 Hours
Hemichorea and other hyperkinetic movement disorders are uncommon presentations of stroke and are usually secondary to deep infarctions affecting the basal ganglia and thalamus. Therefore, temporal ischemic lesions causing hemichorea are rare. We report the cases of two patients with acute ischemic temporal lobe infarct strokes that presented as hemichorea.
Patient 1: An 82-year-old woman presented with a 1-mo history of involuntary movement of the left extremity, which was consistent with hemichorea. Her diffusion-weighted imaging (DWI) revealed an acute ischemic stroke that predominantly affected the right temporal cortex, and magnetic resonance angiography of the head showed significant stenosis of the right middle cerebral artery (MCA). Treatment with 2.5 mg of olanzapine per day was initiated. When she was discharged from the hospital, her symptoms appeared to have improved compared with those previously observed. Twenty-seven days after the first admission, she was readmitted due to acute ischemic stroke. Computed tomogra
When acute hemichorea suddenly appears, temporal cortical ischemic stroke should be considered a possible diagnosis. In addition, hemichorea may be a sign of impending cerebral infarction with MCA stenosis.
Core Tip: Temporal ischemic lesions causing hemichorea are rare in stroke patients, likely delaying proper diagnosis and treatment. We present two cases in which a temporal lobe infarct caused hemichorea. Considering the well-established and time-dependent benefits of reperfusion therapies, we believe that acute onset of hemichorea likely leads to acute stroke. It should be noted that middle cerebral artery stenosis can present with persistent hemichorea, even in the absence of cerebral infarction. Thus, vascular imaging is essential for patients presenting with hemichorea.
- Citation: Wang XD, Li X, Pan CL. Hemichorea in patients with temporal lobe infarcts: Two case reports. World J Clin Cases 2024; 12(4): 806-813
- URL: https://www.wjgnet.com/2307-8960/full/v12/i4/806.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i4.806
Hyperkinetic movement disorders are uncommon in acute stroke patients, with hemichorea being the most common type. Hemichorea is a subtype of chorea and movement disorder that is characterized by continuous, involuntary, and nonrhythmic movements affecting one side of the body[1]. Hemichorea is usually caused by a structural lesion in the contralateral basal ganglia or subthalamic nuclei and can develop as a complication of a neurologic condition, such as hyperglycemia. More recently, cortical ischemic lesions have been observed in locations that cause chorea in stroke patients. Herein, we present the cases of two patients who suffered acute hemichorea secondary to temporal cortical acute ischemic stroke, and we highlight the role of the cortex in acute hyperkinetic disorders. The condition is characterized by unilateral involuntary choreiform movements and confirmed temporal cerebral infarction. In this case, hemichorea completely disappeared after olanzapine treatment and revascularization.
Case 1: An 82-year-old woman with a 1-mo history of involuntary movement of the left extremity.
Case 2: A 76-year-old man presented with a 7-d history of right-upper-sided involuntary movements.
Case 1: The patient presented with a 1-mo history of involuntary movement of the left lower extremity. This involuntary movement of the left lower extremity did not occur during sleep. She had no limb weakness or numbness, aphasia, consciousness disorders, or other discomfort.
Case 2: The patient presented with a 7-d history of involuntary movements of the lips and right upper limb. When stressed, the abnormal movement worsened. The involuntary movement was persistent and unrelated to the patient’s posture. The abnormal movement completely disappeared only during deep sleep.
Case 1: The patient had hypertension (HTN) for 8 years. Her HTN was well controlled (mean systolic blood pressure 140 mmHg) with medication.
Case 2: The patient had no previous abnormalities.
Case 1: The patient had no personal or family history of the disease.
Case 2: The patient had a history of smoking and drinking for 20 years.
Case 1: During neurological examination, the patient was noted to have involuntary, nonrhythmic, and uncontrollable movements of the left lower extremity.
Case 2: On neurological examination, moderately severe abnormal involuntary, nonrhythmic, and uncontrollable movement was evident in the lips and right upper limb, and there were no other signs of neurological impairment.
Case 1: The patient’s laboratory tests revealed that her random blood glucose level was 13.4 mmol/L (normal range: 3.9-11.1 mmol/L) and her glycosylated hemoglobin was 7.1% (normal range: 4%-6%), however, the other test results were normal.
Case 2: The laboratory test results were normal.
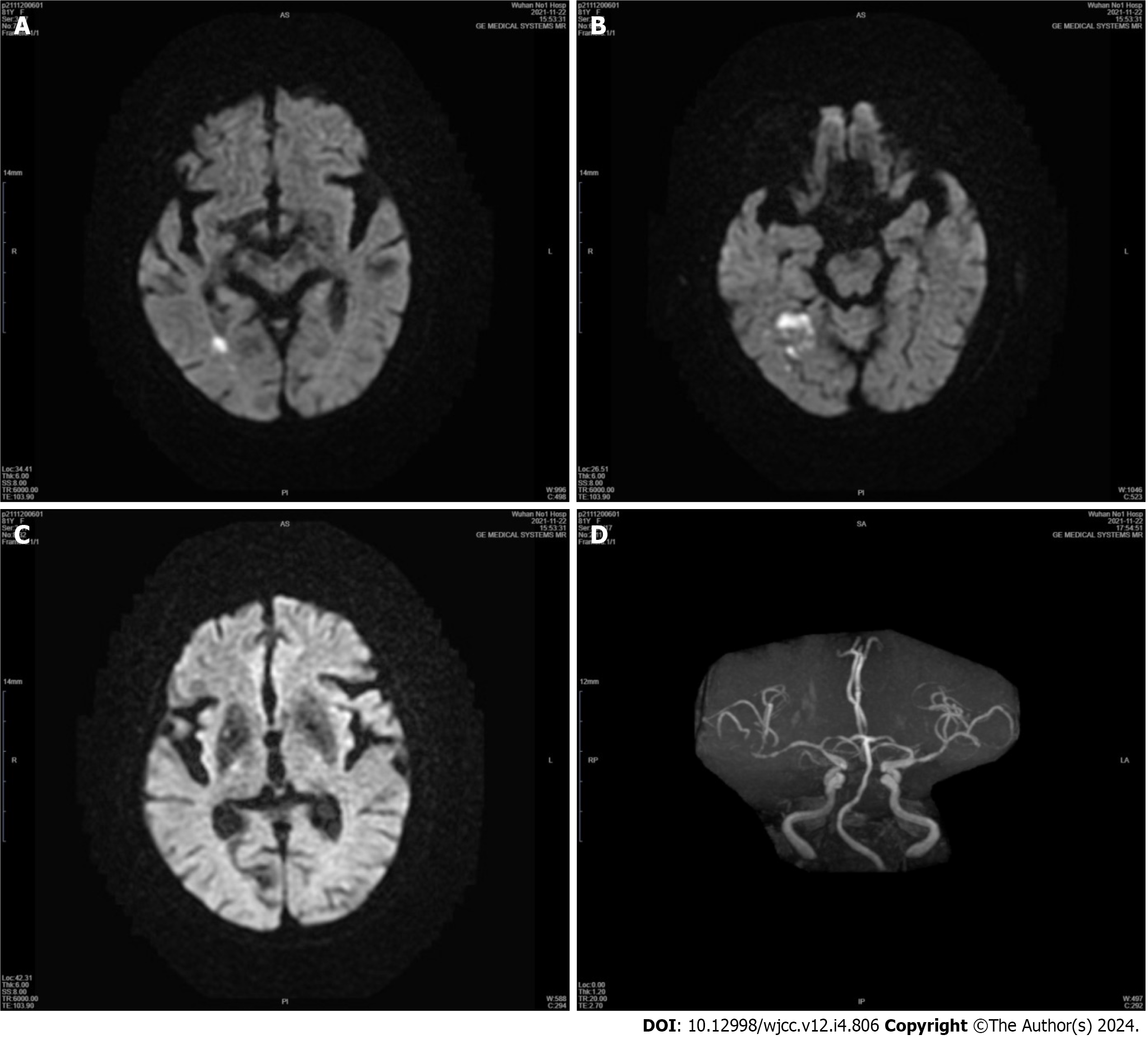
Case 1: An emergency computed tomography (CT) scan of the head showed no high-density lesions. Diffusion-weighted imaging (DWI) revealed an acute ischemic stroke that predominantly affected the right temporal cortex and minimally affected the occipital cortex. The basal ganglia, thalamus, and subthalamic nucleus (STN) were entirely spared (Figure 1). Magnetic resonance angiography of the head showed significant stenosis of the right middle cerebral artery (MCA) and mild stenosis of the left MCA (Figure 1). Twenty-seven days after the first admission, the patient was readmitted due to acute ischemic stroke. CT perfusion (CTP) showed marked hypoperfusion in the right MCA territory (Figure 2). An emergency transfemoral cerebral angiogram was performed and showed severe stenosis in the M1 segment of the right MCA (Figure 2). Two days after the intervention, CTP showed improved perfusion in the right cerebral hemisphere (Figure 2).
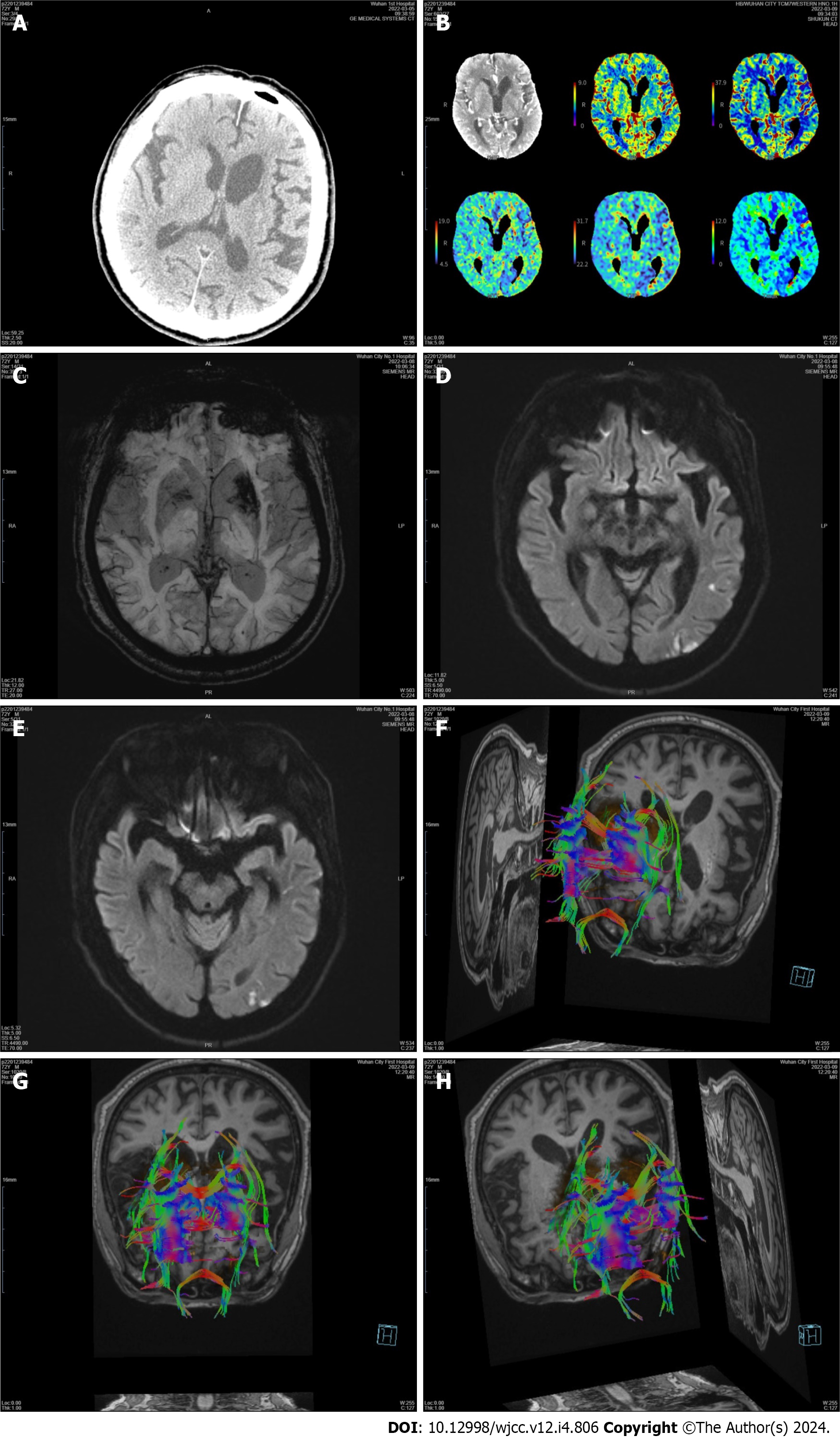
Case 2: A brain CT scan revealed no early ischemic lesions or acute cerebral hemorrhage (Figure 3). There was no proximal occlusion on CT angiography, and CTP during the symptomatic phase showed no abnormalities, but normal perfusion was observed in the basal ganglia (Figure 3). Susceptibility weighted imaging (SWI) revealed low signal intensity in the left basal ganglia (Figure 3). DWI showed an acute patchy ischemic stroke in the left temporal lobe and that the basal ganglia was spared (Figure 3). Subsequent diffusion tensor imaging (DTI) confirmed fewer white matter fiber tracts on the left side than on the opposite side (Figures 3F-H).
Temporal ischemic stroke; hemichorea; severe stenosis of the right MCA.
Temporal ischemic stroke; hemichorea.
During the first hospitalization, although the patient’s blood sugar was controlled to a normal level, her symptoms did not significantly improve. Then, 2.5 mg of olanzapine was administered per day. When she was discharged from the hospital, her symptoms appeared to have improved compared with those previously observed. During the second hospitalization, percutaneous transluminal angioplasty was successfully performed and thus resulted in minimal residual stenosis. Abnormal movements or other neurologic problems did not occur.
Treatment with 2.5 mg of olanzapine per day improved the patient’s condition, and he was discharged.
Postoperative patients recovered well, and no hemichorea was observed after discharge.
After 2 wk, the patient presented for an outpatient follow-up assessment at the neurology clinic without any neurologic sequelae.
Hemichorea is a hyperkinetic movement disorder characterized by irregular, purposeless, involuntary movements that manifest on one side of the extremities[1]. Hemiballism is a form of flinging high-amplitude and coarse hemichorea. We used the term “hemichorea-hemiballism syndrome” because hemichorea and hemiballism originate from the same anatomical location in the brain; moreover, they have a similar pathophysiology and are treated with the same medications[2]. Neuroimaging studies have demonstrated that hemichorea is most commonly associated with the STN, caudate, and putamen[3]. Vascular lesions of the basal ganglia and regions connected to the posterolateral putamen are the most common causes[4]. However, hemichorea has been described in relation to inflammatory, infectious, and neoplastic lesions. Autoimmune, genetic, and metabolic disorders (such as nonketotic hyperglycemia) are also well-known etiologies[5]. In general, the diagnostic workup includes a detailed family and drug history, a general and neurological examination, specific blood tests, molecular genetic testing, and neuroimaging. Most affected individuals are Asian women in their 70s, suggesting the possibility of genetic predisposition. Previous studies indicated that abnormal movements, such as chorea or ballism, in patients with ischemic stroke that specifically impacts the cerebral cortex rather than the basal ganglia[6-10]. Herein, we report two cases of hemichorea secondary to temporal cortical ischemic stroke [as confirmed by perfusion CT scans and DWI-magnetic resonance imaging (MRI)], with no other laboratory or imaging abnormalities indicating alternative causes.
Hemichorea after an ischemic infarct in the temporal lobe is very rare[7]. The pathophysiology underlying the onset of movement disorders after temporal lobe injury is not well known. Several possible pathophysiological mechanisms of movement disorders associated with temporal lobe injury have been suggested. One of these mechanisms may involve hypoperfusion of the basal ganglia. In patient 1, a possible cause of hemichorea may have involved ischemia in the regions connected to the putamen, as this region is supplied by the penetrating branches of the MCA; moreover, ischemic changes were not visible on brain MRI. In previous reports, MCA stenosis was shown to present with hemichorea[11-13]. In this situation, hemichorea was attributed to dysfunctioning neuronal connections between the basal ganglia and the frontal cortical motor regions. Within these networks, the striatal direct and indirect pathways regulate the function of the globus pallidus (GPi) internus, which controls motor facilitation and inhibition. Thus, in the circuit between the basal ganglia and cortical motor regions, the direct pathway offers positive feedback, whereas the indirect pathway provides negative feedback[14]. Our patient developed cerebral infarction only in the right temporal lobe, and CTP analysis after her stroke revealed hypoperfusion of the right MCA territory. In this case, we did not evaluate cerebral blood flow during hemichorea before the patient started cerebral infarction. However, it is hypothesized that the hemichorea was likely due to mild hypoperfusion in the basal ganglia, which did not progress to cerebral infarction. This hypoperfusion may have led to a greater reduction in the activation of the striatal indirect pathway compared to the striatal direct pathway, resulting in increased thalamocortical motor facilitation. Perfusion in the MCA area improved after percutaneous transluminal angioplasty, and the symptoms of hemichorea were significantly relieved. Surgical revascularization may be an effective treatment option. There was complete resolution of hemichorea after revascularization, thus indicating that hemichorea was caused by hypoperfusion. Transient dyskinesias or “limb shaking” spells have been described as symptoms of transient ischemic attacks. In the case described, the persistent hemichorea observed in MCA stenosis is similar to the limb shaking seen in transient ischemic attacks associated with carotid artery stenosis, and they occurred before the cerebral infarction. Vascular imaging (carotid duplex ultrasonography, MR or CT angiography) is essential for patients presenting with hemichorea, regardless of the presence or absence of symptoms, signs, or imaging evidence of cerebrovascular ischemia. Although the blood glucose level was high at admission, CT imaging revealed no abnormalities in the contralateral putamen, GPi, or caudate nucleus. MRI reveals no high signal intensity in the basal ganglia on T1-weighted images[2]. Typically, abnormal movements disappear or markedly diminish once the glucose level is reduced to the normal range. In rare cases, abnormal movements may persist for a certain period of time[15]. For patient 1, a combination of clinical history and neuroimaging excluded hemichorea caused by nonketotic hyperglycemia.
Another hypothetical mechanism, similar to temporal lobe infarct chorea, may be explained by changes in central nervous system plasticity after injury and the reestablishment of functional synapses. There is some brain plasticity present within the basal ganglia anatomical networks, partly as a result of parallel processing and compensatory mechanisms that provide certain resilience and protection against clinically obvious loss of motor control. Following brain injury, dendritic plasticity and alterations in synaptic activity can cause changes in neuronal circuitry. In patient 2, we considered the low signal intensity in the left basal ganglia above the SWI to indicate subacute hemorrhage because no hyperdensity was observed on the brain CT scan. In fact, abnormal involuntary movement onset occurred suddenly on the day of stroke in most patients, and chorea appeared earlier (mean of 4.3 d poststroke), whereas parkinsonism appeared much later (mean of 117.5 d post stroke). As a result, hemichorea cannot be directly caused by subacute hemorrhage. In patient 2, we observed indications of mild cortical hypoperfusion, with the basal ganglia being spared during the symptomatic phase. Therefore, we have evidence of a fundamental contribution of the cortex.
Another mechanism that is not often mentioned involves changes in neuronal firing patterns. Hemichorea can be viewed as a circuit disorder that is not confined to localized structural and/or functional abnormalities of the basal ganglia[16]. Interactions between neurons are far more complicated because they affect each other not only by affecting overall firing rates but also by changing firing patterns. Previous studies have shown that hemichorea patients exhibit aberrant phasic bursting patterns in the interior GPi. A burst pattern caused by the GPi may result in alternative inhibition and disinhibition of the motor cortex and thalamus, thus resulting in ballism or chorea. Because it is apparent that the basal ganglia nuclei are not the only structures that can alter the firing pattern of the GPi, this may explain why temporal lobe injuries can produce hemichorea. As previously reported, patients with cortical infarcts have better outcomes than patients with STN infarcts. Two patients recovered well during the hospital stay.
Several clinical issues warrant further discussion in our cases. First, hemichorea should be distinguished from pseu
Our case highlights the idea that ischemic stroke involving the temporal lobe can result in hemichorea. However, acute stroke rarely manifests with hemichorea, which delays proper diagnosis and prompt treatment. Considering the well-established and time-dependent benefits of reperfusion therapies, we believe that acute onset of hemichorea likely leads to acute stroke. It should be noted that MCA stenosis can present with persistent hemichorea, even in the absence of cerebral infarction. Thus, vascular imaging is essential for patients presenting with hemichorea. Nevertheless, when considering the significance of basal ganglia dysfunction producing involuntary movements, basal ganglionic hypoperfusion still needs to be highlighted. These cases support the theory of a relevant role of the temporal cortex in the origin of abnormal hyperkinetic movements and a possible functional connection with the basal ganglia.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cugy E, France S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Postuma RB, Lang AE. Hemiballism: revisiting a classic disorder. Lancet Neurol. 2003;2:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Hawley JS, Weiner WJ. Hemiballismus: current concepts and review. Parkinsonism Relat Disord. 2012;18:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Tater P, Pandey S. Post-stroke Movement Disorders: Clinical Spectrum, Pathogenesis, and Management. Neurol India. 2021;69:272-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 4. | Laganiere S, Boes AD, Fox MD. Network localization of hemichorea-hemiballismus. Neurology. 2016;86:2187-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Collado-Saenz J, Baeza-Trinidad R. Nonketotic Hyperglycemic Hemichorea. N Engl J Med. 2022;387:e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Cotroneo M, Ciacciarelli A, Cosenza D, Casella C, Dell'Aera C, Grillo F, Fazio MC, La Spina P, Musolino RF. Hemiballism: Unusual clinical manifestation in three patients with frontoparietal infarct. Clin Neurol Neurosurg. 2020;188:105612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Jacob S, Gupta HV. Delayed Hemichorea Following Temporal-Occipital Lobe Infarction. Tremor Other Hyperkinet Mov (N Y). 2016;6:414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Strauss S, Rafie D, Nimma A, Romero R, Hanna PA. Pure Cortical Stroke Causing Hemichorea-Hemiballismus. J Stroke Cerebrovasc Dis. 2019;28:104287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Carbayo Á, Sarto J, Santana D, Compta Y, Urra X. Hemichorea as Presentation of Acute Cortical Ischemic Stroke. Case Series and Review of the Literature. J Stroke Cerebrovasc Dis. 2020;29:105150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Dong H, Zhao J, Lee KY, Shen G. Hemichorea secondary to isolated temporal infarction with severe middle cerebral artery stenosis: a case report and review of literature. BMC Neurol. 2023;23:186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Ueta Y, Kato H, Naito M, Taguchi T, Terashi H, Aizawa H. Persistent Hemichorea as a Preceding Symptom of Cerebral Infarction Due to Middle Cerebral Artery Stenosis. Intern Med. 2021;60:3805-3808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Chung SJ, Lee HS, Yoo HS, Kim KM, Lee KJ, Kim JS, Lee JW, Kim JH, Cho JH, Kim GS, Lee JH, Choi SA. A case of isolated middle cerebral artery stenosis with hemichorea and moyamoya pattern collateralization. J Mov Disord. 2013;6:13-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Irioka T, Ayabe J, Mizusawa H. Hemichorea improved by extracranial-intracranial bypass surgery for middle cerebral artery occlusion. J Neurol. 2010;257:1756-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Park J. Movement Disorders Following Cerebrovascular Lesion in the Basal Ganglia Circuit. J Mov Disord. 2016;9:71-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Jaafar J, Rahman RA, Draman N, Yunus NA. Hemiballismus in Uncontrolled Diabetes Mellitus. Korean J Fam Med. 2018;39:200-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Holtbernd F, Eidelberg D. Functional brain networks in movement disorders: recent advances. Curr Opin Neurol. 2012;25:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Mehanna R, Jankovic J. Movement disorders in cerebrovascular disease. Lancet Neurol. 2013;12:597-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 18. | Cochrane CJ, Ebmeier KP. Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology. 2013;80:857-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |