Published online Dec 26, 2024. doi: 10.12998/wjcc.v12.i36.6926
Revised: September 26, 2024
Accepted: October 15, 2024
Published online: December 26, 2024
Processing time: 123 Days and 21 Hours
Musculoskeletal nontuberculous Mycobacterium (NTM) infections are rare, particularly post-acupuncture therapy, and present diagnostic challenges due to their infrequency and potential severity. Prompt recognition and appropriate manage
We present a case of a chronic intractable NTM-infected wound on the elbow joint that completely healed with conservative wound care and antibiotic treatment. An 81-year-old woman presented with a chronic, ulcerative wound on the right elbow joint where she had undergone repeated acupuncture therapy for chronic intolerable pain. Magnetic resonance imaging revealed synovial thickening, effusion, and subcutaneous cystic lesions. An orthopedic surgeon performed open synovectomy and serial debridement. However, 1 month postoperatively, the wound had not healed and became chronic. A wound culture revealed NTM (Mycobacterium abscessus), and the patient was referred to the Department of Plastic and Reconstructive Surgery. Instead of surgical intervention, conservative wound care with intravenous antibiotics was provided, considering the wound status and the patient’s poor general condition. Complete wound healing was achieved in 12 months, with no impact on the range of motion of the elbow joint.
With clinical awareness, musculoskeletal NTM infection can be treated with conservative wound care and appropriate antimicrobial agents.
Core Tip: This report presents a rare case of a nontuberculous Mycobacterium (NTM)-infected wound involving the right elbow joint after repetitive acupuncture therapy due to chronic intolerable pain. Instead of surgical intervention, which is typically recommended for the treatment of musculoskeletal NTM infections, conservative therapy with wound care and antibiotics was chosen, considering the patient’s overall poor health and intolerance for surgical intervention and anesthesia. We present a clinically significant case where treatment was successful in a patient who was unable to tolerate surgical intervention.
- Citation: Kim JH, Koh IC, Lim SY, Kang SH, Kim H. Chronic intractable nontuberculous mycobacterial-infected wound after acupuncture therapy in the elbow joint: A case report. World J Clin Cases 2024; 12(36): 6926-6934
- URL: https://www.wjgnet.com/2307-8960/full/v12/i36/6926.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i36.6926
The incidence of nontuberculous Mycobacterium (NTM) infections has increased worldwide[1]. NTM infection prevalence has increased because of the frequent use of minor, invasive medical procedures, such as intralesional steroid injections and acupuncture therapies[2], while advancements in diagnostic technologies have also resulted in higher NTM detection rates. Although most NTM infections manifest as pulmonary disease entities, 20%–30% of NTM infections present as extrapulmonary infections[2]. Among extrapulmonary manifestations, skin and soft tissue infections are the most common, whereas musculoskeletal NTM infections involving the joints are less frequent[2,3]. The incidence of musculoskeletal NTM infections after minor invasive procedures, such as acupuncture therapy, is particularly low. This makes diagnosis more challenging, as NTM is rarely suspected, due to the scarcity of reported cases[4]. Delayed treatment of musculoskeletal NTM infections due to a lack of clinical awareness can result in permanent tissue damage and functional impairment of joints. Therefore, early diagnosis based on clinical suspicion and microbiological diagnostic testing is crucial for appropriate treatment[5].
Among the NTM species that cause skin and soft tissue infections, Mycobacterium fortuitum (M. fortuitum), Mycobacterium marinum (M. marinum), Mycobacterium abscessus (M. abscessus) complex, Mycobacterium ulcerans, and Mycobacterium avium complex are the most frequently reported. The major species affecting joints include M. marinum, M. terrae complex, Mycobacterium intracellulare (M. intracellulare), M. abscessus complex, and M. fortuitum[2]. The exact etiology of musculoskeletal NTM infection is unknown; however, tenosynovitis and arthritis are the most common causes[2]. Surgical debridement and NTM-specific antimicrobial therapy are necessary in cases of NTM infections involving the joints[5]. However, these treatments often fail, and relapses accompanied by complications are frequently observed[3]. Herein, we present a rare case of a chronic intractable NTM-infected wound at the elbow joint after repetitive acupuncture therapy. The wound completely healed with nonsurgical conservative wound care and antibiotic therapy, with no complications or limited range of motion (ROM) in the elbow joint.
An 81-year-old female patient presented with intolerable pain in the right elbow joint.
The patient had a chronic, non-healing, ulcerative wound near the right elbow joint after repetitive acupuncture therapy for chronic pain. Following acupuncture, the wound showed progressive deterioration, necessitating referral to a major medical facility. At initial presentation, an orthopedic surgeon performed open synovectomy and antibiotic therapy. However, a wound culture from the elbow revealed NTM (M. abscessus), leading to subsequent serial debridement and antimycobacterial medications. Despite these interventions, no significant improvement was observed in the wound, and the patient was referred to the Department of Plastic and Reconstructive Surgery.
The patient had been undergoing treatment for pulmonary NTM (M. abscessus) infection for 18 months prior to hospital presentation, due to chronic pain in the right elbow. The treatment regimen for pulmonary NTM included intravenous (IV) antibiotics: Azithromycin, imipenem, and amikacin. Following hearing impairment, amikacin was subsequently discontinued and clofazimine was added. Furthermore, imipenem was replaced with cefoxitin, due to nausea and vomiting. During hospitalization for elbow pain, NTM was also identified in the sputum culture. Despite the patient’s advanced age, she had no other underlying disease.
No family history of NTM was reported, and the origin of pulmonary and extrapulmonary NTM infection was unclear. The patient neither smoked nor consumed alcohol.
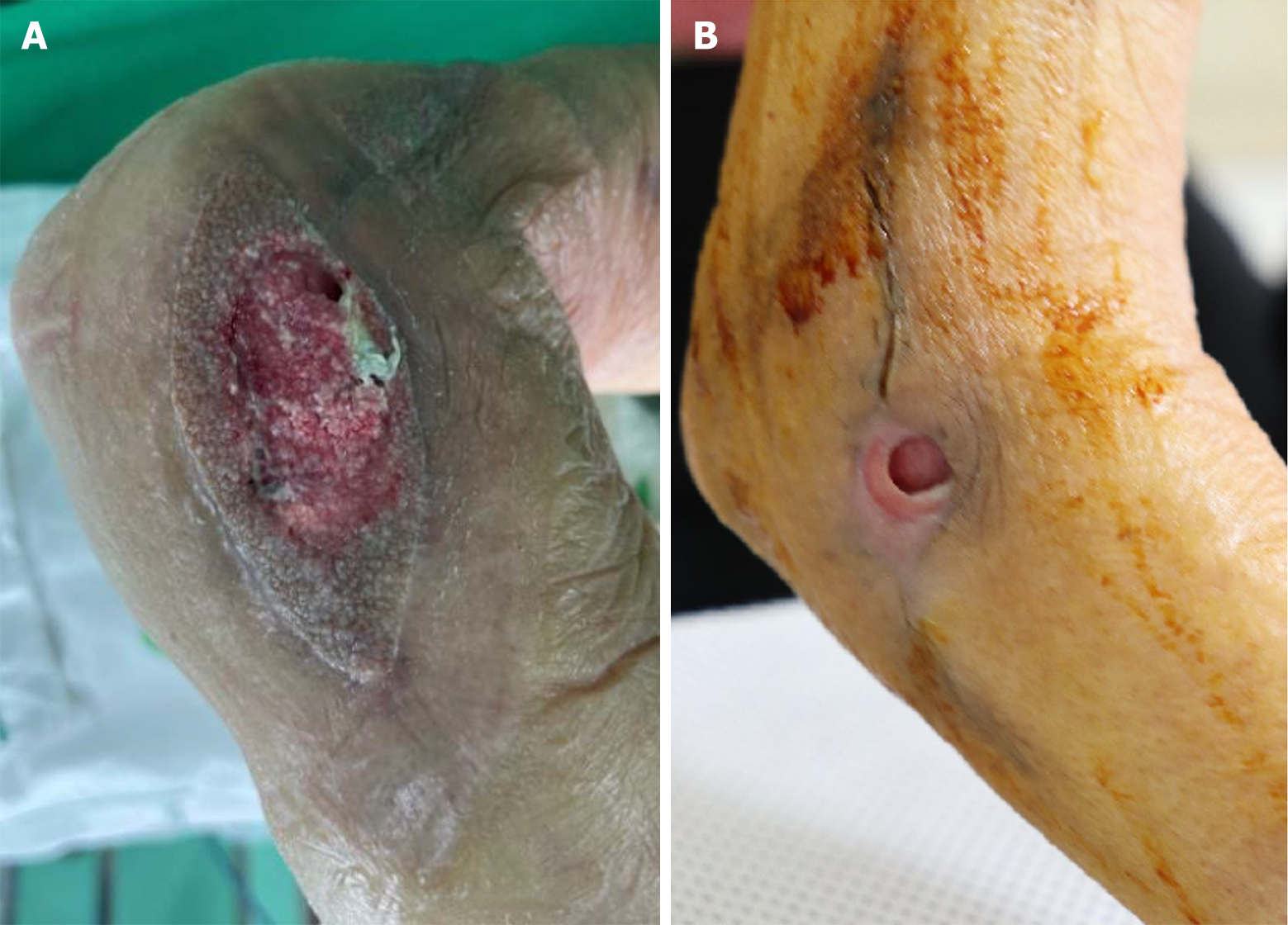
When initially referred to the Department of Plastic and Reconstructive Surgery, the wound was approximately 5.0 cm × 3.0 cm, with overall unhealthy granulation tissue, including a partially necrotic area (Figure 1A).
During open synovectomy, the orthopedic surgeon conducted wound cultures, including acid-fast bacilli liquid culture, considering the history of pulmonary NTM infection treatment 18 months prior. The wound culture revealed NTM (M. abscessus), with negative findings for other bacterial and fungal infections and acid-fast staining. The pathology report suggested acute and chronic synovitis with granulation tissue, consistent with the findings for an atypical mycobacterial infection. When referred to the Department of Plastic and Reconstructive Surgery, laboratory findings showed a high C-reactive protein (CRP) level = 3.0 mg/dL (normal range 0.1–0.5 mg/dL) and normal white blood cell counts = 6500/μL (normal range 4000–10800/μL) and erythrocyte sedimentation rate = 7 mm/h (normal range 0–20 mm/h). Other typical laboratory test results were within normal ranges.
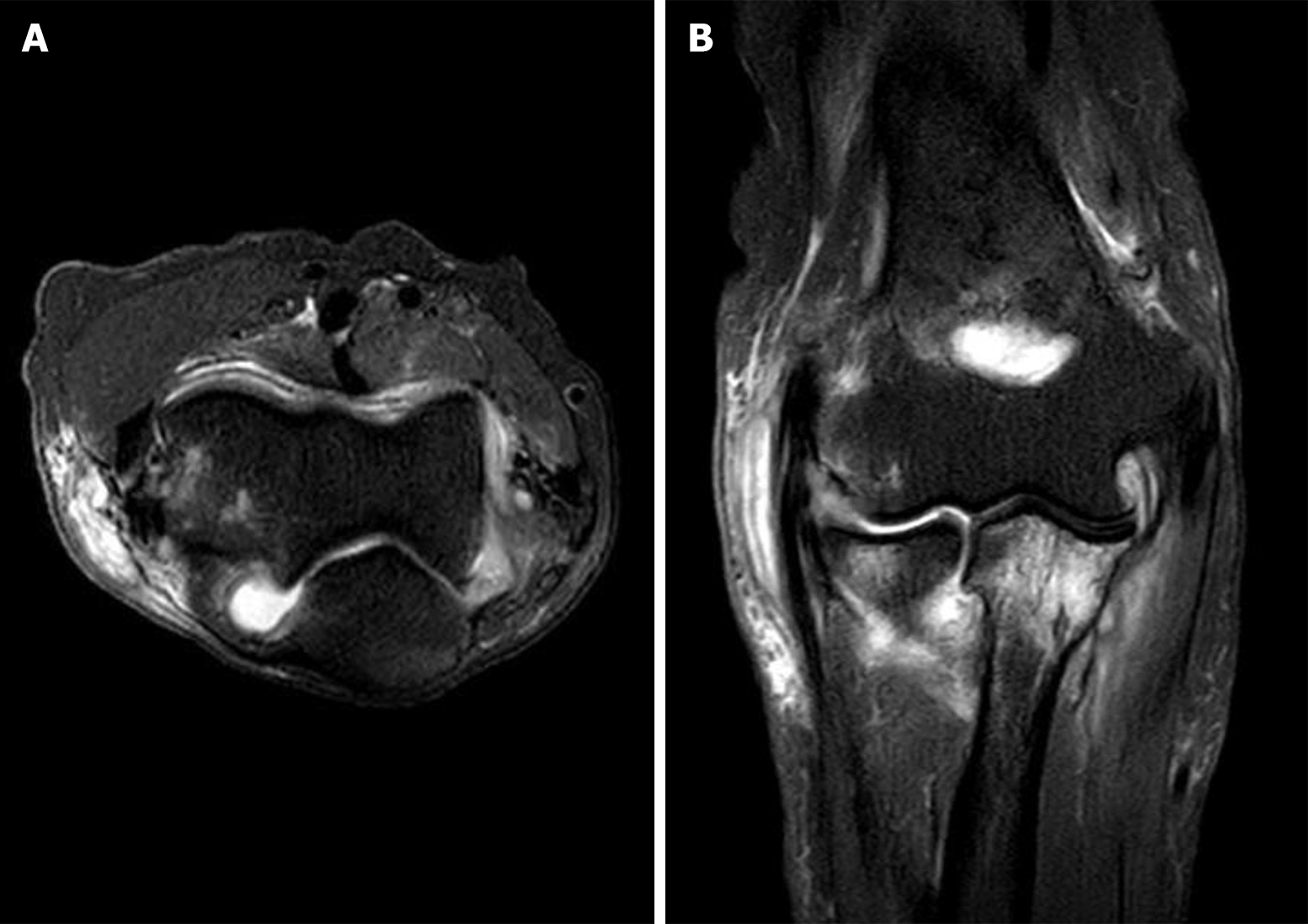
Magnetic resonance imaging performed prior to open synovectomy revealed synovial thickening with effusion in the right elbow joint and subcutaneous cystic lesions (Figure 2).
The patient was diagnosed with a musculoskeletal NTM infection of the right elbow joint.
The patient commenced treatment tailored for musculoskeletal NTM infection. Incremental serial debridement was performed by an orthopedic surgeon with simultaneous IV antibiotic treatment including imipenem 500 mg (twice a day), azithromycin 500 mg (once a day), and clofazimine 100 mg (once a day), according to susceptibility test results and consultation with the infectious disease specialist. However, within a month, the wound had deteriorated and became chronic. Subsequently, the patient was referred to the Department of Plastic and Reconstructive Surgery for coverage of the skin and soft tissue defects 44 days after open synovectomy by an orthopedic surgeon.
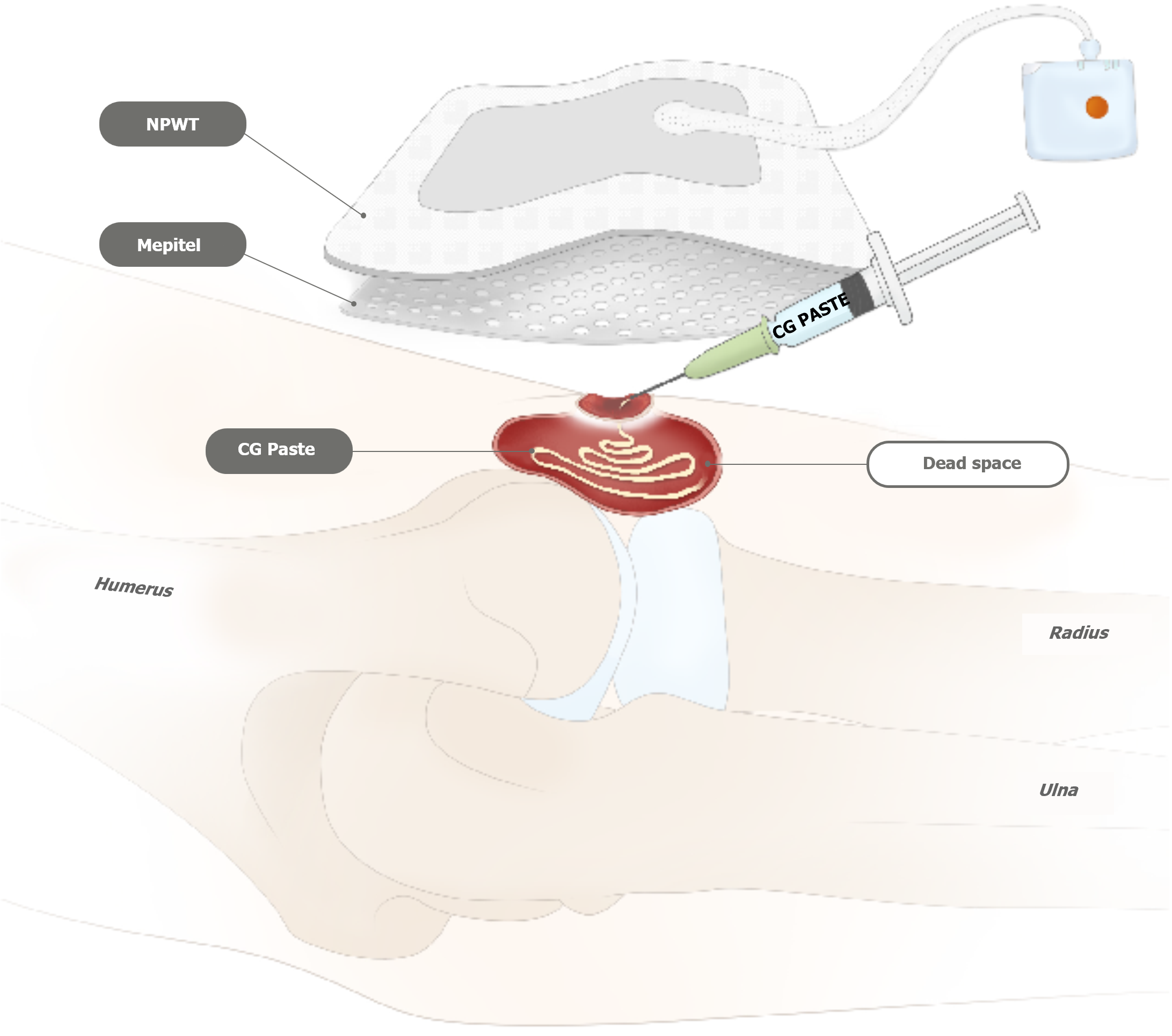
At that time, the patient experienced intolerable side effects, including severe nausea, diarrhea, poor oral nutritional intake, and hearing impairment due to prolonged NTM-targeted therapy. Hence, rather than initiating surgical intervention, we decided to initiate dressing treatment to minimize the bacterial burden. In the early treatment stages, outpatient conservative wound care, including hydrogel with povidone iodine (Repigel), dialchylcarbamoyl chloride-coated dressing, povidone iodine gauze, and intermittent negative pressure wound therapy, was applied twice a week for 4 months to reduce bacterial loading with autolysis of unhealthy granulation tissue. Simultaneously, the infectious disease specialist continuously adjusted the medication regimen, considering the patient's tolerance and drug availability. Additionally, CRP levels were monitored monthly (Table 1). Although CRP levels fluctuated with the patient’s general condition, the wound showed progressive improvement, and CRP levels remained within the normal range after 10 months of treatment. Following 4 months of treatment, all wound cultures, including those for fungi, bacteria, and acid-fast bacilli liquid cultures, showed negative results. At that juncture, despite the reduction in wound size and reduced amount of exudate, relative to the initial stage, further wound recovery was sluggish (Figure 1B). When the mycobacterial load was confirmed to be controlled, an injectable paste-type acellular dermal matrix (CG Paste, CG Bio, Seongnam, Korea) was applied to the joint cavity to facilitate wound healing by mitigating dead space (Figure 3). Subsequently, the wound gradually exhibited reduced raw surface dimensions. Following a discussion with the patient, we decided to continue conservative wound care without additional surgical intervention. At the end of the treatment period (approximately 10 months after treatment initiation), rapid growth of granulation tissue and progressive skin re-epithelialization occurred, and the wound displayed no discharge from the joint cavity.
| Month | Antimycobacterial medication | Dressing material | CRP monitoring (normal range 01–0.5 mg/dL) |
| 0–1 | PO azithromycin 500 mg (once a day) + PO doxycycline 100 mg (twice a day) | Hydrogel with povidone iodine (Repigel) + DACC ribbon (Sorbact ribbon) + povidone iodine-soaked gauze + NPWT (PICO, Smith and Nephew) once a week | 2.6 |
| 1–2 | IV amikacin 500 mg (3 times a week) + PO clofazimine 100 mg (once a day) | Hydrogel with povidone iodine (Repigel) + DACC ribbon (Sorbact ribbon) + povidone iodine-soaked gauze + NPWT (PICO, Smith & Nephew) once a week | 2.4 |
| 2–3 | IV amikacin 500 mg (3 times a week) + PO clofazimine 100 mg (once a day) | Hydrogel with povidone iodine (Repigel) + DACC ribbon (Sorbact ribbon) + povidone iodine-soaked gauze + NPWT (PICO, Smith & Nephew) once a week | 3.0 |
| 3–4 | IV amikacin 500 mg (once a week) + PO clofazimine 100 mg (once a day) | Injectable paste-type acellular dermal matrix (CG Paste, CG Bio, Seongnam, Korea) + DACC ribbon (Sorbact ribbon) + NPWT (PICO, Smith and Nephew) once a week | 3.8 |
| 4–5 | IV amikacin 500 mg (once a week) + PO clofazimine 100 mg (once a day) | Hydrogel with povidone iodine (Repigel) + DACC ribbon (Sorbact ribbon) + povidone iodine-soaked gauze + NPWT (PICO, Smith and Nephew) once a week | 2.8 |
| 5–6 | IV amikacin 500 mg (once a week) + PO clofazimine 100 mg (once a day) | Hydrogel with povidone iodine (Repigel) + DACC ribbon (Sorbact ribbon) + povidone iodine-soaked gauze + NPWT (PICO, Smith and Nephew) once a week | 6.1 |
| 6–7 | IV amikacin 500 mg (once a week) + PO clofazimine 100 mg (once a day) + PO clarithromycin 500 mg (twice a day) | Hydrogel with povidone iodine (Repigel) + DACC ribbon (Sorbact ribbon) + povidone iodine-soaked gauze + NPWT (PICO, Smith and Nephew) once a week | 4.5 |
| 7–8 | PO clofazimine 100 mg (once a day) + PO clarithromycin 500 mg (twice a day) | Hydrogel with povidone iodine (Repigel) + DACC ribbon (Sorbact ribbon) + povidone iodine-soaked gauze + NPWT (PICO, Smith and Nephew) once a week | 2.0 |
| 8–9 | PO clofazimine 100 mg (once a day) + PO clarithromycin 500 mg (twice a day) | Hydrogel with povidone iodine (Repigel) + DACC ribbon (Sorbact ribbon) + povidone iodine-soaked gauze + NPWT (PICO, Smith and Nephew) once a week | 1.1 |
| 9–10 | PO clofazimine 100 mg (once a day) + PO clarithromycin 500 mg (twice a day) | Hydrogel with povidone iodine (Repigel) + DACC ribbon (Sorbact ribbon) + povidone iodine-soaked gauze + NPWT (PICO, Smith and Nephew) once a week | 0.6 |
| 10–11 | PO clofazimine 100 mg (once a day) + PO clarithromycin 500 mg (twice a day) | Hydrogel with povidone iodine (Repigel) + DACC ribbon (Sorbact ribbon) + povidone iodine-soaked gauze + NPWT (PICO, Smith and Nephew) once a week | 0.3 |
| 11–12 | PO clofazimine 100 mg (once a day) + PO clarithromycin 500 mg (twice a day) | Hydrogel with povidone iodine (Repigel) + DACC film (Sorbact surgical dressing) | 0.3 |
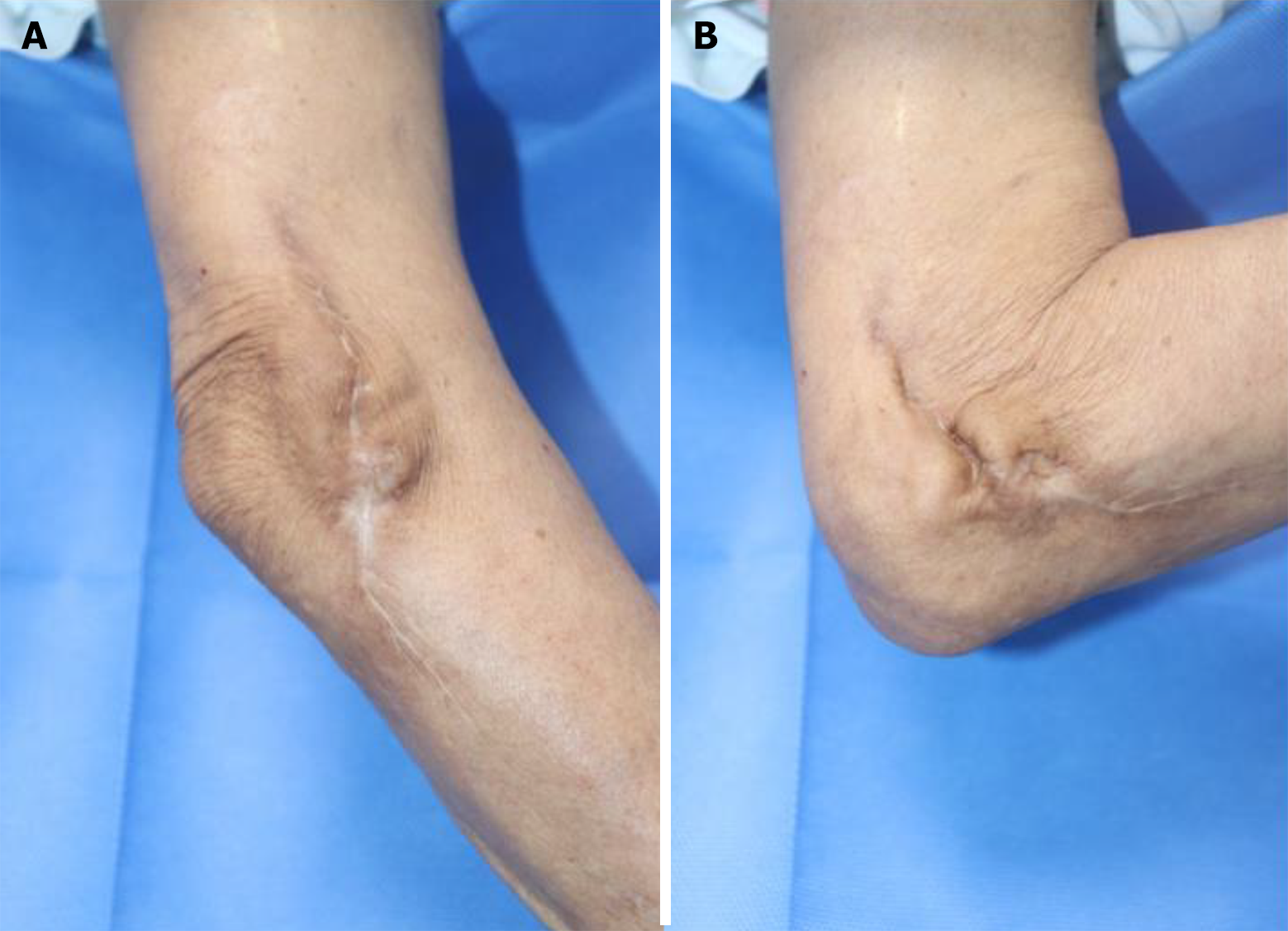
Ultimately, after approximately 12 months of treatment without surgical intervention, the wound had completely healed with intact ROM in the joint. Ten months after complete recovery, no signs of recurrence of infection were observed in the affected area (Figure 4). The patient expressed high satisfaction with the outcome following wound healing, as there were no residual sequelae, such as joint motion limitations due to scar contracture, or complaints of pain or stiffness during full ROM. Additionally, the patient’s quality of life significantly improved after ceasing medication.
Musculoskeletal NTM infections are rare and an accurate incidence is unknown, as NTM infections do not require public health reporting[6]. Although more than 120 different NTM species can potentially infect humans, systematic studies of these diseases are scarce. Furthermore, because the diagnosis of such infections is not easily made through routine bacterial culture, NTM infections can be easily overlooked if not appropriately suspected and are often missed, due to lack of physician awareness, the indolent nature of the disease, misinterpretation of pathological reports, and limitations of mycobacterial culture[7].
Deep soft tissue and musculoskeletal NTM infections can occur when pathogens enter the body through superficial wounds. In our patient, M. abscessus was identified in the right elbow joint. The M. abscessus complex is a group of rapidly growing bacteria commonly found in soil and water. The treatment of NTM infection is challenging because of antimicrobial drug resistance[8,9]. Approximately 45% of extrapulmonary infections caused by M. abscessus occur post-surgery[10] and NTM infections related to acupuncture therapy, mesotherapy, intravenous injections, pedicure sessions, and tattooing have also been reported worldwide[11-14].
In our case, the patient received multiple acupuncture therapies at a traditional Korean medicine clinic for the treatment of chronic pain. Typically, infections related to acupuncture therapy are mild (e.g., cellulitis) but can also lead to serious infections, resulting in life-threatening outcomes such as necrotizing fasciitis, osteomyelitis, and septic shock[15]. Most previously reported post-acupuncture NTM infection cases were limited to cutaneous infections, and the infection sites mainly involved the trunk, lower extremities, and shoulders[4,11,16]. To the best of our knowledge, a case like ours, involving the invasion of the deep elbow joint cavity after acupuncture, leading to synovitis accompanied by joint fluid leakage, has not yet been reported. To elaborate further, most cases of NTM infection associated with acupuncture reported in previous reviews presented as erythematous papules or nodules with mild symptoms such as burning pain and pruritus[4,16]. Our case, however, exhibited an extensively ulcerative appearance with purulent discharge and involvement of the deep elbow joint. Hence, the diagnosis may have been delayed if NTM had not been suspected, as the pathogen would not have been identified through routine bacterial cultures. Fortunately, given our patient's history of treatment for pulmonary NTM infection, extrapulmonary NTM infection could be considered relatively easily. Without this history, the rarity of musculoskeletal NTM infection following acupuncture therapy makes missing an NTM infection diagnosis more likely which can lead to unnecessary and inefficient treatment[4,16].
Common clinical manifestations of M. abscessus complex infection include localized pain, swelling, pus-like discharge, ulcerations, and draining sinuses[17,18]. Our patient displayed an ulcerative lesion with surrounding necrotic tissues and purulent discharge accompanying dead space in the right elbow joint. In cases of suspected chronic intractable wound infection, computed tomography (CT) or magnetic resonance imaging (MRI) should be performed to assess the characteristics, extent, and depth of the lesion. If inflammation has spread to deep tissue and a sinus tract formation is suspected, surgical intervention should be considered, along with a deep tissue biopsy. In addition, acid-fast bacilli stain, aerobic and anaerobic cultures, fungal cultures, as well as mycobacterial culture and mycobacterial polymerase chain reaction should also be considered[18,19]. Magnetic resonance imaging typically exhibits hypointensity or isointensity on T1-weighted images and hyperintensity on T2-weighted images[17,18]. Medication therapies for musculoskeletal infections caused by M. abscessus complex depend on antibiotic susceptibility and commonly involve multidrug therapy for at least 6 months[18]. Such key antibiotics include amikacin, imipenem/cefoxitin, and clofazimine, which are highly effective[2,6]. Macrolide antibiotics are commonly used for NTM infection treatment[2,5].
The recommended treatment regimen for musculoskeletal NTM infections includes a combination of surgical debridement to lessen the overall infection load and NTM-specific antimicrobial therapy to eliminate residual infection[5,19]. Previous reviews have also reported that most cases of musculoskeletal NTM infections include surgical debridement as an essential part of treatment[3,5,19]. However, even with complete debridement and long-term appropriate medi
As previously reported, combination drug therapies are considered essential; however, the choice of drugs and duration of treatment depends not only on the results of drug-susceptibility testing, but also on the patient's tolerance to drug toxicity. In particular, when patient intolerance is prominent, selecting the appropriate medication can be challenging[3,6]. In such cases, appropriate medication adjustments should be made through ongoing consultation with an infectious disease specialist, aiming to minimize side effects while preserving the potential therapeutic effects of the medication on the wound. In our case, the patient received a combination drug therapy of per oral (PO) azithromycin and doxycycline for one month until the NTM drug sensitivity test results became available. The use of imipenem was also initiated but discontinued after a short period due to the patient’s complaints of nausea and vomiting. The drug sensitivity test results showed resistance to most medications, and azithromycin, suspected to be the cause of the patient's hearing impairment, along with doxycycline, which showed drug resistance, were discontinued. For the following 6 months, the patient was treated with IV amikacin, considering drug susceptibility and PO clofazimine, considering its proven effectiveness in recent reports[2,9,18]. Subsequently, due to a supply shortage of amikacin at the hospital, the patient was treated with a combination of PO clofazimine and clarithromycin for 5 months. Appropriate selection of dressing materials is also essential to reduce NTM burden while promoting wound healing and to avoid surgical intervention.
In clinical practice, for cases of ulcerative stubborn wounds occurring in the elbow joint area, a thorough history assessment is essential for identifying the underlying cause. If a subacute to chronic infection is suspected following acupuncture, CT or MRI should be performed. If deep space inflammation is detected, the possibility of musculoskeletal NTM infection should be considered. When surgical debridement is performed, it is essential to include deep tissue cultures along with mycobacterial culture to enable the earliest possible diagnosis. If a musculoskeletal NTM infection is diagnosed, serial surgical debridement should be performed. However, if the patient is unable to undergo surgical treatment, antimicrobial medication therapy should be adjusted based on NTM drug sensitivity test results and the patient's tolerance, in consultation with an infectious disease specialist. Concurrently, conservative management should be implemented. Dressing materials for inflammation control may include hydrogel with povidone iodine (Repigel), antibiotic ointment, Dialchylcarbamoyl chloride-coated ribbon (Sorbact ribbon), and povidone iodine-soaked gauze, along with negative pressure wound therapy. Once culture studies confirm the absence of NTM organisms, a paste-type acellular dermal matrix can be used to quickly fill the dead space and promote wound healing.
Our patient had also been undergoing treatment for a pulmonary NTM (M. intracellulare/abscessus) infection that occurred 18 months prior to the onset of the right elbow joint infection. To the best of our knowledge, no cases of pulmonary and extrapulmonary M. abscessus infections have been reported. The identification of the same NTM species in both pulmonary and extrapulmonary infections in our case suggests that the NTM species causing the pulmonary infection acted as the source for the musculoskeletal NTM infection. Revealing this association poses another formidable challenge.
We present a rare case of a musculoskeletal NTM infection in the elbow joint after acupuncture. Despite contraindications for surgical intervention, nonsurgical conservative dressing treatment and appropriate adjustment of antimycobacterial medications facilitated complete wound healing with no complications. Our report underscores the importance of promptly suspecting NTM in patients with chronic intractable wound infections following acupuncture therapy. Rapid diagnosis, appropriate antibiotic therapy, and wound care with adjustments, as needed, are paramount, particularly for patients for whom surgical intervention is challenging due to a deteriorating general condition.
| 1. | Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med. 2015;36:13-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 655] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 2. | Wi YM. Treatment of Extrapulmonary Nontuberculous Mycobacterial Diseases. Infect Chemother. 2019;51:245-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Goldstein N, St Clair JB, Kasperbauer SH, Daley CL, Lindeque B. Nontuberculous Mycobacterial Musculoskeletal Infection Cases from a Tertiary Referral Center, Colorado, USA. Emerg Infect Dis. 2019;25:1075-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Song JY, Sohn JW, Jeong HW, Cheong HJ, Kim WJ, Kim MJ. An outbreak of post-acupuncture cutaneous infection due to Mycobacterium abscessus. BMC Infect Dis. 2006;6:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Park HY, Yoon JO, Park J, Yoon J, Kim JS. Diagnosis and Treatment for Deep Nontuberculous Mycobacteria Infection of the Hand and Wrist. J Korean Soc Surg Hand. 2015;20:119. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Hogan JI, Hurtado RM, Nelson SB. Mycobacterial Musculoskeletal Infections. Infect Dis Clin North Am. 2017;31:369-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Smidt KP, Stern PJ, Kiefhaber TR. Atypical Mycobacterial Infections of the Upper Extremity. Orthopedics. 2018;41:e383-e388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Lagune M, Kremer L, Herrmann JL. Mycobacterium abscessus, a complex of three fast-growing subspecies sharing virulence traits with slow-growing mycobacteria. Clin Microbiol Infect. 2024;30:726-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 9. | Abdelaal HFM, Chan ED, Young L, Baldwin SL, Coler RN. Mycobacterium abscessus: It's Complex. Microorganisms. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Chadha R, Grover M, Sharma A, Lakshmy A, Deb M, Kumar A, Mehta G. An outbreak of post-surgical wound infections due to Mycobacterium abscessus. Pediatr Surg Int. 1998;13:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Gnatta JR, Kurebayashi LF, Paes da Silva MJ. Atypical mycobacterias associated to acupuncuture: an integrative review. Rev Lat Am Enfermagem. 2013;21:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Uslan DZ, Kowalski TJ, Wengenack NL, Virk A, Wilson JW. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Ricciardo B, Weedon D, Butler G. Mycobacterium abscessus infection complicating a professional tattoo. Australas J Dermatol. 2010;51:287-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Mougari F, Guglielmetti L, Raskine L, Sermet-Gaudelus I, Veziris N, Cambau E. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Kim YJ, Kim SH, Lee HJ, Kim WY. Infectious Adverse Events Following Acupuncture: Clinical Progress and Microbiological Etiology. J Korean Med Sci. 2018;33:e164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Tang P, Walsh S, Murray C, Alterman C, Varia M, Broukhanski G, Chedore P, DeKoven J, Assaad D, Gold WL, Ghazarian D, Finkelstein M, Pritchard M, Yaffe B, Jamieson F, Henry B, Phillips E. Outbreak of acupuncture-associated cutaneous Mycobacterium abscessus infections. J Cutan Med Surg. 2006;10:166-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Wongkitisophon P, Rattanakaemakorn P, Tanrattanakorn S, Vachiramon V. Cutaneous Mycobacterium abscessus Infection Associated with Mesotherapy Injection. Case Rep Dermatol. 2011;3:37-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Holt MR, Kasperbauer S. Management of Extrapulmonary Nontuberculous Mycobacterial Infections. Semin Respir Crit Care Med. 2018;39:399-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Kwan M, Tupler R. Recurrent Nontuberculous Mycobacterial Tenosynovitis. Ochsner J. 2021;21:86-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |