Published online Dec 26, 2024. doi: 10.12998/wjcc.v12.i36.6905
Revised: September 29, 2024
Accepted: October 15, 2024
Published online: December 26, 2024
Processing time: 197 Days and 13.8 Hours
Spinal meningiomas (SMs) are common benign tumors that are typically treated with surgical resection. The choice of surgical approach may vary depending on the location of dural attachment of the SM, with a posterior approach being the traditional preference. However, there is limited research available on the impact of dural attachment location on outcomes following posterior approach for SM resection.
To investigate the outcomes of posterior approach for SM resection, and compare the results among different dural attachment location subgroups.
Between January 2013 and February 2023, a total of 34 SM patients were included in the study. Various clinical and radiologic features, functional states before and after surgery, operating time, intraoperative blood loss, tumor recurrence, and perioperative complications were assessed and compared.
The average age of the included 34 patients’ (10 males and 24 females) age was 62.09 years. Mean follow-up duration was 22.65 months. The location of SM was the thoracic spine in 32 cases, with only 2 in the cervical spine. On average, intraoperative blood loss was 520.59 mL, and operating time was 176.76 minutes. Thirty three cases had successful outcomes while only 1 experienced an unexpe
The posterior approach for SM resection is safe and effective, yielding comparable surgical and neurological outcomes regardless of the dural attachment location.
Core Tip: This retrospective study aimed to assess the safety and efficacy of the posterior approach for spinal meningiomas resection, and compare the outcomes among different dural attachment location subgroups. Thirty four patients with an average follow-up time of 22.65 months were included. The average operating time was 176.76 min, with intraoperative blood loss of 520.59 mL. Satisfactory outcomes were observed in 97.06% of cases and the tumor recurrence rate was 2.94%. There were no significant differences in operating time, intraoperative blood loss, neurological function, and recurrence rates among three distinct dural attachment location subgroups.
- Citation: Chen H, Fu YN, Fu CD. Safety and efficacy of posterior approach for resection of spinal meningioma: Impact of dural attachment location. World J Clin Cases 2024; 12(36): 6905-6915
- URL: https://www.wjgnet.com/2307-8960/full/v12/i36/6905.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i36.6905
Spinal meningiomas (SMs) originate from meningothelial arachnoid cap cells and account for 25%-45% of all spinal tumors[1]. These slow-growing intradural extramedullary lesions can develop anywhere along the spine, with a higher prevalence in the thoracic region (67%-84%), followed by the cervical (14%-27%) and lumbar (2%-14%) regions[2]. SMs are most commonly found in women aged between 40 and 70 years, with a female-to-male ratio of 4:1[3].
Fortunately, the majority of SMs are histologically benign or classified as World Health Organization (WHO) grade 1, with only a small percentage considered atypical (WHO grade 2) (5%-25%) or anaplastic (WHO grade 3) (1%-5%)[4]. The most common histological subtypes of SMs include meningothelial, psammomatous, and transitional meningiomas, all of which are classified as WHO grade 1[5]. Patients with SM may present with a range of symptoms from asymptomatic to severe neurological impairments, depending on the extent of spinal cord compression. Typical symptoms include pain, sensory loss, weakness, and bowel or urinary dysfunction[6].
Surgical resection is the primary treatment for SMs, with generally favorable outcomes and a low recurrence rate (1.3%-6.4%)[7]. The choice of surgical approach for resection depends on the tumor site and dural attachment location, typically favoring a posterior approach[8,9]. However, challenges arise when managing with SMs attached ventrally or ventrolaterally. Some surgeons have attempted anterior approaches for these cases but note a higher complication rate[10,11]. Others argue that posterior approaches can provide sufficient exposure for safe removal of ventral or ventrolateral SMs[12-14]. The optimal method for resecting SMs with ventral or ventrolateral dural attachment remains a topic of ongoing debate.
This study aimed to evaluate the safety and efficacy of using a posterior approach for resecting SMs with different sites and dural attachments locations. Additionally, the study examined whether different dural attachments influenced outcomes.
This study retrospectively identified patients who underwent SM resection in the Department of Orthopedics at 903 Hospital of the Joint Logistic Support Force of the People’s Liberation Army from January 2013 to February 2023. Inclusion criteria were: SM was confirmed by pathology; the patient underwent a posterior approach; had complete medical records and imaging data. Exclusion criteria were: Presence of other neurogenic tumors; did not undergo a posterior approach; had severe cardiovascular or cerebrovascular disease and could not tolerate anesthesia and surgery. Finally, 34 patients were included in the study.
The data collected included information on age, sex, tumor level and length, dural attachment location, preoperative symptoms, duration of symptoms, operative time, intraoperative blood loss, instrumented fusion, perioperative complications, hospital length of stay, time to follow-up, and recurrence. Additionally, 3-dimensional computed tomography and magnetic resonance imaging (MRI) were used to assess tumor size, calcification, spinal cord compression, and dural attachment location.
The modified McCormick grade (MMG) scale was used to classify neurological function, visual analogue scale (VAS) scores were used to assess pain, the Simpson grading scale was used to grade resection extension, and WHO tumor grade was used for histological evaluation.
All patients underwent surgery using the median posterior approach while in the prone position. A partial or total laminectomy was carried out to access the tumor. Subsequently, the tumor was grossly resected after making a midline incision in the dura. The dural attachment was either removed or cauterized using bipolar coagulation forceps. Following this, the dura was either continuously sutured or covered with artificial dura. In cases where total laminectomy was performed, pedicle screw fixation was performed, followed by posterolateral bone graft fusion to stabilize the spine.
The data were analyzed using SPSS 25 (IBM Corp., Armonk, NY, United States). Categorical variables were reported as count and percentage, while continuous variables were presented as mean ± SD. The Kolmogorov-Smirnov test was used to assess normal distribution. Fisher’s exact test was used to compare categorical variables, the Student’s t test to evaluate normally distributed variables between 2 groups, the Mann-Whitney U test to compare non-normally distributed variables between 2 groups, and the Kruskal-Wallis H test to compare 3 groups. Statistical significance was set at a P value < 0.05.
The study included a total of 34 patients, consisting of 24 females and 10 males with a sex ratio of 2.4:1. The average age was 62.09 years (ranging from 27 to 83 years). The average hospital stay was 24.76 days (ranging from 9 to 39 days), and the average follow-up time after surgery was 22.65 months (ranging from 6 to 36 months). Of these cases, 31 patients presented with pain and/or myelopathy, while 3 cases were incidentally found to have meningiomas. Preoperative symptoms included neck/back/radicular pain (23.53%), sensory deficit (73.53%), motor deficit (70.59%), and urinary dysfunction (5.88%), with an average duration of 15.12 months (ranging from 0 to 72 months). SMs were located in the cervical spine in 2 cases and in the thoracic spine in 32 cases. The location of dural attachments of the tumors were 8 dorsal, 5 dorsolateral, 3 ventral, 11 ventrolateral and 7 lateral. Tumor length was less than 1 cm in 2 cases, between 1 and 2 cm in 24 cases, and greater than 2 cm in 8 cases. Patient information is listed in Table 1.
| Case number | Age (years) | Sex | Clinical presentation | Duration of symptoms (months) | Spinal segment | Dural attachment location | Calcification | Tumor length (cm) |
| 1 | 68 | Male | Pain; Sensory and motor deficit | 4 | T8 | Ventrolateral | No | 1.66 |
| 2 | 73 | Male | No | 0 | T8/9 | Dorsolateral | Yes | 1.88 |
| 3 | 71 | Female | Pain | 24 | T4 | Dorsolateral | Yes | 2.26 |
| 4 | 60 | Female | Pain; Sensory deficit | 12 | T7-9 | Dorsolateral | Yes | 1.66 |
| 5 | 57 | Female | No | 0 | T9-11 | Ventrolateral | No | 2.00 |
| 6 | 68 | Female | Pain; Sensory and motor deficit | 24 | T4 | Ventrolateral | Yes | 1.35 |
| 7 | 54 | Female | Sensory and motor deficit | 5 | T9/10 | Ventral | No | 1.69 |
| 8 | 45 | Male | No | 0 | T12 | Lateral | No | 1.57 |
| 9 | 61 | Female | Pain; Sensory deficit | 12 | T11/12 | Dorsal | Yes | 1.63 |
| 10 | 69 | Female | Sensory and motor deficit | 12 | T3 | Ventral | No | 1.70 |
| 11 | 58 | Female | Sensory and motor deficit; Urinary dysfunction | 72 | T6/7 | Ventrolateral | Yes | 1.66 |
| 12 | 63 | Female | Sensory and motor deficit | 24 | T11/12 | Dorsal | No | 2.63 |
| 13 | 69 | Female | Sensory and motor deficit | 4 | T5/6 | Ventrolateral | No | 1.61 |
| 14 | 53 | Male | Sensory and motor deficit | 12 | T9/10 | Dorsal | No | 1.26 |
| 15 | 71 | Female | Sensory and motor deficit | 12 | C5/6 | Lateral | Yes | 1.74 |
| 16 | 57 | Male | Pain; Sensory deficit | 12 | T5/6 | Lateral | No | 1.39 |
| 17 | 58 | Female | Sensory and motor deficit | 12 | T7/8 | Lateral | No | 1.46 |
| 18 | 76 | Female | Sensory and motor deficit | 2 | T7/8 | Dorsolateral | No | 1.20 |
| 19 | 71 | Male | Sensory and motor deficit | 60 | T4/5 | Dorsal | No | 1.34 |
| 20 | 58 | Female | Sensory and motor deficit | 36 | T7/8 | Ventrolateral | No | 2.22 |
| 21 | 50 | Male | Sensory and motor deficit | 6 | T7/8 | Dorsal | No | 1.46 |
| 22 | 49 | Female | No | 0 | T10/11 | Dorsal | No | 1.44 |
| 23 | 72 | Female | Sensory and motor deficit | 24 | T9 | Ventral | No | 1.39 |
| 24 | 73 | Female | Motor deficit; Urinary dysfunction | 12 | T7/8 | Lateral | No | 1.54 |
| 25 | 50 | Female | Sensory and motor deficit | 12 | T4 | Ventrolateral | No | 0.94 |
| 26 | 56 | Female | Pain; Sensory and motor deficit | 3 | T3/4 | Ventrolateral | No | 2.51 |
| 27 | 68 | Male | Sensory deficit | 2 | T4 | Dorsal | No | 2.25 |
| 28 | 70 | Female | Pain; Sensory deficit | 48 | T8/9 | Dorsal | Yes | 1.93 |
| 29 | 58 | Female | Sensory and motor deficit | 1 | T10/11 | Ventrolateral | Yes | 1.56 |
| 30 | 61 | Male | Sensory and motor deficit | 12 | T1/2 | Ventrolateral | No | 1.45 |
| 31 | 27 | Female | Sensory and motor deficit | 7 | C3-6 | Ventrolateral | No | 4.05 |
| 32 | 83 | Male | Motor deficit | 12 | T6/7 | Dorsolateral | No | 1.60 |
| 33 | 71 | Female | Motor deficit | 12 | T11/12 | Lateral | No | 2.50 |
| 34 | 63 | Female | Sensory and motor deficit | 24 | T5/6 | Lateral | No | 0.80 |
Neurological function and pain were assessed using the MMG and VAS scores respectively. Preoperatively, MMG classifications I, II, III, IV, and V were observed in 4, 2, 6, 14, and 8 cases, respectively, with a mean VAS score of 4.03 (range 0-7) (Table 2).
| Variable | Value |
| Cohort size (n) | 34 |
| Age (years) | 62.09 ± 10.8 (27-83) |
| Sex | |
| Male | 10 |
| Female | 24 |
| Tumor level | |
| Cervical | 2 |
| Thoracic | 32 |
| Dural attachment location | |
| Dorsal/dorsolateral | 13 |
| Ventral/ventrolateral | 14 |
| Lateral | 7 |
| Tumor length | |
| < 1 cm | 2 |
| 1-2 cm | 24 |
| > 2 cm | 8 |
| Preoperative MMG | |
| I/II | 6 |
| III/IV/V | 28 |
| Symptoms | |
| Pain/myelopathy | 31 |
| Incidental | 3 |
| Duration of symptoms (months) | 15.12 ± 16.86 (0-72) |
| Hospital length of stay (days) | 19.68 ± 7.91 (9-39) |
| Follow-up duration (months) | 22.65 ± 12 (6-36) |
| Operative time (minutes) | 176.76 ± 50.22 (80-310) |
| Intraoperative blood loss (mL) | 520.59 ± 380.41 (100-1700) |
| Calcification | |
| No | 25 |
| Yes | 9 |
| Histological types | |
| Meningothelial | 17 |
| Psammomatous | 10 |
| Transitional | 4 |
| Fibrous | 2 |
| Atypical | 1 |
| WHO grade | |
| 1 | 33 |
| 2 | 1 |
| Simpson grade | |
| 1 | 5 |
| 2 | 29 |
| Instrument fusion | |
| No | 11 |
| Yes | 23 |
| Perioperative complications | |
| CSF leakage | 3 |
| Pneumonia | 1 |
| Urinary tract infection | 1 |
| Recurrence | 1 |
As illustrated in Table 2, a posterior approach was utilized for all cases, with 11 undergoing partial laminectomy without internal fixation and 23 undergoing total laminectomy with instrumented fusion. Of these cases, 5 underwent Simpson grade 1 tumor resection, while the remaining 29 cases underwent Simpson grade 2 resection. The average operating time was 176.76 minutes (ranging from 80 to 310 minutes), and the average intraoperative blood loss was 520.59 mL (ranging from 100 to 1700 mL). Of these cases, 25 tumors were non-calcified and 9 were calcified. The histological type of 33 cases was WHO grade 1 (meningothelial 17, psammomatous 10, transitional 4, and fibrous 2), with only 1 case classified as WHO grade 2 (atypical).
The distribution of MMG grades postoperatively (1 week after surgery) were as follows: 5 cases were MMG I, 9 cases were MMG II, 12 cases were MMG III, 7 cases were MMG IV, and 1 case was MMG V. At the final follow-up, 26 cases were classified as MMG I, 7 cases as MMG II, and 1 case as MMG III. Satisfactory outcomes, defined as no or minimal function deficit at follow-up (MMG I or II), were observed in 97.06% of cases, while unsatisfactory outcomes, defined as no change in dysfunction or postoperative MMG III-IV, were found in 2.94% of cases. The mean VAS score decreased from 2.03 postoperatively to 0.41 at the final follow-up. All cases showed improvement in neurological function and significant pain relief at the final follow-up compared to preoperative and postoperative assessments (P < 0.05). Perioperative complications included cerebral spinal fluid (CSF) leakage in 3 cases, pneumonia in 1 case, and urinary tract infection in 1 case. Three patients with CSF leakage had drainage tubes placed in the surgical area for 5-7 days to ensure adequate CSF drainage; 1 patient with pneumonia and 1 patient with urinary tract infection received antibiotics to control the infection. All complications were resolved before discharge, and had no long-term effects on patient outcomes. Recurrence occurred 9 years after surgery in a 27-year-old woman with a tumor located ventrolateral to the spinal cord in the cervical spine, presenting with a long dural tail. The initial surgery involved Simpson grade 2 resection of a WHO grade I meningothelial tumor, and the patient subsequently underwent Simpson grade 1 resection followed by radiotherapy. The recurrence rate was 2.94%.
Patients with dorsal or dorsolateral dural attachment were categorized as the dorsal/dorsolateral subgroup (n = 13), those with ventral or ventrolateral dural attachment were categorized as the ventral/ventrolateral subgroup (n = 14), and those with lateral dural attachment were categorized as the lateral subgroup (n = 7). There were no significant differences in age, sex, preoperative MMG and VAS scores, degree of tumor calcification, Simpson grades, and follow-up duration between these subgroups (P > 0.05), indicating that the data among the 3 subgroups were comparable. Following surgery and at the final follow-up, improvements in neurological function and pain relief were observed in all subgroups. Additionally, the MMG and VAS scores, operative time, intraoperative blood loss, perioperative complications, and recurrence rates were similar across the 3 subgroups (P > 0.05) (Table 3).
| Variable | Dorsal/dorsolateral (n = 13) | Ventral/ventrolateral (n = 14) | Lateral (n = 7) | P value |
| Age (years) | 65.23 ± 10.35 | 58.93 ± 11.37 | 62.57 ± 10.1 | 0.314 |
| Sex | ||||
| Male | 6 | 2 | 2 | 0.208 |
| Female | 7 | 12 | 5 | |
| Preoperative MMG | ||||
| I/II/III | 6 | 4 | 2 | 0.996 |
| IV/V | 7 | 10 | 5 | |
| Postoperative MMG | ||||
| I/II/III | 12 | 9 | 5 | 0.495 |
| IV/V | 1 | 5 | 2 | |
| Final follow-up MMG | ||||
| I/II/III | 12 | 11 | 4 | 0.208 |
| IV/V | 1 | 3 | 3 | |
| Preoperative VAS score | 3.54 ± 2.67 | 4.64 ± 1.6 | 3.71 ± 2.81 | 0.732 |
| Postoperative VAS score | 1.62 ± 1.33 | 2.57 ± 1.09 | 1.71 ± 1.25 | 0.11 |
| Final follow-up VAS score | 0.38 ± 0.65 | 0.5 ± 0.65 | 0.29 ± 0.49 | 0.738 |
| Calcification | ||||
| No | 8 | 11 | 6 | 0.538 |
| Yes | 5 | 3 | 1 | |
| Operative time (min) | 185.38 ± 53.29 | 174.29 ± 33.62 | 165.71 ± 73.68 | 0.751 |
| Intraoperative blood loss (mL) | 569.23 ± 449.79 | 478.57 ± 254.74 | 514.29 ± 491.35 | 0.8 |
| Simpson grade | ||||
| 1 | 4 | 1 | 0 | 0.189 |
| 2 | 9 | 13 | 7 | |
| Perioperative complications | ||||
| CSF leakage | 2 | 1 | 0 | |
| Urinary tract infection | 0 | 1 | 0 | 0.602 |
| Pneumonia | 0 | 0 | 1 | |
| Follow-up duration (months) | 19.31 ± 12.53 (6-36) | 24 ± 11.6 (6-36) | 26.14 ± 12.08 (6-36) | 0.34 |
| Recurrence | ||||
| No | 13 | 13 | 7 | 1 |
| Yes | 0 | 1 | 0 |
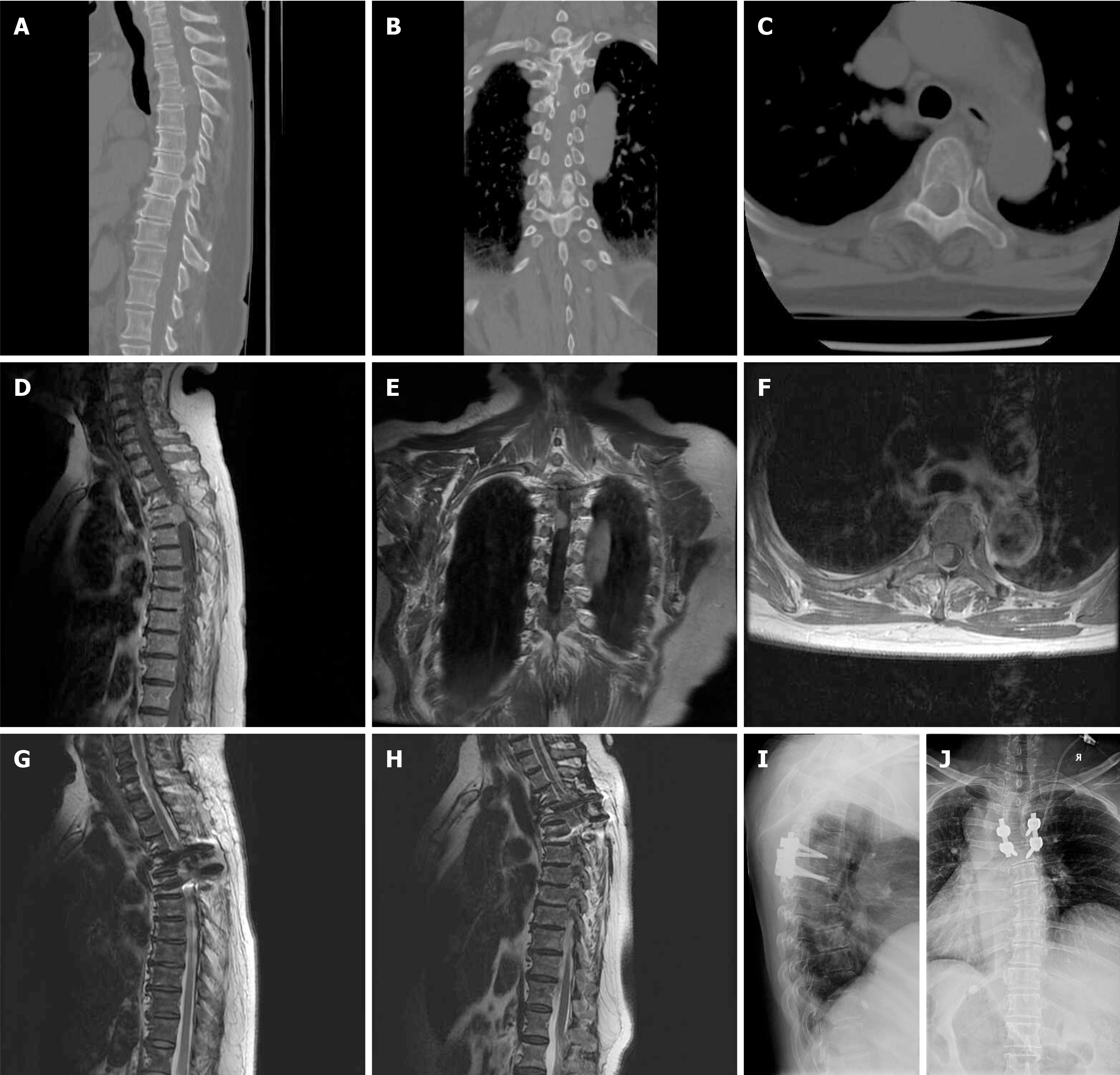
Two representative cases are presented in Figure 1 and Figure 2. Case 1 involved a 68-year-old woman who had been experiencing pain, numbness, and weakness in both lower limbs for 2 years. Prior to surgery, her neurological function was assessed as MMG IV and she had a VAS score of 6. Imaging revealed a calcified tumor in the thoracic spine with ventrolateral dural attachment measuring 1.35 cm in length. The patient underwent a posterior approach for total laminectomy and a Simpson grade 2 resection. The surgery lasted 230 minutes with an intraoperative blood loss of 1200 mL. Pedicle screw fixation and posterolateral bone graft fusion were performed for spinal stabilization. The histological type of the tumor was WHO grade 1 (psammomatous). There were no perioperative complications during her hospital stay. Postoperatively, the patient's MMG score improved to III and VAS score decreased to 3. At the final follow-up 3 years after surgery, the patient's MMG score was I and VAS score was 0 (Figure 1).
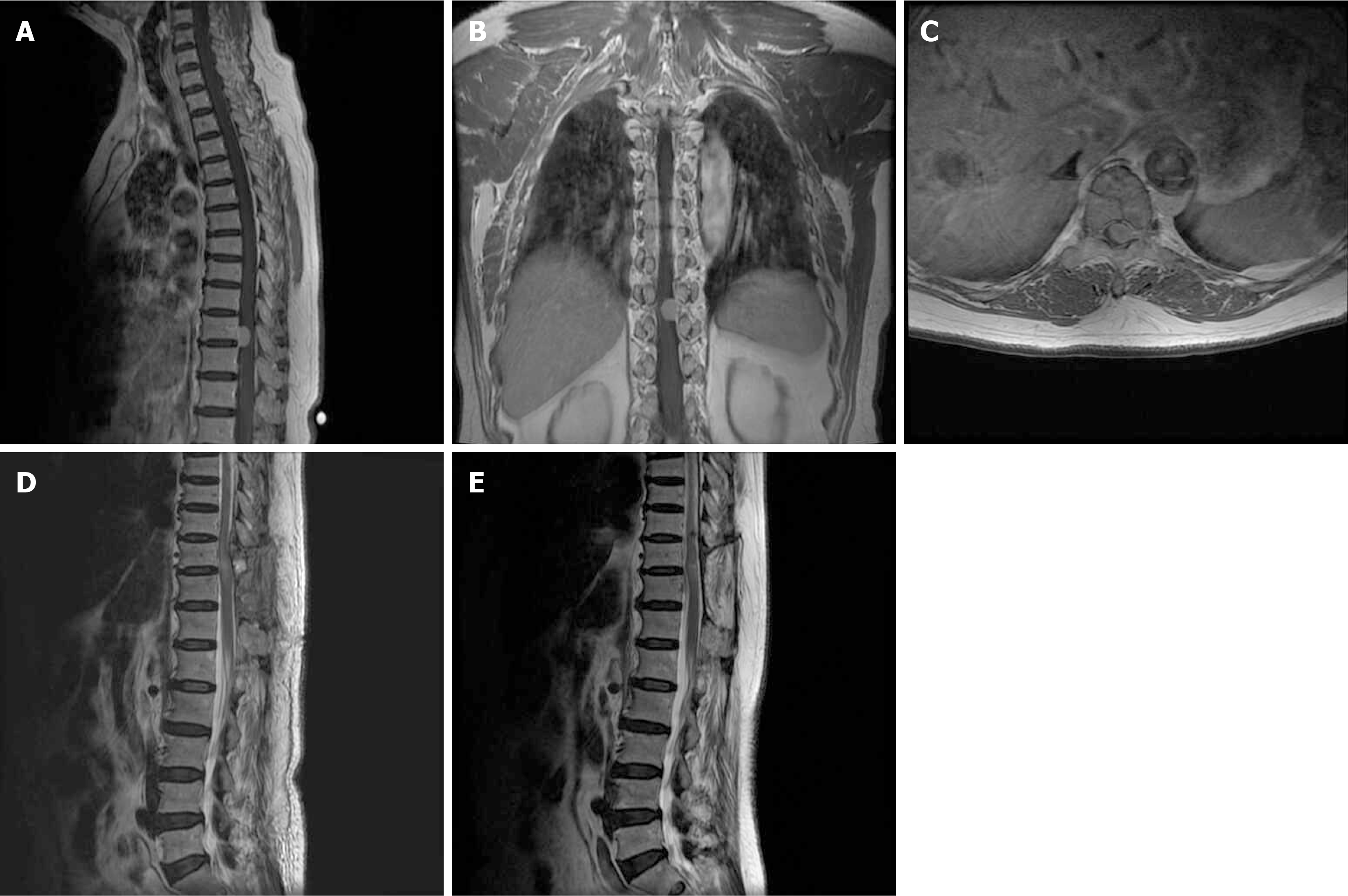
Case 2 involved a 58-year-old female patient who presented with pain and numbness in both lower limbs, along with difficulty walking steadily for a month. Prior to surgery, her neurological function was classified as MMG III and she reported a VAS score of 4. MRI results indicated a tumor in the thoracic spine with ventrolateral dural attachment, measuring 1.56 cm in length. The surgical procedure involved a posterior approach for partial laminectomy and a Si
Among the 34 patients analyzed in this study, 70.59% were female. The majority of patients exhibited symptoms such as local/radicular pain, sensory/motor deficit, and urinary tract dysfunction. Most SMs were found in the thoracic spine and were non-calcified, with prevalent histological types being meningothelial, psammomatous, and transitional. The epidemiology, clinicoradiologic characteristics, and histological types observed in this study closely resembled those documented in previous reports[15].
The primary treatment for symptomatic SMs was maximal surgical resection, with adjuvant treatments including radiotherapy and chemotherapy. All patients in this study underwent Simpson grade 1 or 2 resection, resulting in good functional and neurological outcomes during the follow-up period. Only 1 patient experienced relapse 9 years post-surgery. Sarikaya et al[16] reported recurrence in 2 patients under 18 years old with cervical SMs and long dural tails after Simpson grade 2 resection[16]. These patients underwent Simpson grade 1 resection upon recurrence and remained in remission. The authors suggested that young patients with cervical SMs and long dural tails might be at higher risk of recurrence. The characteristics of the recurrent patient in our study aligned with those reported by Sarikaya et al[16] supporting their hypothesis. Misra et al[17] proposed a classification system for SMs and emphasized the importance of instrumented fusion to prevent delayed spinal deformity or instability post-tumor excision[17]. Total laminectomy with facet joint resection was identified as a predictor of such issues, with patients typically undergoing laminoplasty with micro-titanium plate fixation for spinal stability. Posterolateral fusion with pedicle screw fixation was also utilized in our study, with no instances of internal fixation failure or spinal deformity during follow-up. Following the dural opening and subsequent repair, CSF leakage was the most common complication, with a reported incidence ranging from 0% to 4%. Additional perioperative complications can include pneumonia, urinary tract infection, surgical site infection, spinal cord edema, deep venous thrombosis, pulmonary embolism and myocardial infarction. CSF leakage, pneumonia and urinary tract infection were observed in our study, with the incidence of CSF leakage consistent with previous literature[18]. Spinal cord edema is a prevalent complication that can occur following spinal cord decompression. Clinically, this condition is characterized by deterioration in neurological function rather than improvement. To mitigate or reduce the discomfort associated with spinal cord edema, we administered glucocorticoids during surgery after spinal cord decompression, followed by a regimen of glucocorticoids and nerve dehydration medications for 2-3 days postoperatively.
SMs are often detected due to symptoms of spinal cord compression, but some cases are found incidentally. MRI is considered the gold standard for detection, with SMs appearing isointense to the spinal cord on T1-weighted images and isointense or hypointense on T2-weighted images. T1-weighted post-contrast-enhanced images typically show homo
The most direct approaches for SMs resection were based on the dural attachment locations, which include anterior, lateral, and posterior approaches. SMs in the cervical spine are best approached anteriorly via corpectomy for removal, but dural repair can be challenging in this scenario. For SMs with anterior or lateral dural attachments at T3-L2, a lateral extracavitary approach or costotransversectomy can be utilized for excision. Posterior fixation may be necessary with these approaches due to extensive pedicle removal and facetectomy, with attention given to the great vessels and radicular arteries. While the posterior approach has traditionally been the preferred method for SM resection, the safety and efficacy of this approach for SMs with ventral or ventrolateral dural attachments are still under debate. In our study, some surgeons believed that they were familiar with the posterior approach and this approach could successfully remove the tumor; thus, some patients with SMs on the ventral or ventrolateral side were selected to undergo the posterior approach. The results from this study show that there were no significant differences in outcomes among patients with dorsal/dorsolateral, ventral/ventrolateral and lateral dural attachments who underwent the posterior approach for resection, indicating that the posterior approach may be adequate for any dural attachments.
Although a myriad of new technological advances such as surgical microscopes, intraoperative ultrasound, ultrasonic tumor aspirators, intraoperative neurological monitoring and microscope-based augmented reality have been used to enhance the resection of SMs and minimize neural tissue injury[21,22], many hospitals do not have these new medical devices. Our retrospective study suggested that posterior approach resection of SMs accompanied by detailed preo
Given the retrospective nature of this study, it is important to note the limitations such as the lack of extensive follow-up for certain patients due to changes in medical providers and electronic medical records. Moreover, all patients in the study were selected to undergo the posterior approach by their respective surgeons, which may have introduced a selection bias. Additionally, the small sample size restricts the ability to draw robust conclusions on the safety and efficacy of resecting SM with any dural attachment location through a posterior approach.
Despite these constraints, this study stands out as one of the few that investigated the outcomes of patients with SM and compares these outcomes across various dural attachment subgroups in individuals who underwent the posterior approach for resection. It is recommended that future studies should include prospective trials, with multi-center collaborations and larger patient cohorts with longer follow-up durations.
SMs are benign tumors with favorable prognoses after surgical resection. However, resecting SMs with a ventral or ventrolateral dural attachment using a posterior approach can be challenging. The impact of dural attachment location on outcomes following posterior resection of SMs remains poorly understood. This study found that posterior resection of SMs with various dural attachment locations resulted in good outcomes, with no significant differences in neurological outcomes, Simpson grade, complications, or recurrence rates among the different subgroups. These findings highlight the feasibility of successfully resecting any SM through via posterior approach, with consistently positive outcomes regardless of the dural attachment location.
| 1. | Hachem LD, Nater A, Fehlings MG. Spinal Meningiomas. Adv Exp Med Biol. 2023;1416:69-78. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Hohenberger C, Hau P, Schebesch KM, Kölbl O, Riemenschneider MJ, Pohl F, Proeschold M, Schmidt NO. Spinal meningiomas. Neurooncol Adv. 2023;5:i112-i121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 3. | Ravindra VM, Schmidt MH. Spinal Meningiomas: Diagnosis, Surgical Management, and Adjuvant Therapies. Neurosurg Clin N Am. 2023;34:425-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Serratrice N, Lameche I, Attieh C, Chalah MA, Faddoul J, Tarabay B, Bou-Nassif R, Ali Y, Mattar JG, Nataf F, Ayache SS, Abi Lahoud GN. Spinal meningiomas, from biology to management - A literature review. Front Oncol. 2022;12:1084404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | El-Hajj VG, Pettersson Segerlind J, Burström G, Edström E, Elmi-Terander A. Current knowledge on spinal meningiomas: a systematic review protocol. BMJ Open. 2022;12:e061614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Elsamadicy AA, Reeves BC, Craft S, Sherman JJZ, Koo AB, Sayeed S, Sarkozy M, Kolb L, Lo SL, Shin JH, Sciubba DM, Mendel E. A current review of spinal meningiomas: epidemiology, clinical presentation and management. J Neurooncol. 2023;161:395-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Tariq A, Sohail A, Shah Z, Bakhshi S, Shamim MS. Spinal meningiomas: Management and outcomes. J Pak Med Assoc. 2023;73:1548-1550. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Wang X, Wang J, Wang L, Lin Y, Yang M, Chen X, Teng L, Guo H, Chen X. Surgical Resection of Dorsal Spinal Meningiomas with the Inner Dura Layer-An Improved Preservation Technique of Spinal Dura in 40 Cases. World Neurosurg. 2022;160:e250-e255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Said W, Maragno E, Leibrandt L, Spille D, Schipmann S, Stummer W, Gallus M, Schwake M. A Retrospective Cohort Study Evaluating the Comparative Effectiveness of Unilateral Hemilaminectomy and Bilateral Laminectomy in the Resection of Spinal Meningiomas. Oper Neurosurg (Hagerstown). 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 10. | Lonjon N, Russo V, Barbarisi M, Choi D, Allibone J, Casey A. Spinal Cervical Meningiomas: The Challenge Posed by Ventral Location. World Neurosurg. 2016;89:464-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Payer M. The anterior approach to anterior cervical meningiomas: review illustrated by a case. Acta Neurochir (Wien). 2005;147:555-60; discussion 560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Haddad AF, Safaee MM, Pereira MP, Oh JY, Lau D, Tan LA, Clark AJ, Chou D, Mummaneni PV, Ames CP. Posterior-based resection of spinal meningiomas: an institutional experience of 141 patients with an average of 28 months of follow-up. J Neurosurg Spine. 2023;38:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Onken J, Obermüller K, Staub-Bartelt F, Meyer B, Vajkoczy P, Wostrack M. Surgical management of spinal meningiomas: focus on unilateral posterior approach and anterior localization. J Neurosurg Spine. 2019;30:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Chang HS. Posterior Paramedian Approach to Ventrally Located Spinal Meningioma. World Neurosurg. 2017;105:755-759. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | DiGiorgio AM, Virk MS, Mummaneni PV. Spinal meningiomas. Handb Clin Neurol. 2020;170:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 16. | Sarıkaya C, Ramazanoğlu AF, Yaltırık CK, Etli MU, Önen MR, Naderi S. Short-Term Results of Simpson Grade 2 Resection in Spinal Meningiomas. World Neurosurg. 2023;171:e792-e795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 17. | Misra SN, Morgan HW. Avoidance of structural pitfalls in spinal meningioma resection. Neurosurg Focus. 2003;14:e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Gottfried ON, Gluf W, Quinones-Hinojosa A, Kan P, Schmidt MH. Spinal meningiomas: surgical management and outcome. Neurosurg Focus. 2003;14:e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Chung JY, Lee JJ, Kim HJ, Seo HY. Characterization of magnetic resonance images for spinal cord tumors. Asian Spine J. 2008;2:15-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Krishnan P. Gingko Leaf Sign: A Classical Imaging Finding in Spinal Meningiomas. Asian J Neurosurg. 2023;18:228-229. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Dang DD, Mugge LA, Awan OK, Gong AD, Fanous AA. Spinal Meningiomas: A Comprehensive Review and Update on Advancements in Molecular Characterization, Diagnostics, Surgical Approach and Technology, and Alternative Therapies. Cancers (Basel). 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Pojskić M, Bopp M, Saß B, Nimsky C. Single-Center Experience of Resection of 120 Cases of Intradural Spinal Tumors. World Neurosurg. 2024;187:e233-e256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |