Published online Feb 6, 2024. doi: 10.12998/wjcc.v12.i4.746
Peer-review started: October 11, 2023
First decision: December 8, 2023
Revised: December 17, 2023
Accepted: January 8, 2024
Article in press: January 8, 2024
Published online: February 6, 2024
Processing time: 106 Days and 1.3 Hours
While primary intestinal lymphangiectasia (PIL) is considered a rare condition, there have been several reported cases in adults. Nevertheless, the absence of clear guidance from diagnosis to treatment and prognosis poses challenges for both physicians and patients.
To enhance understanding by investigating clinical presentation, diagnosis, treatment, complications, and prognoses in adult PIL cases.
We enrolled adult patients diagnosed with PIL between March 2016 and September 2021. The primary outcome involved examining the diagnosis and treatment process of these patients. The secondary outcomes included identifying complications (infections, thromboembolism) and assessing prognoses (frequency of hospitalization and mortality) during the follow-up period.
Among the 12 included patients, peripheral edema (100%) and diarrhea (75%) were the main presenting complaints. Laboratory tests showed that all the pati
PIL can be diagnosed in adults across various age groups, with different severity and treatment responses among patients, leading to diverse complications and prognoses. Consequently, tailored treatments will be necessary. We anticipate that our findings will contribute to the management of PIL, an etiology of protein-losing enteropathy.
Core Tip: We reviewed the diagnosis, treatment, and prognosis of adult patients diagnosed with primary intestinal lymphangiectasia and found varying severities. Hence, tailored treatments are essential for adult patients with primary intestinal lymphangiectasia given the diverse presentations and responses.
- Citation: Na JE, Kim JE, Park S, Kim ER, Hong SN, Kim YH, Chang DK. Experience of primary intestinal lymphangiectasia in adults: Twelve case series from a tertiary referral hospital. World J Clin Cases 2024; 12(4): 746-757
- URL: https://www.wjgnet.com/2307-8960/full/v12/i4/746.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i4.746
Primary intestinal lymphangiectasia (PIL), or Waldmann's disease, is a rare lymphatic system disorder that predominantly affects the gastrointestinal tract[1]. Lymphatic capillaries exist within the intestinal villi, following the submucosal lymphatic vessels, and ultimately converging into lymphatic vessels or lymph nodes within the mesentery. This gathered lymph drains into the thoracic duct and enters the venous circulation at the same as the left subclavian vein[2]. The lymphatic system of the gut plays a significant role in regulating interstitial fluid, absorbing long-chain fatty acids through chylomicrons, and housing T and B cell lymphocytes, thus serving an essential function within the immune system[3]. In the PIL, the dilation or rupture of the lymphatic system of the gut results in the leakage of lymph, leading to lymphopenia and hypogammaglobulinemia. Furthermore, increased protein excretion in the stool causes hypoproteinemia[1]. Clinical manifestations of this condition encompass various symptoms, including peripheral edema, diarrhea, and abdominal pain[4]. Pathological diagnosis serves as the confirmation method, yet the absence of guidance regarding the diagnostic process and treatment poses challenges for clinicians and patients, even given the rare nature of the PIL.
Although PIL has been reported in children[5,6], its mechanisms and prevalence remain poorly understood, with some reports suggesting possible genetic alterations. Moreover, several cases with detailed PIL occurrences in adults have also been documented[4,7-11].
Thus, this study aims to elucidate the clinical presentation, diagnosis, treatment strategies, complications, and prognoses associated with PIL cases diagnosed in adults. By sharing these experiences, we aim to provide valuable insights for the effective management of this condition.
A retrospective screening was conducted on patients aged 18 and above diagnosed with PIL (I89.0) based on medical records from March 2016 to September 2021. A medical fee reduction policy in South Korea is accessible specifically for extremely rare diseases. PIL falls within this category, allowing for the utilization of the disease code for screening purposes. Exclusions were made for patients who lacked treatment records at our institution or were followed up for less than six months.
Applying the criteria for special coding of extremely rare diseases and following the diagnostic criteria utilized at our institution required meeting all three of the following criteria: First, distinct clinical symptoms must be present, including hypoproteinemia, peripheral edema, lymphocytopenia, chronic diarrhea, steatorrhea, abdominal pain, nausea, vomiting, chyle ascites or chylothorax, or alpha-1 antitrypsin (AAT) clearance exceeding 27 mL/24 h. Second, relevant findings must be verified through imaging tests [endoscopy, ultrasonography, abdominopelvic computed tomography (CT), etc.]. Such findings encompassed a snowflake appearance, low attenuation thickening of the small bowel wall, or ascites. Third, histological confirmation of dilated lymphatics within the intestine was required, observed in the mucosa, submucosa, serosa, subserosa, or mesentery.
Following the diagnostic criteria, basic blood tests were conducted, including complete blood count, chemistry profile, electrolyte levels, lipid profile, immunoglobulin (Ig) A/G/M/E, prothrombin time/activated partial thromboplastin time, fibrinogen, ferritin, and C-reactive protein (CRP). For confirming protein-losing enteropathy, serum AAT (mg/dL) was assessed, along with stool concentration of AAT (mg/dL) and AAT clearance calculated following a 24-h stool collection (grams). Radiologically, chest X-rays and abdominopelvic CT scans were performed as standard imaging procedures. Endoscopically, esophagogastroduodenoscopy (EGD), colonoscopy, and capsule endoscopy were performed. Depending on the physician's assessment, oral or anal enteroscopy could be chosen instead of EGD and colonoscopy. A corresponding diagnosis code was assigned based on the comprehensive results of these tests and their alignment with the specified diagnostic criteria.
The primary outcome was to disseminate diagnostic findings and the treatment course for adult PIL. The secondary outcome was to convey experiences regarding complications and prognoses encountered during the follow-up period.
The study data were retrospectively acquired through the review of medical records. Demographic information included age, gender, body mass index, and comorbidities at diagnosis. Diagnostic findings were categorized into symptoms (peripheral edema, diarrhea, abdominal pain, nausea/vomiting), laboratory results (leukocyte count, lymphocyte count, hemoglobin, albumin, IgG, ferritin, CRP, AAT clearance), radiological findings (small bowel wall edema, ascites, pleural effusion, ileus), endoscopic observations (snowflake appearance), and pathological findings. Treatment was classified into medical treatment and interventions. Medical treatment includes diet therapy, albumin administration, octreotide, tranexamic acid, steroids, sirolimus, and everolimus. The sequence of drug selection, treatment continuation status, and reasons for discontinuation were outlined for each case. The execution and reasons for interventions [such as lung mediastinum magnetic resonance imaging (MRI), lymphangiography, or lymphatic embo
A total of 18 patients aged 18 and above diagnosed with PIL were screened. Among them, 12 patients were included in the case series, following the exclusion of those with no treatment records or a follow-up period of fewer than six months (6 patients). The age range was from 28 yr to 76 yr, with a median age of 48 yr. The gender distribution was well-balanced, with seven males and five females. Among the existing comorbidities, cerebrovascular disease, diabetes mellitus, and cancer were the most prevalent, each accounting for 3 cases out of 12 (25%) (Table 1).
| Demographic information | |
| Total number of patients | 12 |
| Age, yr | 48 (32, 63) |
| Male/female | 7/5 (58/42) |
| BMI | 22 (21, 23) |
| Comorbidities | |
| Cerebrovascular disease | 3 (25) |
| Hypertension | 2 (17) |
| Cardiovascular disease (unstable angina, MI, CHF) | 1 (8) |
| Chronic obstructive pulmonary disease | 0 (0) |
| Diabetes mellitus | 3 (25) |
| Chronic kidney disease | 1 (8) |
| Cancer | 3 (25) |
| Follow-up duration, mo | 30 (24, 37) |
Peripheral edema was observed in all 12 patients, diarrhea diagnosed in 9 patients (75%), and abdominal pain in 4 patients (33%) (Table 2). No patients reported feeling nausea or vomiting. Lymphocytopenia was observed in 5 patients (42%). The median albumin level was 1.8 g/dL, highlighting hypoalbuminemia across all patients. IgG levels were also low, with a median level of 391 mg/dL (normal range 600-1600 mg/dL). Except for two missing values, AAT clearance exceeding 27 mL/24 h was confirmed in all ten patients.
| Diagnostic findings | |
| Symptoms | |
| Peripheral edema | 12/12 (100) |
| Diarrhea | 9/12 (75) |
| Abdominal pain | 4/12 (33) |
| Nausea/vomiting | 0 (0) |
| Laboratory findings | |
| Leukocyte, 109/L | 4.8 (3.9, 6.6) |
| Lymphocyte, 109/L | 1.1 (0.7, 1.4) |
| Lymphocytopenia, < 1.0 × 109/L | 5/12 (42) |
| Hemoglobin, g/dL | 11.9 (10.7, 13.4) |
| Albumin, g/dL | 1.8 (1.7, 2.4) |
| IgG, mg/dL | 391 (343, 474) |
| Ferritin, ng/mL | 130 (54, 256) |
| Ferritin < 30 (male) or 13 (female) ng/mL (two missing value) | 1/10 (10) |
| CRP, mg/dL | 0.16 (0.08, 0.49) |
| Alpha-1 antitrypsin clearance, mL/24 h | 566 (211, 704) |
| Alpha-1 antitrypsin clearance > 27 mL/24 h (two missing value) | 10/10 (100) |
| Radiologic findings | |
| Small bowel wall thickening and edema | 8/12 (67) |
| Ascites | 7/12 (58) |
| Pleural effusion | 3/12 (25) |
| Ileus | 0/12 (0) |
| Endoscopic findings | |
| Snowflake appearance | 9/12 (75) |
| Pathological findings | |
| Dilation of the lymphatics in mucosa or submucosa | 12/12 (100) |
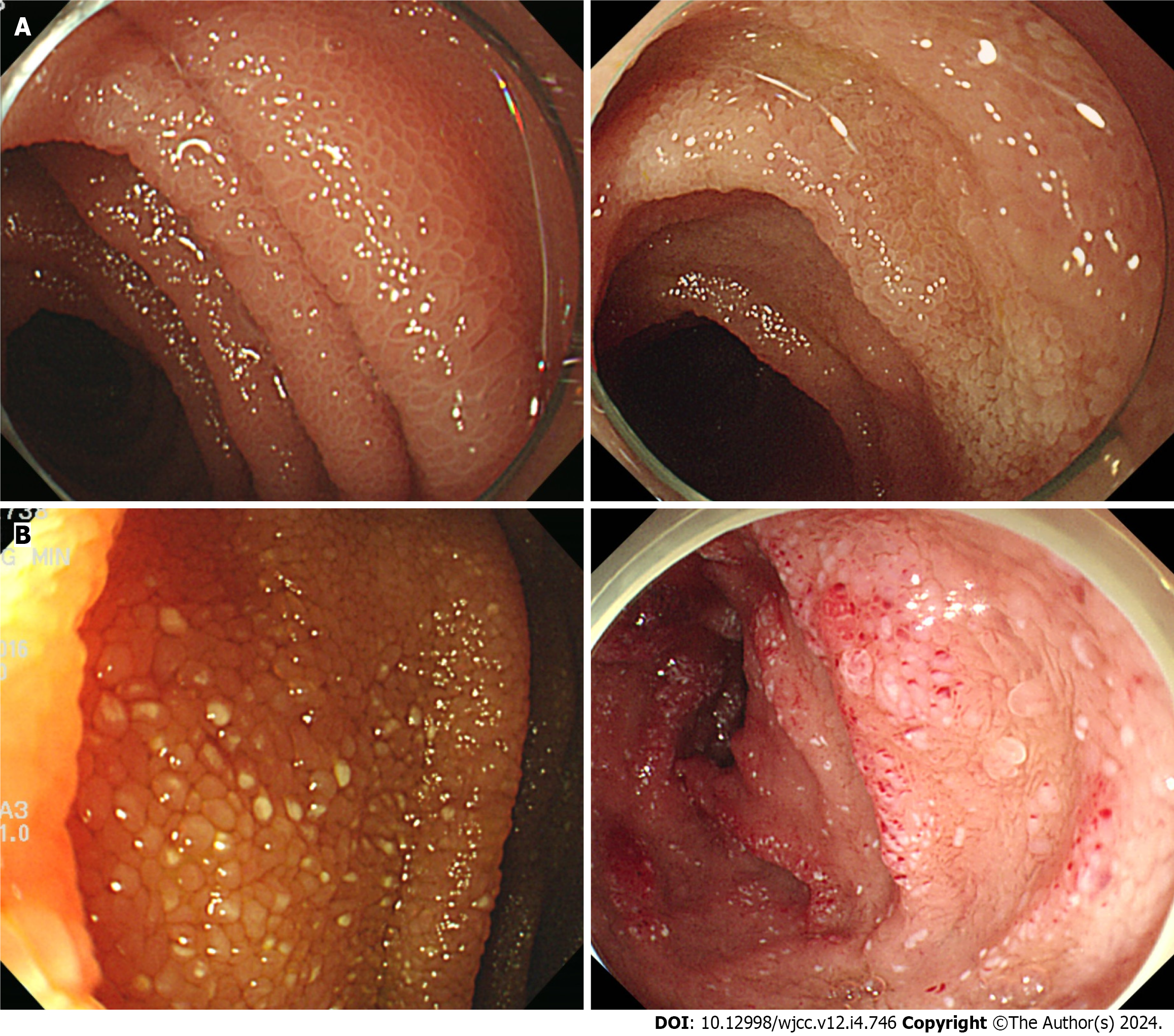
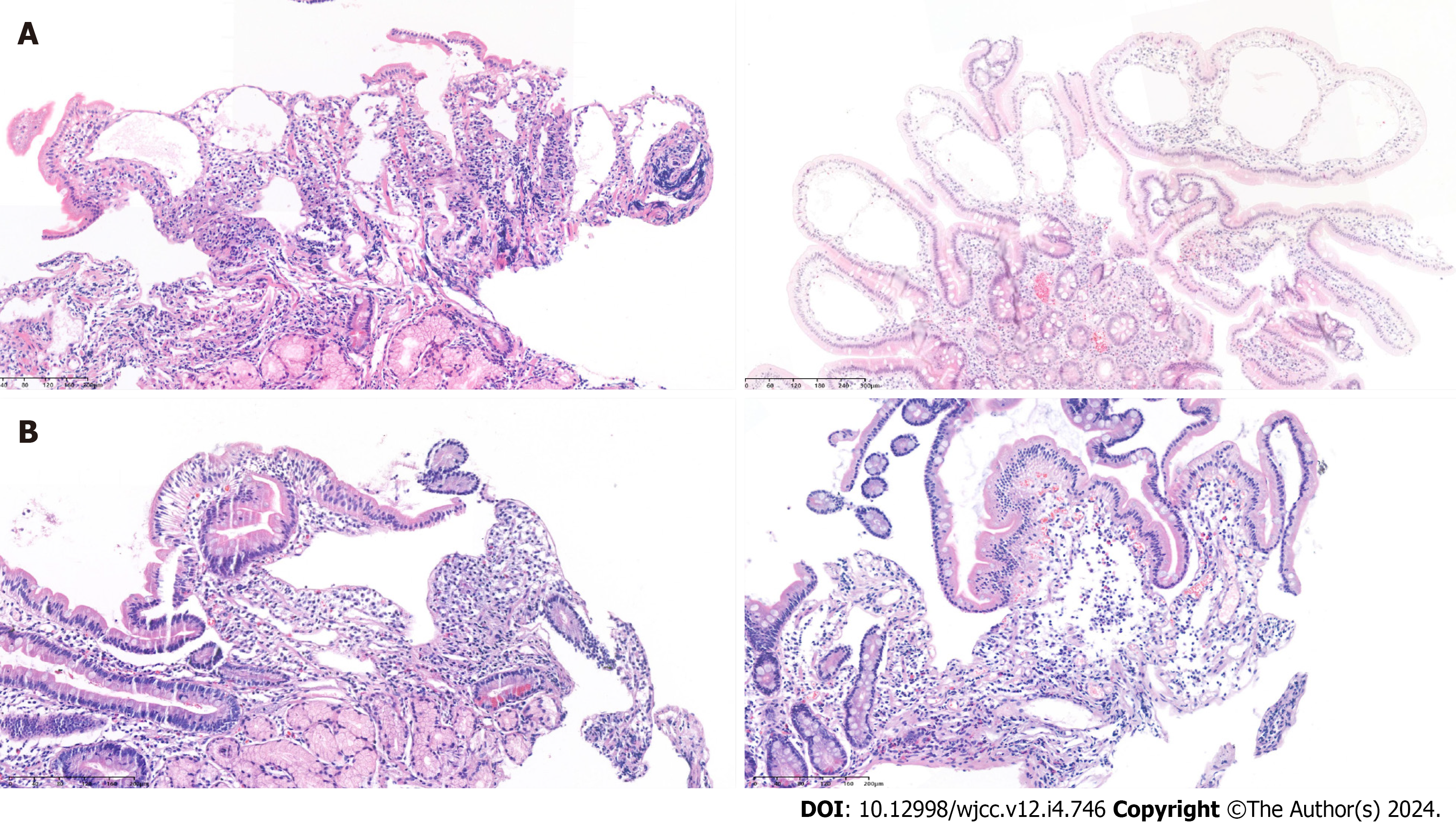
Radiologically, bowel wall thickening and edema were observed in 8 patients (67%), and ascites were detected in 7 patients (58%). Pleural effusion was present in 3 cases (25%). Endoscopic findings revealed edematous and whitish delineated villi observed in all patients (Figure 1). The snowflake appearance (whitish spots) was noted in 9 patients (75%). Pathological confirmation was observed in all patients, illustrating lymphangiectasia in the mucosa and submucosal layers (Figure 2).
Dietary education was provided to all patients, focusing on a low-fat and high-protein diet incorporating medium-chain triglycerides (MCT) oil. Tailored to individual symptoms, periodic albumin replacement was recommended, and most patients underwent albumin infusion within the outpatient hospital setting. Over a median follow-up duration of 30 mo, in conjunction with foundational dietary education and albumin replacement, the subsequent medications were used for treatment: octreotide, tranexamic acid, steroids, sirolimus, and everolimus. In each case, the drug selection sequence and the concurrent administration periods were delineated using the numbers 1 to 5 (Table 3). Among the 12 patients, eight (#2, 4, 5, 6, 8, 10, 11, and 12) continued long-term treatment with octreotide or sirolimus, either as monotherapy or in combination. However, two patients (#1 and #9) exhibited enhanced albumin levels and ameliorated clinical symptoms during maintenance treatment with octreotide (#1) and sirolimus (#9), allowing them to maintain regular follow-ups after discontinuing the medications. One patient (#7) had mild clinical symptoms and was only monitored using diet and albumin replacement. Patient (#3) was ineligible for PIL treatment due to concurrent cancer treatment.
| Case number | Age/sex | Albumin | Octreotide | Tranexamic acid | Steroid | Sirolimus | Everolimus | Outcome of treatment | Lng medias | Lymphangiography | Lymphatic embolization |
| Periodic injection intravenously | Subcutaneous; Dose; Frequency; Duration | Oral; Dose; Frequency; Duration | Oral; Dose; Frequency; Duration | Oral; Dose; Frequency; Duration | Oral; Dose; Frequency; Duration | No/yes (reason) | No/yes (reason) | No/yes (reason) | |||
| 1 | 30/M | Yes | (5) 100-200 mcg qd-bid for 6 month | (4) 500 mg tid for 3 month | (2) 40 mg tapered for 2 month | (3)-(4) 1-2 mg qd for 6 month | (1) 2.5 mg qd for 2 month | Improvement and observation after stopping medical treatment | |||
| 2 | 70/M | Yes | (1)-(3) 50-100 mcg qd-bid for continuously | (2) 500 mg tid for 3 month | (3) 1 mg qd for 6 month | Octreotide continuously | |||||
| 3 | 63/F | Yes | Unable to treat appropriately due to underlying colon cancer | ||||||||
| 4 | 54/M | Yes | (1) 2-3 mg qd for continuously | Sirolimus continuously | |||||||
| 5 | 28/F | Yes | (1)-(2) 100 mcg qd for 6 month | (2) 1-2 mg qd for continuously | Sirolimus continuously | ||||||
| 6 | 67/F | Yes | (1) 100 mcg bid for 3 month | (2) 250 mg tid for continuously | (2) 2 mg qd for continuously | Tranexamic acid and sirolimus continuously | Yes (pleural effusion) | Yes (pleural effusion) | Yes (pleural effusion) | ||
| 7 | 32/M | Yes | Regular albumin replacement | No | No | No | |||||
| 8 | 65/M | Yes | (1) 1-2 mg qd for continuously | Sirolimus continuously | No | No | No | ||||
| 9 | 41/F | Yes | (1) 40 mg tapered for 4 wk | (2) 1-2 mg qd for 1.5 yr | Improvement and observation after stopping medical treatment | Yes (pleural effusion) | No | No | |||
| 10 | 35/M | Yes | (1)-(2) 50-200 mcg qd-bid for 2 yr | (4) 250 mg tid for 6 month | (3) 30 mg tapered for 1 month | (2)-(4) 1 mg qd for continuously | Sirolimus continuously | Yes (pleural effusion) | Yes (pleural effusion) | No | |
| 11 | 32/M | Yes | (2) 100 mcg qd-bid for continuously | (1)-(2) 1-4 mg qd for continuously | Octreotide and sirolimus continuously | No | No | No | |||
| 12 | 76/F | Yes | (2) 100 mcg qd-bid for continuously | (1) 1 mg qd-bid for 6 month | Octreotide continuously | No | No | No |
Three patients (#6, 9, and 10) exhibited pleural effusion and underwent lung mediastinal MRI. Among these cases, two patients (#6 and #10) underwent lymphangiography to validate the presence of lymphatic leakage. Within this subset, one patient (#6) received confirmation of lymphatic leakage and consequently underwent embolization.
The yearly frequency of hospitalizations ranged from 0 to as high as 14 occurrences (Table 4). Infections were identified in 6 patients (#1, 3, 6, 9, 10, and 12), encompassing bacterial infections such as urinary tract infections, pneumonia, spontaneous bacterial peritonitis, cellulitis, and catheter-related infections. Furthermore, a diverse array of infections, including tuberculosis, herpes zoster, and invasive pulmonary aspergillosis, were observed. Symptoms of thromboembolism were observed in 3 cases involving pulmonary thromboembolism in patients #1 and #10 and cerebral infarction in patients #6. Mortality was witnessed in 2 cases, specifically in patients #3 and #6. Among these cases, patient #6 encountered a deteriorating course attributed to uncontrolled PIL, resulting in their death.
| Case number | Age/sex | Hospitalization after diagnosis, frequency per year | Any infection, no/yes (disease) | Any thromboembolism, no/yes (disease) | Mortality, no/yes (cause) |
| 1 | 30/M | 14 | Yes (invasive pulmonary aspergillosis, zoster, tuberculosis) | Yes (pulmonary thromboembolism) | No |
| 2 | 70/M | 2 | No | No | No |
| 3 | 63/F | 6 | Yes (pneumonia, cellulitis, urinary tract infection) | No | Yes (underlying cancer) |
| 4 | 54/M | 1 | No | No | No |
| 5 | 28/F | 1 | No | No | No |
| 6 | 67/F | 6 | Yes (pneumonia) | Yes (cerebral infarction) | Yes (primary intestinal lymphangiectasia) |
| 7 | 32/M | 2 | No | No | No |
| 8 | 65/M | 1 | No | No | No |
| 9 | 41/F | 2 | Yes (zoster) | No | No |
| 10 | 35/M | 7 | Yes (catheter-related infection) | Yes (pulmonary thromboembolism) | No |
| 11 | 32/M | 0 | No | No | No |
| 12 | 76/F | 3 | Yes (spontaneous bacterial peritonitis, pneumonia) | No | No |
This study observed that PIL can be diagnosed across a range of age groups in adults, between the twenties the seventies, with most of the patients primarily presenting with peripheral edema and diarrhea. Hypoalbuminemia and hypogammaglobulinemia were observed in blood tests, often accompanied by lymphopenia of less than half. These findings are consistent with previous reports[4]. Protein-losing enteropathy was confirmed in all patients, excluding those with missing data, based on AAT clearance. Radiologic images showed frequent small bowel wall edema, aligning with prior studies[12,13]. Over half of the patients presented moderate to significant amounts of ascites. Of all 12 patients, four patients underwent enteroscopy instead of EGD and colonoscopy. A characteristic snowflake appearance was observed in 75% of cases. Edematous villi and whitish delineated villi were observed in all patients. It is important to be aware that such distinctive findings can be observed, and histological examination is necessary to distinguish them from normal features. Pathological confirmation was possible in all patients using biopsies targeting suspicious lesions and random biopsies. All PIL patients received fundamental dietary education about the low-fat, high-protein, and MCT diet. Octreotide and sirolimus demonstrated the most promising treatment persistence effects. However, the occurrence of complications and treatment outcomes varied significantly among patients. While two patients displayed improvement and were ready to discontinue treatment, one patient experienced complications that ultimately led to death. This emphasizes that even in cases of PIL diagnosed in adults; however, prognosis can vary greatly depending on the severity of the condition and the response to treatment.
This study has significant importance as it provides the first insights into the diagnosis, treatment, complications, and prognosis of adult patients with PIL. Given the rare nature of PIL and the limited number of case reports in adults, there was a lack of guidance for diagnosis and treatment. This study presented the institutional protocols for diagnosing PIL, a cause of protein-losing enteropathy, among patients exhibiting clinical symptoms and blood test results. Sharing such protocols has the potential to aid physicians encountering similar patients, offering them a valuable point of reference.
The low-fat, high-protein, MCT oil diet has been reported to contribute to the recovery of PIL by lowering lymphatic pressure through bypassing absorption via the gut lymphatic system[14,15]. Therefore, dietary education on this approach is considered fundamental for initiating treatment. The potential efficacy of drugs such as octreotide, sirolimus, everolimus, tranexamic acid, and steroids has been suggested, but studies regarding their long-term maintenance treatment effects are limited[16]. Octreotide is believed to exhibit therapeutic effects by targeting gut lymphatics flow to reduce fat absorption in the intestine[17-19]. One case report observed sustained treatment effects for over six months[19]. In our study, among seven patients who received octreotide treatment, three patients showed no effect and discontinued, one patient improved and subsequently stopped, and among the remaining three patients who received consistent therapy, one continued octreotide monotherapy for four years (#2), and another patient received combination treatment with sirolimus for two years (#11). This suggests that long-term treatment might also be anticipated in patients with a favorable response. Sirolimus acts on lymphatic endothelial cells by inhibiting the mammalian target of rapamycin signaling, thereby suppressing lymphatic proliferation. This mechanism has been utilized in the treatment of lymphangiectasia and lymphangioleiomyomatosis[20,21]. Among four pediatric patients, two patients with lymphangiectasia confined to the intestine reported an effect after 3-4 mo of treatment, though there has been no report in adults[22]. In our case series, among the ten patients who received sirolimus treatment, three patients experienced limited efficacy and discontinued, one improved and subsequently discontinued, and six patients showed sustained effects lasting from around 1 yr to 4 yr, leading to maintenance treatment. This suggests that long-term therapy may also yield effectiveness in patients who respond well to sirolimus. Considering the mechanistic distinctions, where octreotide predominantly impacts the flow within the lymphatic system, and sirolimus acts on lymphatic endothelial cells, it is conceivable that patients who did not respond well to octreotide might respond to sirolimus and vice versa.
This suggests that the efficacy of these two drugs could differ based on the primary pathology of each patient, and combination therapy could also be considered an option. Everolimus[23], as the 2nd generation of sirolimus, and tranexamic acid[24,25], which aims to reduce intestinal protein loss by decreasing plasma fibrinolytic activity, did not demonstrate long-term efficacy in this study.
In cases where chyle ascites or chylothorax is identified, non-invasive tests such as lymphatic scintigraphy[26,27] or MR lymphangiography[28] can be conducted to assess problems within the lymphatic drainage system. However, their role in PIL is limited. At our institution, lung mediastinal MRI can be utilized to examine potential leakage or rupture in thoracic lymphatics. Consequently, we conducted this examination in three cases. While lymphangiography is an invasive procedure, it was performed for two patients in this study based on the outcomes of preceding non-invasive tests and consultation with specialized radiologists, leading to embolization in one case. While the available tests might vary among different medical facilities, it is worth considering the flow of the lymphatic system, where lymphatic capillaries connect to lymphatic vessels and nodes within the submucosal and mesentery regions that ultimately drain into the thoracic duct2. This investigation could help illuminate whether chyle ascites or chylothorax is linked to structural abnormalities within the subserosal lymphatics[29].
Mechanistically, PIL can induce changes in the immune system due to lymphatic loss, leading to lymphocytopenia and hypogammaglobulinemia[1]. Although reports on infections in adult PIL are limited, this study observed that half of the patients experienced various infections. One patient suffered from a severe fungal infection, such as invasive pulmonary aspergillosis. Therefore, it is necessary to exercise caution regarding infectious complications in adult patients with PIL. Venous thromboembolism was observed in 2 out of 12 patients. Prior research has established an association between hypoalbuminemia and venous thromboembolism in various conditions, such as nephrotic syndrome, hospitalized patients, and inflammatory bowel disease[30-34]. This connection is linked to the anticoagulant properties of albumin[35,36], its role as a negative acute-phase reactant indicating a hyperinflammatory state[37,38], and the compensatory hepatic synthesis of coagulation factors[39]. Similarly, caution is warranted concerning the potential association between hypoalbuminemia and venous thromboembolism within the context of PIL.
The present study had several limitations. Firstly, owing to the rare nature of the disease, the number of patients included in this study was constrained. Nonetheless, as a tertiary referral center, the primary objective was to share comprehensive experience spanning from diagnosis to prognosis of PIL. Secondly, we did not provide guidance on which treatment approach to choose first, how long to observe, or the appropriate dosage. However, we described the dosages and durations of medications in detail with their treatment effects. Thirdly, the details of albumin replacement, including dosage and frequency, were not fully available for all patients. Fourthly, lymphatic scintigraphy was not feasible within this institution, and consequently, it was not incorporated into this study.
While the clinical presentation and laboratory findings in adults diagnosed with PIL were like previous reports, considerable variability exists in the severity of the disease, treatment response, and prognosis among patients. This suggests the need for individually tailored and proactive treatment approaches in adult PIL.
This thesis explores Primary Intestinal Lymphangiectasia (PIL), a rare disorder affecting the gastrointestinal tract. PIL involves the dilation or rupture of the gut's lymphatic system, leading to lymph leakage, lymphopenia, hypogammaglobulinemia, and other symptoms like peripheral edema and abdominal pain. Despite its rarity, PIL has been reported in both children and adults, with unclear mechanisms and prevalence.
The research seeks to fill gaps in knowledge, offering insights for more effective management of adult PIL patients.
The study aims to enhance understanding by investigating clinical presentation, diagnosis, treatment, complications, and prognoses in adult PIL cases.
The research employed a retrospective screening of adult patients diagnosed with PIL from March 2016 to September 2021. Utilizing the specific disease code for extremely rare diseases in South Korea, medical records were examined, excluding those with insufficient treatment history or follow-up less than six months. Diagnosis criteria involved clinical symptoms, imaging tests, and histological confirmation. Various tests, including blood, radiological, and endoscopic examinations, were conducted to confirm the diagnosis. Data collected included demographic information, diagnostic findings, treatment details, and outcomes. Treatment options ranged from medical therapies (diet, albumin administration, octreotide, etc.) to interventions (magnetic resonance imaging, lymphangiography, embolization). Complications and prognoses were tracked through annual hospitalization frequency, infections, thromboembolism, and mortality. Patients were followed from diagnosis to the last outpatient visit or date of death.
In this study, 12 out of 18 adult patients diagnosed with PIL were included in the case series. The patients, aged 28 yr to 76 yr, exhibited a well-balanced gender distribution, with prevalent comorbidities such as cerebrovascular disease, diabetes mellitus, and cancer. Diagnostic findings revealed common symptoms of PIL, including peripheral edema in all patients, diarrhea in 75%, and abdominal pain in 33%. Lymphocytopenia was observed in 42% of patients. Radiological and endoscopic examinations consistently confirmed features of PIL, such as bowel wall thickening, ascites, pleural effusion, and a distinctive snowflake appearance. Treatment involved dietary education focusing on a low-fat, high-protein diet with medium-chain triglycerides, along with periodic albumin replacement. Medications like octreotide, tranexamic acid, steroids, sirolimus, and everolimus were employed, with individualized sequences and durations. Eight patients continued long-term treatment, while some achieved symptom improvement and discontinued medication. Complications included infections in six patients, thromboembolism in three cases, and mortality in two patients. Pleural effusion cases underwent lung mediastinal magnetic resonance imaging and lymphangiography, with one patient undergoing embolization. The study contributes valuable insights into the clinical presentation, treatment, and outcomes of adult PIL cases, highlighting the challenges and complexities associated with the management of this rare disorder. Remaining issues include optimizing treatment strategies, addressing complications, and improving long-term prognoses.
The study enhances our understanding of adult PIL, emphasizing the need for personalized treatment approaches and proactive management strategies. The findings contribute valuable knowledge to the limited existing literature on this rare condition, offering insights that can guide clinicians in the diagnosis and treatment of adult patients with PIL.
Future research in PIL should focus on refining treatment protocols, exploring combination therapies, conducting larger multi-center studies, investigating the role of diet, and addressing immune system changes. These efforts will contribute to improving the management and outcomes of adult PIL patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gu Y, China S-Editor: Gao CC L-Editor: A P-Editor: Zhao S
| 1. | Vignes S, Bellanger J. Primary intestinal lymphangiectasia (Waldmann's disease). Orphanet J Rare Dis. 2008;3:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (36)] |
| 2. | Cifarelli V, Eichmann A. The Intestinal Lymphatic System: Functions and Metabolic Implications. Cell Mol Gastroenterol Hepatol. 2019;7:503-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (35)] |
| 3. | Hokari R, Tomioka A. The role of lymphatics in intestinal inflammation. Inflamm Regen. 2021;41:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (9)] |
| 4. | Huber R, Semmler G, Mayr A, Offner F, Datz C. Primary intestinal lymphangiectasia in an adult patient: A case report and review of literature. World J Gastroenterol. 2020;26:7707-7718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (6)] |
| 5. | Lopez RN, Day AS. Primary intestinal lymphangiectasia in children: A review. J Paediatr Child Health. 2020;56:1719-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (32)] |
| 6. | Braamskamp MJ, Dolman KM, Tabbers MM. Clinical practice. Protein-losing enteropathy in children. Eur J Pediatr. 2010;169:1179-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (32)] |
| 7. | Hokari R, Kitagawa N, Watanabe C, Komoto S, Kurihara C, Okada Y, Kawaguchi A, Nagao S, Hibi T, Miura S. Changes in regulatory molecules for lymphangiogenesis in intestinal lymphangiectasia with enteric protein loss. J Gastroenterol Hepatol. 2008;23:e88-e95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (3)] |
| 8. | Martins CR, Gagnaire A, Rostain F, Lepage C. Waldmann's disease: a rare cause of protein losing enteropathy in an adult patient. Rev Esp Enferm Dig. 2017;109:385-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (35)] |
| 9. | Balaban VD, Popp A, Grasu M, Vasilescu F, Jinga M. Severe Refractory Anemia in Primary Intestinal Lymphangiectasia. A Case Report. J Gastrointestin Liver Dis. 2015;24:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (36)] |
| 10. | Freeman HJ, Nimmo M. Intestinal lymphangiectasia in adults. World J Gastrointest Oncol. 2011;3:19-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (39)] |
| 11. | Cappell MS, Edhi A, Amin M. Case report of primary intestinal lymphangiectasia diagnosed in an octogenarian by ileal intubation and by push enteroscopy after missed diagnosis by standard colonoscopy and EGD. Medicine (Baltimore). 2018;97:e9649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Yang DM, Jung DH. Localized intestinal lymphangiectasia: CT findings. AJR Am J Roentgenol. 2003;180:213-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Crutzen B, Poncelet PA. Protein-Losing Enteropathy in Primary Lymphangiectasia. J Belg Soc Radiol. 2020;104:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Jeffries GH, Chapman A, Sleisenger MH. Low-fat diet in intestinal lymphangiectasia. Its effect on albumin metabolism. N Engl J Med. 1964;270:761-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Alfano V, Tritto G, Alfonsi L, Cella A, Pasanisi F, Contaldo F. Stable reversal of pathologic signs of primitive intestinal lymphangiectasia with a hypolipidic, MCT-enriched diet. Nutrition. 2000;16:303-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Correction to: BST-104, a Water Extract of Lonicera japonica, Has a Gastroprotective Effect via Antioxidant and Anti-Inflammatory Activities, by Bang BW, Park D, Kwon KS, Lee DH, Jang M-J, Park SK, and Kim J-Y. J Med Food. 2019;22:140-151. DOI: 10.1089/jmf.2018.4231. J Med Food. 2019;22:433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Sari S, Baris Z, Dalgic B. Primary intestinal lymphangiectasia in children: is octreotide an effective and safe option in the treatment? J Pediatr Gastroenterol Nutr. 2010;51:454-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Suehiro K, Morikage N, Murakami M, Yamashita O, Hamano K. Late-onset primary intestinal lymphangiectasia successfully managed with octreotide: a case report. Ann Vasc Dis. 2012;5:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Kuroiwa G, Takayama T, Sato Y, Takahashi Y, Fujita T, Nobuoka A, Kukitsu T, Kato J, Sakamaki S, Niitsu Y. Primary intestinal lymphangiectasia successfully treated with octreotide. J Gastroenterol. 2001;36:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Hu S, Wu X, Xu W, Tian X, Yang Y, Wang ST, Liu S, Xu X, Xu KF. Long-term efficacy and safety of sirolimus therapy in patients with lymphangioleiomyomatosis. Orphanet J Rare Dis. 2019;14:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Baluk P, Yao LC, Flores JC, Choi D, Hong YK, McDonald DM. Rapamycin reversal of VEGF-C-driven lymphatic anomalies in the respiratory tract. JCI Insight. 2017;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Kwon Y, Kim ES, Choe YH, Kim MJ. Individual approach for treatment of primary intestinal lymphangiectasia in children: single-center experience and review of the literature. BMC Pediatr. 2021;21:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Kirchner GI, Meier-Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet. 2004;43:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 383] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 24. | Mine K, Matsubayashi S, Nakai Y, Nakagawa T. Intestinal lymphangiectasia markedly improved with antiplasmin therapy. Gastroenterology. 1989;96:1596-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | MacLean JE, Cohen E, Weinstein M. Primary intestinal and thoracic lymphangiectasia: a response to antiplasmin therapy. Pediatrics. 2002;109:1177-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Kang HR, Cho YK, Jo YJ, Jung YY, Kim EK. Primary intestinal lymphangiectasia diagnosed by chylous ascites. Kjg. 2016;67:116-118. [DOI] [Full Text] |
| 27. | So Y, Chung JK, Seo JK, Ko JS, Kim JY, Lee DS, Lee MC. Different patterns of lymphoscintigraphic findings in patients with intestinal lymphangiectasia. Nucl Med Commun. 2001;22:1249-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Cholet C, Delalandre C, Monnier-Cholley L, Le Pimpec-Barthes F, El Mouhadi S, Arrivé L. Nontraumatic Chylothorax: Nonenhanced MR Lymphography. Radiographics. 2020;40:1554-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Lizaola B, Bonder A, Trivedi HD, Tapper EB, Cardenas A. Review article: the diagnostic approach and current management of chylous ascites. Aliment Pharmacol Ther. 2017;46:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 30. | Chi G, Gibson CM, Liu Y, Hernandez AF, Hull RD, Cohen AT, Harrington RA, Goldhaber SZ. Inverse relationship of serum albumin to the risk of venous thromboembolism among acutely ill hospitalized patients: Analysis from the APEX trial. Am J Hematol. 2019;94:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Gyamlani G, Molnar MZ, Lu JL, Sumida K, Kalantar-Zadeh K, Kovesdy CP. Association of serum albumin level and venous thromboembolic events in a large cohort of patients with nephrotic syndrome. Nephrol Dial Transplant. 2017;32:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Imbrizi MR, Magro DO, Secundo TML, Cunha-Silva M, Kotze PG, Montes CG, Almeida JRS, Cabral VLR. Hypoalbuminemia as a risk factor for thromboembolic events in inflammatory bowel disease inpatients. Intest Res. 2019;17:63-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Folsom AR, Lutsey PL, Heckbert SR, Cushman M. Serum albumin and risk of venous thromboembolism. Thromb Haemost. 2010;104:100-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol. 2012;7:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 245] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 35. | Mikhailidis DP, Ganotakis ES. Plasma albumin and platelet function: relevance to atherogenesis and thrombosis. Platelets. 1996;7:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 36. | Jøorgensen KA, Stoffersen E. Heparin like activity of albumin. Thromb Res. 1979;16:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Castell JV, Gómez-Lechón MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12:1179-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 531] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 38. | Perlmutter DH, Dinarello CA, Punsal PI, Colten HR. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986;78:1349-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 421] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 39. | Kanfer A, Kleinknecht D, Broyer M, Josso F. Coagulation studies in 45 cases of nephrotic syndrome without uremia. Thromb Diath Haemorrh. 1970;24:562-571. [PubMed] |