Published online Dec 26, 2024. doi: 10.12998/wjcc.v12.i36.6892
Revised: September 28, 2024
Accepted: October 21, 2024
Published online: December 26, 2024
Processing time: 133 Days and 18.1 Hours
Intravenous (IV) vasopressors are essential in the management of hypotension and shock. Initiation of oral vasoactive agents to facilitate weaning of IV vasopressors to liberate patients from the intensive care unit is common despite conflicting evidence regarding the benefits of this practice. While midodrine appears to be the most frequently studied oral vasoactive agent for this purpose, its adverse effect profile may preclude its use in certain populations. In addition, some patients may require persistent use of IV vasopressors for hypotension refractory to midodrine. The use of additional and alternative oral vasoactive agents bearing different mechanisms of action is emerging. This article provides a comprehensive review of the pharmacology, clinical uses, dosing strategies, and safety considerations of oral vasoactive agents and their application in the inten
Core Tip: This paper sets to explore the evolving role of oral blood pressure augmenting agents in the intensive care setting. With their ability to increase blood pressure through direct or indirect vasoconstriction, these agents have been increasingly utilized to facilitate weaning from intravenous (IV) vasopressors. Use of these agents may have a role in reducing IV vasopressor exposure, intensive care unit lengths of stay, and overall healthcare resource utilization. Despite being commonly used, their role in practice should be tempered by lack of demonstrable efficacy in major trials and important adverse effect considerations.
- Citation: Robinson JC, ElSaban M, Smischney NJ, Wieruszewski PM. Oral blood pressure augmenting agents for intravenous vasopressor weaning. World J Clin Cases 2024; 12(36): 6892-6904
- URL: https://www.wjgnet.com/2307-8960/full/v12/i36/6892.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i36.6892
Arterial hypotension is amongst the most commonly encountered conditions in critically ill persons, with incidence rates ranging between 36% to 75%[1,2]. Vasopressors increase arterial blood pressure through direct vascular smooth muscle constriction and are an essential component of hypotension and shock management in intensive care settings[1,2]. While guidance on the initiation of vasopressors has long been established including agent selection, initiation timing, and indications[3-5], far less evidence on strategies for weaning and discontinuation of vasopressors exists[6,7]. Additionally, vasopressor use has become more common in the management of various etiologies of shock with an estimated use among 27% of intensive care unit (ICU) admissions[8] as increased evidence reveals improved outcomes with early initiation of vasopressor therapy[9,10]. Hence, the need to explore the evidence around vasopressor weaning and discontinuation becomes essential.
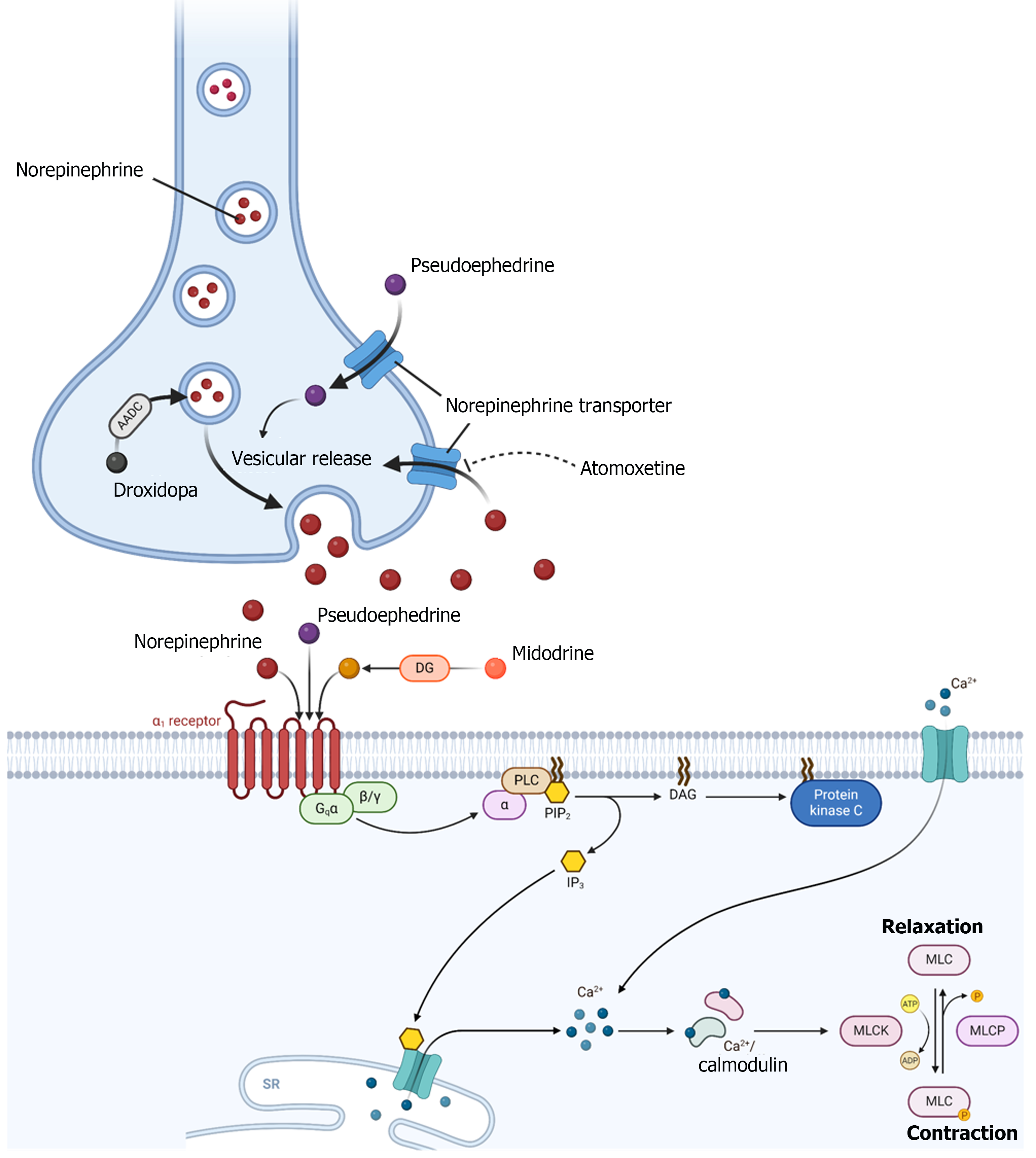
Once underlying shock pathologies have been corrected, continued vasopressor administration may constitute unnecessary intensive care resource utilization and confer undue risks[11,12]. To this end, there has been increasing interest in the application of oral blood pressure agents to facilitate separation from intravenous (IV) vasopressors as a mechanism to expedite intensive care discharge and bridge to recovery following shock. In this review, we evaluate various oral blood pressure augmenting agents that have been used to facilitate IV vasopressor weaning, including their pharmacology, physiologic mechanisms, clinical uses reported in the literature, and dosing and safety considerations for use. The specific agents that have been used for vasopressor weaning and reviewed herein include midodrine, droxidopa, pseudoephedrine, atomoxetine, and desmopressin.
Oral vasoactive agents exert their hemodynamic effects through a variety of mechanisms. Most of these agents increase systemic vascular resistance through direct and/or indirect activation of α1-adrenergic receptors (Figure 1). Agonism of α1-receptors in vascular smooth muscle causes Gq-activation of phospholipase C, which generates inositol-1,4,5-triphosphate and diacylglycerol, secondary messengers that lead to mobilization of intracellular calcium, an influx of calcium, and subsequent smooth muscle contraction (Figure 1)[13].
Midodrine is rapidly metabolized by deglycination to its active metabolite, desglymidodrine, a selective, peripherally acting, α1-receptor agonist[14,15]. Intraneuronally, droxidopa is converted to norepinephrine by aromatic amino acid decarboxylase, which is then released upon neuronal depolarization[16]. The conversion of droxidopa to norepinephrine also occurs in non-neuronal tissues leading to an increase in circulating norepinephrine[16,17]. In presynaptic neurons, pseudoephedrine stimulates vesicular release of endogenous norepinephrine[18]. Pseudoephedrine also directly stimulates α1 and β-adrenergic receptors[18]. Atomoxetine is a selective norepinephrine transporter inhibitor that increases availability of norepinephrine in the synaptic cleft[19]. Therefore, droxidopa, pseudoephedrine, and atomoxetine exert their blood pressure raising effects through endogenous norepinephrine. Oral desmopressin, a synthetic analogue of vasopressin with more antidiuretic activity and minimal pressor activity[20] is generally not considered an oral vasoactive agent but was used to wean norepinephrine in one prospective trial[21].
In addition to having different mechanisms of action, oral vasoactive agents also vary based on their pharmacokinetic properties (Table 1)[17,22-25]. Since midodrine, droxidopa, and pseudoephedrine have relatively short elimination half-lives, more frequent dosing is required to achieve the desired pharmacodynamic effects. Midodrine’s active metabolite, desglymidodrine[22], and pseudoephedrine[23] are renally eliminated and dose adjustments should be considered in the setting of renal dysfunction. Atomoxetine, on the other hand, has a longer half-life and is typically dosed once daily with dose reductions required in the setting of hepatic dysfunction[24].
| Agent | Onset | Half-life | Metabolism | Special considerations |
| Midodrine, desglymidodrine | 1 hour | 0.5 hour, 3-4 hours | Rapid deglycination to desglymidodrine | Avoid during bradycardia. Dose reductions necessary in renal dysfunction |
| Droxidopa | 1 hour | 2-3 hours | Metabolized to norepinephrine via the catecholamine pathway | Recent data suggests that capsule may be opened and administered via feeding tube |
| Pseudoephedrine | 0.5 hour | 3-16 hours1 | Not substantially metabolized | Dose reductions should be considered in renal dysfunction |
| Atomoxetine | 1 hour | 5-24 hours2 | Hepatic, cytochrome P4502D6 (major) and cytochrome P450 family 2 subfamily C member 19 (minor) | Avoid during agitated delirium. Dose reductions necessary in hepatic dysfunction |
Midodrine is approved by the United States Food and Drug Administration for treatment of orthostatic hypotension and dysautonomia[26]. Unlike other agents, midodrine is unique in that it is effective through parenteral and enteral routes with 90% bioavailability allowing for oral formulation use. It is a prodrug that undergoes conversion to its active metabolite desglymidodrine through the liver. This metabolite is a selective α1-receptor agonist that acts through vasoconstriction of venous and arteriolar systems. Plasma concentrations of the prodrug peak in 30 minutes, while the active metabolite’s plasma concentrations peak in about 2 hours (with a half-life of 3-4 hours).
Midodrine has been explored in the management of intra-dialytic hypotension, a phenomenon observed in a subset of dialysis patients that is thought to be related to the complex interplay between patient factors such as cardiac output and vascular tone and dialysis factors such as ultrafiltration rate and dialysate choice. A systematic review and meta-analysis of 10 studies and 117 patients found that the use of midodrine was associated with reduced hypotension during dialysis by improved blood pressure of 13.3 mmHg (95%CI: 8.6–18.0) in the midodrine group[27]. While that review was limited due to a small patient population, another extensive observational study matched 1046 patients who received midodrine for intra-dialytic hypotension to 2037 unexposed controls. This study found a higher incidence rate ratio of mortality (1.37, 95%CI: 1.15-1.62) and hospitalization (1.31, 95%CI: 1.19-1.43) with midodrine use[28].
Midodrine has been used off-label in the management of hepatorenal syndrome associated with acute renal failure[29,30]. When used alone, no positive outcomes were found[31]. Additionally, the combination of midodrine and octreotide was found to be less effective in clinical outcomes than using terlipressin and albumin[32].
Midodrine is the most studied oral agent for vasopressor weaning in the intensive care setting (Table 2)[21,25,33-49]. In 2008, the use of midodrine as an alternative to IV vasopressors was well tolerated and associated with lower cost of hospitalization in a case series of patients with hypotension associated carotid artery stenting[33]. Five years later, the use of midodrine to facilitate IV vasopressor weaning was evaluated in a prospective study including 20 surgical ICU patients receiving IV vasopressors that otherwise met criteria for ICU discharge. In this study, the rate of vasopressor discontinuation was significantly faster after initiation of midodrine[34], but there was no control group to evaluate the potential impact on ICU length of stay (LOS). In a retrospective study of 188 patients (94 midodrine and 94 control), the median time to IV vasopressor discontinuation after midodrine initiation was 1.2 days. However, this outcome was not compared to the control group. Discharge from the ICU occurred sooner after IV vasopressor discontinuation in the midodrine group (0.8 days) compared to the control group (1.5 days), P = 0.01. However, there was no difference in ICU LOS; and hospital LOS was longer in the midodrine group[35]. Despite equivocal results from multiple observational studies, midodrine was increasingly used as an oral vasoactive weaning agent[50] and an interventional study was warranted.
| Drug | Ref. | Study type | Population | Drug dosing | Duration of IVV | ICU LOS | Adverse reactions | Outcomes |
| Midodrine | Sharma et al[33], 2008 | Case series | Coronary artery stenting (n = 55), midodrine (n = 4), dopamine (n = 11) | 10 mg Q8H x 3 doses | 15 hours in dopamine group and 0 hour in midodrine group | 2 days vs 0 day | None | Midodrine was well tolerated. Cost of hospitalization was higher in the dopamine group because of the need for ICU admission |
| Levine et al[34], 2013 | Prospective | Surgical ICU (n = 20) | 5-20 mg Q8H | Median 17 hours after midodrine initiation | NR | NR | The change in IVV rate decreased from -0.62 to –2.20 mcg/minutes/hour of phenylephrine equivalents following the initiation midodrine, P = 0.01 | |
| Poveromo et al[35], 2016 | Retrospective | Mixed ICU (n = 188) | 10 mg Q8H | Median 1.2 days (0.5–2.8 days) after midodrine initiation (not compared to control group) | No difference | Bradycardia in 12.8% midodrine group vs 0% in control group | Discharge from the ICU occurred sooner after IVV discontinuation in the midodrine group compared to the control group (0.8 days vs 1.5 days, P = 0.01). There was no difference in ICU LOS; and hospital LOS was longer in the midodrine group | |
| Whitson et al[45], 2016 | Retrospective | MICU, septic shock (n = 275), IVV only (n = 140), IVV plus midodrine (n = 135) | 10 mg Q8H | Mean duration 3.8 vs 2.9 days, P < 0.001 | 9.4 vs 7.5 days, P = 0.02 | Bradycardia requiring midodrine discontinuation in 1 patient | Midodrine decreased duration of IVV use and ICU LOS | |
| Rizvi et al[48], 2019 | Observational | ICU patients that received midodrine and survived to discharge (n = 1119) | 5-30 mg Q8H | NA | NA | NA | The study assessed continuation of midodrine during transition of care and 34% of patients were continued on midodrine at hospital discharge | |
| Tremblay et al[47], 2020 | Retrospective | Cardiac surgery patients (n = 148), IVV only (n = 74), IVV plus midodrine (n = 74) | 10 mg Q8H | Median duration 44 hours (26-66 hours) vs 63 hours (40-86.5 hours), P = 0.05 | 68 hours (48-99 hours) vs 99 hours (68-146 hours), P < 0.01 | No significant difference in acute kidney injury | Negative results with midodrine use association with an increased ICU LOS | |
| Santer et al[36], 2020 | RCT | ICU patients (n = 132), midodrine (n = 66), placebo (n = 66) | 20 mg Q8H | Median duration 23.5 hours (10.0–54.0 hours) vs 22.5 hours (10.4–40.0 hours), P = 0.62 | 6.0 days (5.0–8.0 days) vs 6.0 days (4.0–8.0 days), P = 0.46 | Bradycardia 5 (7.6%) vs 0 (0%), P = 0.02 | No difference in IVV use duration or ICU LOS | |
| Macielak et al[46], 2021 | Observational | ICU and floor patients, (n = 44), received IVV (n = 23) | 5-20 mg Q6H | NR | 12 days (5–27 days) | The 1 case of mesenteric ischemia | NEE requirements decreased from 0.1 to 0.05 norepinephrine equivalents at 24 hours after midodrine was ordered for Q6H | |
| Lal et al[49], 2021 | RCT | MICU (n = 32), midodine (n = 17), placebo (n = 15) | 10 mg Q8H | Median duration 14.5 hours ± 8.1 hours vs 18.8 hours ± 7.1 hours, P = 0.19 | 2.29 days vs 2.45 days, P = 0.36 | No major adverse events | Study not powered to detect statistically significant results | |
| Adly et al[37], 2022 | RCT | Septic shock (n = 60) | 10mg Q8H | Median duration NE 4 days with midodrine vs 6 days control, P < 0.01 | NR | NR | Use of midodrine resulted in reduced IVV duration and improved mortality rates | |
| Droxidopa | Zundel et al[38], 2016 | Case report | 53F with vasoplegic shock post cardiac transplant | Titrated to 600 mg 4× daily. | Not weaned off despite 10 days of droxidopa | NR | No apparent adverse effects | Droxidopa temporarily increased MAP and reduction in vasoactive requirements after midodrine was not effective. Patient died on post-op day 60 with multiorgan failure |
| Hong et al[40], 2022 | Retrospective | The 64M with SS and midodrine-induced bradycardia. The 73M with SS and midodrine-induced bradycardia | 100 mg TID, 100 mg BID titrated up to 600 mg TID | The 1 day after droxidopa initiation and MAP goal reduction. The 9 days after starting droxidopa and 5 days after restarting midodrine | NR | NR | Droxidopa was weaned off prior to patient discharge. Droxidopa in combination with midodrine led to avoidance of pacemaker placement. Patient was discharged to rehabilitation on droxidopa and midodrine | |
| Noble et al[39], 2023 | Case report | The 57F with persistent hypotension despite midodrine | 100 mg TID titrated to 500 mg TID | 3 days after droxidopa initiation | NR | NR | After initiation of droxidopa the patient was weaned off IVV and continuous renal replacement therapy and ultimately discharged home on droxidopa | |
| Webb et al[25], 2024 | Retrospective | n = 21 (80.5% cardiac ICU) with persistent hypotension despite midodrine | Most frequent starting dose 100 mg TID. Max dose: 600 mg TID | Median time to discontinuation 87 hours (interquartile range 34-175) | NR | Similar rates of tachycardia pre- and post-initiation | NEE requirements were lower after initiation of droxidopa in patients that were trial on alternative oral vasoactive agents prior to droxidopa. About half of the patients died in the hospital. The number of patients discharged on droxidopa was not reported | |
| Pseudoephedrine | Patterson et al[41], 2008 | Case report | The 77F with idiopathic autonomic dysfunction | 60 mg Q8H and tapered to 60 mg Q6H | NR | NR | NR | Norepinephrine was weaned off temporarily within 14 hours of PSE initiation, but frequency was increased to Q6H to permanently liberate patient from IVV |
| Curran et al[42], 2023 | Case report | Male in 40s with autonomic dysfunction and refractory bradycardia and hypotension on midodrine at home | 60 mg Q6H that was weaned off within 7 days | < 24 hours | NR | NR | Addition of PSE facilitated IVV weaning in a patient with bradycardia and hypotension refractory to midodrine | |
| Wood et al[43], 2014 | Case series | n = 38 SS | Daily PSE dose varied widely (30–720 mg) | The mean time to discontinuation of vasopressors and/or atropine was 8 days | Mean: 39 days | No adverse events directly attributed to PSE therapy were documented | ‘Success’: Vasopressor discontinuation or decreased use of atropine was achieved in 82% of patients | |
| Atomoxetine | Lessing et al[44], 2024 | Retrospective | Congenital tracheal stenosis patients with midodrine-refractory hypotension (n = 45), atomoxetine (n = 18), droxidopa (n = 17), both (n = 10) | Starting daily dose: Atomoxetine 10-20 mg; droxidopa 300 mg. Maximum daily dose: Atomoxetine 40 mg; droxidopa 1800 mg | Median time to discontinuation 21.9 days vs 8 days vs 13.9 days, P = 0.26 | Median: 30 days vs 18 days vs 35 days | Hypertension requiring treatment in 2 patients receiving atomoxetine and in 2 patients receiving both. Ischemic bowel complication in 1 patient receiving atomoxetine and 1 patient receiving both. New onset arrhythmia in 3 patients receiving atomoxetine and 2 patients receiving droxidopa and 1 patient receiving both | There was no difference in IVV duration or LOS. A higher percentage of patients who survived to hospital discharge received both study medications or droxidopa alone (90% vs 76.5%) compared to atomoxetine alone (44.4%, P = 0.03) |
| Desmopressin | Ahmed et al[21], 2022 | RCT | SS, control (n = 30), midodrine (n = 30), minirin (n = 30) | Midodrine 10 mg Q8H. Minirin 60 mcg Q8H | Control 6.93 days, midodine3.3 days, minirin 4.8 days, P <0.01 | 9.03 days, 5.13 days, 5.5 days, P < 0.01 | NR | Midodrine and minirin use significantly reduced duration of pressors and ICU LOS. Duration of pressors was shorter in midodrine group compared to midodrine, but ICU LOS was similar between these two groups |
In 2020, the landmark MIDAS trial was published with results that halted some of the enthusiasm with using midodrine to wean IV vasopressors[36]. The primary outcome revealed no significant benefit in median time to vasopre
Two recent RCTs[21,37] revealed a reduction in IV vasopressor duration with the use of midodrine. Though limitations of these trials include their single center designs and increased risk of bias due to lack of blinding. Most recently, a meta-analysis that included four RCTs and 314 patients did not show any significant differences in total IV vasopressor duration or time-to-IV vasopressor discontinuation between the midodrine and control groups[51].
Droxidopa is available in an oral capsule formulation and is approved for the treatment of neurogenic orthostatic hypotension. Droxidopa has been used to facilitate the weaning of IV vasopressors, but literature is limited to case reports and retrospective case series. In one case report, droxidopa was utilized in a patient with persistent vasoplegic syndrome post-cardiac transplant that was unresponsive to midodrine. In this case, droxidopa reduced vasoactive requirements and was well tolerated with a dose titrated to 600 mg four times per day[38]. Droxidopa was also used to facilitate IV vasopressor weaning and liberation from continuous renal replacement therapy in a patient with persistent hypotension and renal failure[51]. Additionally, the use of droxidopa was described in two patients with spinal shock and midodrine-induced bradycardia[40].
The package labeling of droxidopa states that capsules should be administered whole. However, the administration of droxidopa via gastric tube was recently described in a retrospective case series of 954 droxidopa doses that were administered via gastric tube to 21 patients (81% cardiac ICU) for the purpose of vasopressor weaning[25]. When compared to 24 hours prior to droxidopa initiation, there were similar rates of tachycardia, less hypotension, and lower mean norepinephrine requirements following droxidopa. Additionally, at least one other oral vasoactive agent was trialed prior to droxidopa initiation and the median time to vasopressor discontinuation was 87 hours. In contrast to midodrine, a pure α1-receptor agonist, droxidopa is converted to norepinephrine, which also acts on cardiac β1 receptors, which could be advantageous for weaning IV vasoactive support in cardiac surgery patients or in patients with sinus bradycardia.
Pseudoephedrine is available for the treatment of temporary relief of symptomatic nasal congestion[52,53]. This agent has also been used off-label for the treatment of hyperlactation[54] and is notoriously abused in the clandestine manufacturing of illicit methamphetamine[55]. Successful use of pseudoephedrine to wean IV vasopressors in patients with autonomic dysfunction has been described in case reports[41,42]. The largest study to date evaluating the use of pseudoephedrine as an oral vasopressor agent is a case series of 38 patients with neurogenic shock after spinal cord injury[43]. In this study, the effect of pseudoephedrine was classified as: (1) ‘Success’: Vasopressor discontinuation or decreased use of atropine; (2) ‘Failure’: Not meeting one of the success criteria; and (3) ‘Inconclusive’: Presence of confounding factors such as restarting vasopressors for another indication. Pseudoephedrine was successful in 82% of patients, failed in 5% of patients, and was inconclusive in 13% of patients. The mean time to discontinuation of vasopressors and/or atropine was 8 days. Additionally, the mean duration of pseudoephedrine use was 32 days. Information was collected through 7 days after pseudoephedrine was discontinued or until the date the patient was discharged; and most surviving patients (64.5%) were discharged on pseudoephedrine. No adverse effects related to pseudoephedrine were reported in this study[43]. Pseudoephedrine is readily accessible and since it agonizes β adrenergic receptors, it may be considered for use in the setting of sinus bradycardia.
Atomoxetine is approved for the treatment of attention-deficit/hyperactivity disorder[56]. This agent has also been used off label for the treatment of neurogenic orthostatic hypotension[57,58]. Literature evaluating the use of atomoxetine as an adjunctive agent to wean IV vasopressors is sparse. A recent retrospective cohort reported critically ill cardiothoracic surgery patients with refractory hypotension despite midodrine who were treated with either atomoxetine (n = 18), droxidopa (n = 17), or both (n = 10)[44]. The median time to discontinuation of IV vasoactive agents was not different between the groups (21.9 days vs 8.0 days vs 13.9 days, respectively; P = 0.26). There were no differences in ICU or hospital lengths of stay. Of note, baseline total daily midodrine doses were significantly different between groups (45 mg vs 60 mg vs 15 mg, respectively; P < 0.01), which could confound the results of this study.
Desmopressin acetate is available in an oral tablet formulation used for treating central diabetes insipidus[20], refractory nocturia[59], and primary nocturnal enuresis[60-62] due to its water-retaining effects. Desmopressin has minimal pressor activity due to substitution of D-arginine for L-arginine in position 8 of arginine vasopressin which prevents engagement of vasopressin V1a receptors[20].
Desmopressin has been assessed as a strategy to wean norepinephrine in patients with spinal shock in a recent randomized trial[21]. In this trial, hemodynamically stable patients without renal failure on low-dose norepinephrine (< 8 mcg/minute) for at least 24 hours were randomized to receive midodrine 10 mg every 8 hours, minirin (oral desmo
The most commonly reported adverse reactions with the various oral blood pressure augmenting agents are those associated with vasoconstriction including supine hypertension and severe hypertension requiring pharmacologic intervention[36,44,63]. Additionally with its unopposed alpha agonism, bradycardia is commonly reported when midodrine is used for vasopressor weaning[36,40,45,64]. In a meta-analysis, midodrine was found to have over 5-fold greater risk of bradycardia compared to placebo (relative risk = 5.56, 95%CI: 1.54-20.05, P = 0.01)[51]. Oral pseudoephedrine[42] and droxidopa[40] have been used for persistent bradycardia in the setting of high dose midodrine. Importantly, potentially due to excessive constriction of microcirculatory beds, ischemic bowel complications have been reported with midodrine[46,47,50], droxidopa[44], pseudoephedrine[65,66], and atomoxetine[44]. Other reported adverse events include tachyarrhythmias[25,36,44,47] and specifically with atomoxetine, agitation and delirium requiring drug cessation[44]. Additionally, the main adverse reaction of desmopressin is dilutional hyponatremia secondary to its antidiuretic effect[67].
There are many purported cost benefits of employing oral blood pressure augmenting agents, namely sparing central catheters and invasive hemodynamic monitoring, as well as reduced hospital expenses from shorter lengths of stay. Although some have reported cost reductions, such claims are implied based on minimization or avoidance of ICU admission rather than direct cost analysis[33,38,39]. Overall, cost benefits remain theoretical and formal economic evaluations of oral blood pressure augmenting agents are lacking. The LIBERATE trial, a placebo randomized comparator assessing midodrine in those with stable or decreasing vasopressors will include a comprehensive health economic analysis[68].
When oral agents are used for the purposes of weaning IV vasopressors, the goal should remain to taper, and discontinue those oral agents as quickly as possible. In one cohort study of 1010 started on midodrine to wean from vasopressors, about two-thirds were discharged from the ICU still on midodrine and one-third were discharged from the hospital still on midodrine[48]. There are several safety considerations with discharging patients out of the ICU and hospital while still on oral blood pressure augmenting agents. Outside of the care of intensive care specialists, there may be less attention to hemodynamic monitoring, and subclinical hypoperfusion may persist despite the use of oral agents, prompting the need for additional workup to identify uncorrected pathologies[69]. Additionally, the ability to monitor for supine hy
Midodrine dosing is not standardized in the critically ill and thus studies have applied widely variable dosing strategies. In contrast to typical dosing for orthostatic hypotension of 2.5 mg to 10 mg thrice daily (upon awakening, at midday, and in the late afternoon)[72,73], midodrine dosing for the purpose of weaning vasoactive support is much higher and with a consistent, around-the-clock interval. Based on available RCTs, the most common dosing strategy is 10 mg every 8 hours, with the MIDAS clinical trial utilizing a dosing strategy of 20 mg every 8 hours[36,70]. However, even with the higher dosing strategy used in the MIDAS trial, midodrine use was not significantly associated with reductions in ICU/hospital LOS nor time to discontinuation of IV vasopressors, which represents the most common reason for midodrine use in the ICU[70]. One clinical trial from Egypt did find a significant reduction in median time to IV vasopressor cessation while also finding a significant reduction in ICU LOS[21]. However, this trial and others have all used fixed dosing strategies which may be a reason why most trials found no significant associations with midodrine use and vasopressor weaning and/or ICU LOS. A titratable dosing strategy is more compatible with the application of IV vasopressors in the ICU which are adjusted to a specific MAP[74] and thus, this strategy may yield different results. Another reason for lack of benefit in the ICU population may be enteral drug formulation. Oral bioavailability is altered in the critically ill and likely unpredictable, thus limiting the clinical efficacy of orally administered medications to this population[75]. Moreover, the majority of studies assessing midodrine in the ICU have been evaluated in a heterogenous group of ICU patients. Perhaps, specific subsets of ICU patients may benefit more from midodrine use than others, as demonstrated by the MIDAS trial in which patients with neurogenic vasoplegia from epidural analgesia had significantly shorter durations of IV vasopressor therapy with the use of midodrine.
Finally, clinical trials that have failed to show a benefit for midodrine use in the ICU regarding cessation of vasopressor therapy or ICU LOS may be a result of inadequate dosing frequency given the drug’s short half-life of 3-4 hours. Therefore, a dosing strategy that is more frequent and consistent with its pharmacokinetic profile may result in a beneficial effect on weaning vasopressors or ICU LOS. For example, retrospective studies that have used more frequent (every 6 hours or four-times daily) dosing of midodrine have demonstrated significant reductions in vasopressor requirements (median reduction of 97.3%[34] and mean reduction of 40%[46] at 24 hours post drug initiation). One retrospective study using doses as high as 40 mg every 8 hours demonstrated reductions in IV vasopressor duration and ICU LOS[45]. Although higher doses may prove to be useful in the future, these higher doses may be hindered by resulting bradycardia, which has been documented in the MIDAS trial.
Future areas of focus for oral vasoactive agents in the ICU population include timing of use (early vs late), optimal dose, dosing frequency, patient selection, titration protocols for specific MAP goal, adverse effects of higher dosing strategies, and additional long-term outcomes of oral vasoactive agents in the ICU.
The application of oral vasoactive agents to facilitate weaning of IV vasopressors and expedite ICU discharge is common practice despite equivocal results regarding their efficacy in randomized trials. Midodrine is the oral vasoactive agent with the most evidence in this setting, although not consistently positive and its association with bradycardia may limit its use in certain patients. The use of midodrine-alternative oral vasoactive agents has emerged, including multiple medications that exert their hemodynamic effects through norepinephrine, which may be ideal in certain populations. Additional prospective research is warranted to determine the optimal dosing of oral vasoactive agents and to define the role of midodrine-alternative agents.
| 1. | Vincent JL, Nielsen ND, Shapiro NI, Gerbasi ME, Grossman A, Doroff R, Zeng F, Young PJ, Russell JA. Mean arterial pressure and mortality in patients with distributive shock: a retrospective analysis of the MIMIC-III database. Ann Intensive Care. 2018;8:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Terwindt LE, Schuurmans J, van der Ster BJP, Wensing CAGCL, Mulder MP, Wijnberge M, Cherpanath TGV, Lagrand WK, Karlas AA, Verlinde MH, Hollmann MW, Geerts BF, Veelo DP, Vlaar APJ. Incidence, Severity and Clinical Factors Associated with Hypotension in Patients Admitted to an Intensive Care Unit: A Prospective Observational Study. J Clin Med. 2022;11:6832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Scheeren TWL, Bakker J, De Backer D, Annane D, Asfar P, Boerma EC, Cecconi M, Dubin A, Dünser MW, Duranteau J, Gordon AC, Hamzaoui O, Hernández G, Leone M, Levy B, Martin C, Mebazaa A, Monnet X, Morelli A, Payen D, Pearse R, Pinsky MR, Radermacher P, Reuter D, Saugel B, Sakr Y, Singer M, Squara P, Vieillard-Baron A, Vignon P, Vistisen ST, van der Horst ICC, Vincent JL, Teboul JL. Current use of vasopressors in septic shock. Ann Intensive Care. 2019;9:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 4. | Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3352] [Cited by in RCA: 4008] [Article Influence: 501.0] [Reference Citation Analysis (0)] |
| 5. | McIntyre WF, Um KJ, Alhazzani W, Lengyel AP, Hajjar L, Gordon AC, Lamontagne F, Healey JS, Whitlock RP, Belley-Côté EP. Association of Vasopressin Plus Catecholamine Vasopressors vs Catecholamines Alone With Atrial Fibrillation in Patients With Distributive Shock: A Systematic Review and Meta-analysis. JAMA. 2018;319:1889-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 6. | Wu M, Ghassemi M, Feng M, Celi LA, Szolovits P, Doshi-Velez F. Understanding vasopressor intervention and weaning: risk prediction in a public heterogeneous clinical time series database. J Am Med Inform Assoc. 2017;24:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Wu Z, Zhang S, Xu J, Xie J, Huang L, Huang Y, Yang Y, Qiu H. Norepinephrine vs Vasopressin: Which Vasopressor Should Be Discontinued First in Septic Shock? A Meta-Analysis. Shock. 2020;53:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Thongprayoon C, Cheungpasitporn W, Harrison AM, Carrera P, Srivali N, Kittamongkolchai W, Erdogan A, Kashani KB. Temporal trends in the utilization of vasopressors in intensive care units: an epidemiologic study. BMC Pharmacol Toxicol. 2016;17:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Ospina-Tascón GA, Hernandez G, Alvarez I, Calderón-Tapia LE, Manzano-Nunez R, Sánchez-Ortiz AI, Quiñones E, Ruiz-Yucuma JE, Aldana JL, Teboul JL, Cavalcanti AB, De Backer D, Bakker J. Effects of very early start of norepinephrine in patients with septic shock: a propensity score-based analysis. Crit Care. 2020;24:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 10. | Permpikul C, Tongyoo S, Viarasilpa T, Trainarongsakul T, Chakorn T, Udompanturak S. Early Use of Norepinephrine in Septic Shock Resuscitation (CENSER). A Randomized Trial. Am J Respir Crit Care Med. 2019;199:1097-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 11. | Self WH, Liu D, Strayer N, Russ S, Ward MJ, Shapiro NI, Rice TW, Semler MW. Charge Reductions Associated With Shorter Time to Recovery in Septic Shock. Chest. 2019;155:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Lewis T, Merchan C, Altshuler D, Papadopoulos J. Safety of the Peripheral Administration of Vasopressor Agents. J Intensive Care Med. 2019;34:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 13. | Piascik MT, Perez DM. Alpha1-adrenergic receptors: new insights and directions. J Pharmacol Exp Ther. 2001;298:403-410. [PubMed] |
| 14. | Pittner H, Stormann H, Enzenhofer R. Pharmacodynamic actions of midodrine, a new alpha-adrenergic stimulating agent, and its main metabolite, ST 1059. Arzneimittelforschung. 1976;26:2145-2154. [PubMed] |
| 15. | Kolassa N, Schützenberger WG, Wiener H, Krivanek P. Plasma level of the prodrug midodrine and its active metabolite in comparison with the alpha-mimetic action in dogs. Arch Int Pharmacodyn Ther. 1979;238:96-104. [PubMed] |
| 16. | Kaufmann H, Norcliffe-Kaufmann L, Palma JA. Droxidopa in neurogenic orthostatic hypotension. Expert Rev Cardiovasc Ther. 2015;13:875-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Goldstein DS. L-Dihydroxyphenylserine (L-DOPS): a norepinephrine prodrug. Cardiovasc Drug Rev. 2006;24:189-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Głowacka K, Wiela-Hojeńska A. Pseudoephedrine-Benefits and Risks. Int J Mol Sci. 2021;22:5146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Shibao CA, Palma JA, Celedonio JE, Martinez J, Kaufmann H, Biaggioni I. Predictors of the Pressor Response to the Norepinephrine Transporter Inhibitor, Atomoxetine, in Neurogenic Orthostatic Hypotension. Hypertension. 2021;78:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Robinson AG. DDAVP in the treatment of central diabetes insipidus. N Engl J Med. 1976;294:507-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 96] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Ahmed Ali AT, Abd El-Aziz MA, Mohamed Abdelhafez A, Ahmed Thabet AM. Effect of Oral Vasopressors Used for Liberation from Intravenous Vasopressors in Intensive Care Unit Patients Recovering from Spinal Shock: A Randomized Controlled Trial. Crit Care Res Pract. 2022;2022:6448504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Blowey DL, Balfe JW, Gupta I, Gajaria MM, Koren G. Midodrine efficacy and pharmacokinetics in a patient with recurrent intradialytic hypotension. Am J Kidney Dis. 1996;28:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Kanfer I, Dowse R, Vuma V. Pharmacokinetics of Oral Decongestants. Pharmacotherapy. 1993;13:116S-128S. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Chalon SA, Desager JP, Desante KA, Frye RF, Witcher J, Long AJ, Sauer JM, Golnez JL, Smith BP, Thomasson HR, Horsmans Y. Effect of hepatic impairment on the pharmacokinetics of atomoxetine and its metabolites. Clin Pharmacol Ther. 2003;73:178-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Webb A, Lauren Casal G, Culshaw J, Northam K, Solomon E, Beargie S, Johnson R, Lopez N, Newman K, Hayes B, Roberts R. 940: Safety and effectiveness of droxidopa administration for persistent hypotension via gastric tube. Crit Care Med. 2024;52:S442-S442. [DOI] [Full Text] |
| 26. | Jankovic J, Gilden JL, Hiner BC, Kaufmann H, Brown DC, Coghlan CH, Rubin M, Fouad-Tarazi FM. Neurogenic orthostatic hypotension: a double-blind, placebo-controlled study with midodrine. Am J Med. 1993;95:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 241] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 27. | Prakash S, Garg AX, Heidenheim AP, House AA. Midodrine appears to be safe and effective for dialysis-induced hypotension: a systematic review. Nephrol Dial Transplant. 2004;19:2553-2558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Brunelli SM, Cohen DE, Marlowe G, Van Wyck D. The Impact of Midodrine on Outcomes in Patients with Intradialytic Hypotension. Am J Nephrol. 2018;48:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Karwa R, Woodis CB. Midodrine and octreotide in treatment of cirrhosis-related hemodynamic complications. Ann Pharmacother. 2009;43:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Pomier-Layrargues G, Paquin SC, Hassoun Z, Lafortune M, Tran A. Octreotide in hepatorenal syndrome: a randomized, double-blind, placebo-controlled, crossover study. Hepatology. 2003;38:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Angeli P, Volpin R, Piovan D, Bortoluzzi A, Craighero R, Bottaro S, Finucci GF, Casiglia E, Sticca A, De Toni R, Pavan L, Gatta A. Acute effects of the oral administration of midodrine, an alpha-adrenergic agonist, on renal hemodynamics and renal function in cirrhotic patients with ascites. Hepatology. 1998;28:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Cavallin M, Kamath PS, Merli M, Fasolato S, Toniutto P, Salerno F, Bernardi M, Romanelli RG, Colletta C, Salinas F, Di Giacomo A, Ridola L, Fornasiere E, Caraceni P, Morando F, Piano S, Gatta A, Angeli P; Italian Association for the Study of the Liver Study Group on Hepatorenal Syndrome. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology. 2015;62:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 259] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 33. | Sharma S, Lardizabal JA, Bhambi B. Oral midodrine is effective for the treatment of hypotension associated with carotid artery stenting. J Cardiovasc Pharmacol Ther. 2008;13:94-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Levine AR, Meyer MJ, Bittner EA, Berg S, Kalman R, Stanislaus AB, Ryan C, Ball SA, Eikermann M. Oral midodrine treatment accelerates the liberation of intensive care unit patients from intravenous vasopressor infusions. J Crit Care. 2013;28:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Poveromo LB, Michalets EL, Sutherland SE. Midodrine for the weaning of vasopressor infusions. J Clin Pharm Ther. 2016;41:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Santer P, Anstey MH, Patrocínio MD, Wibrow B, Teja B, Shay D, Shaefi S, Parsons CS, Houle TT, Eikermann M; MIDAS Study Group. Effect of midodrine versus placebo on time to vasopressor discontinuation in patients with persistent hypotension in the intensive care unit (MIDAS): an international randomised clinical trial. Intensive Care Med. 2020;46:1884-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 37. | Adly DHE, Bazan NS, El Borolossy RM, Anan IF, Fakher MA, El Wakeel LM. Midodrine improves clinical and economic outcomes in patients with septic shock: a randomized controlled clinical trial. Ir J Med Sci. 2022;191:2785-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 38. | Zundel MT, Boettcher BT, Feih JT, Gaglianello N, Pagel PS. Use of Oral Droxidopa to Improve Arterial Pressure and Reduce Vasoactive Drug Requirements During Persistent Vasoplegic Syndrome After Cardiac Transplantation. J Cardiothorac Vasc Anesth. 2016;30:1624-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Noble M, Sunjic K, Ferguson K. 774: Use of droxidopa for hemodynamic support and vasopressor weaning in the intensive care unit. Crit Care Med. 2023;51:377-377. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 40. | Hong CS, Effendi MK, Ammar AA, Owusu KA, Ammar MA, Koo AB, Lamsam LA, Elsamadicy AA, Kuzmik GA, Laurans M, DiLuna ML, Landreneau ML. Use of droxidopa for blood pressure augmentation after acute spinal cord injury: case reports. Acute Crit Care. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 41. | Patterson A, Bourgault AM, Crawford BR. Correction of Severe Hypotension by Oral Pseudoephedrine in a Patient With Idiopathic Autonomic Dysfunction. Am J Crit Care. 2008;17:484-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 42. | Curran C, Davoudi F, Foster G, Gordan P. Refractory bradycardia and hypotension in patients with autonomic dysfunction treated with pseudoephedrine. BMJ Case Rep. 2023;16:e253274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 43. | Wood GC, Boucher AB, Johnson JL, Wisniewski JN, Magnotti LJ, Croce MA, Swanson JM, Boucher BA, Fabian TC. Effectiveness of pseudoephedrine as adjunctive therapy for neurogenic shock after acute spinal cord injury: a case series. Pharmacotherapy. 2014;34:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Lessing JK, Kram SJ, Levy JH, Grecu LM, Katz JN. Droxidopa or Atomoxetine for Refractory Hypotension in Critically Ill Cardiothoracic Surgery Patients. J Cardiothorac Vasc Anesth. 2024;38:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 45. | Whitson MR, Mo E, Nabi T, Healy L, Koenig S, Narasimhan M, Mayo PH. Feasibility, Utility, and Safety of Midodrine During Recovery Phase From Septic Shock. Chest. 2016;149:1380-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 46. | Macielak SA, Vollmer NJ, Haddad NA, Nabzdyk CGS, Nei SD. Hemodynamic Effects of an Increased Midodrine Dosing Frequency. Crit Care Explor. 2021;3:e0405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Tremblay JA, Laramée P, Lamarche Y, Denault A, Beaubien-Souligny W, Frenette AJ, Kontar L, Serri K, Charbonney E. Potential risks in using midodrine for persistent hypotension after cardiac surgery: a comparative cohort study. Ann Intensive Care. 2020;10:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Rizvi MS, Nei AM, Gajic O, Mara KC, Barreto EF. Continuation of Newly Initiated Midodrine Therapy After Intensive Care and Hospital Discharge: A Single-Center Retrospective Study. Crit Care Med. 2019;47:e648-e653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Lal A, Trivedi V, Rizvi MS, Amsbaugh A, Myers MK, Saleh K, Kashyap R, Gajic O. Oral Midodrine Administration During the First 24 Hours of Sepsis to Reduce the Need of Vasoactive Agents: Placebo-Controlled Feasibility Clinical Trial. Crit Care Explor. 2021;3:e0382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Rizvi MS, Trivedi V, Nasim F, Lin E, Kashyap R, Andrijasevic N, Gajic O. Trends in Use of Midodrine in the ICU: A Single-Center Retrospective Case Series. Crit Care Med. 2018;46:e628-e633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 51. | Hamed M, Elseidy SA, Elkheshen A, Maher J, Elmoghrabi A, Zaghloul A, Panakos A, Panaich S, Saad M, Elbadawi A. The Use of Midodrine as an Adjunctive Therapy to Liberate Patients from Intravenous Vasopressors: A Systematic Review and Meta-analysis of Randomized Controlled Studies. Cardiol Ther. 2023;12:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 52. | Jawad SS, Eccles R. Effect of pseudoephedrine on nasal airflow in patients with nasal congestion associated with common cold. Rhinology. 1998;36:73-76. [PubMed] |
| 53. | Eccles R, Jawad MSM, Jawad SSM, Angello JT, Druce HM. Efficacy and Safety of Single and Multiple Doses of Pseudoephedrine in the Treatment of Nasal Congestion associated with Common Cold. Am J Rhinol. 2005;19:25-31. [PubMed] [DOI] [Full Text] |
| 54. | Johnson HM, Eglash A, Mitchell KB, Leeper K, Smillie CM, Moore-Ostby L, Manson N, Simon L; Academy of Breastfeeding Medicine. ABM Clinical Protocol #32: Management of Hyperlactation. Breastfeed Med. 2020;15:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Presley B, Bianchi B, Coleman J, Diamond F, McNally G. Efficiency of extraction and conversion of pseudoephedrine to methamphetamine from tamper-resistant and non-tamper-resistant formulations. J Pharm Biomed Anal. 2018;156:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Adler LA, Spencer T, Brown TE, Holdnack J, Saylor K, Schuh K, Trzepacz PT, Williams DW, Kelsey D. Once-daily atomoxetine for adult attention-deficit/hyperactivity disorder: a 6-month, double-blind trial. J Clin Psychopharmacol. 2009;29:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 57. | Byun JI, Kim DY, Moon J, Shin HR, Sunwoo JS, Lee WJ, Lee HS, Park KI, Lee ST, Jung KH, Jung KY, Kim M, Lee SK, Chu K. Efficacy of atomoxetine versus midodrine for neurogenic orthostatic hypotension. Ann Clin Transl Neurol. 2020;7:112-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Ramirez CE, Okamoto LE, Arnold AC, Gamboa A, Diedrich A, Choi L, Raj SR, Robertson D, Biaggioni I, Shibao CA. Efficacy of atomoxetine versus midodrine for the treatment of orthostatic hypotension in autonomic failure. Hypertension. 2014;64:1235-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 59. | Ebell MH, Radke T, Gardner J. A systematic review of the efficacy and safety of desmopressin for nocturia in adults. J Urol. 2014;192:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Stenberg A, Läckgren G. Desmopressin Tablets in the Treatment of Severe Nocturnal Enuresis in Adolescents. Pediatrics. 1994;94:841-846. [PubMed] [DOI] [Full Text] |
| 61. | Skoog SJ, Stokes A, Turner KL. Oral Desmopressin: A Randomized Double-Blind Placebo Controlled Study of Effectiveness in Children With Primary Nocturnal Enuresis. J Urol. 1997;158:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Schulman SL, Stokes A, Salzman PM. The efficacy and safety of oral desmopressin in children with primary nocturnal enuresis. J Urol. 2001;166:2427-2431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | McDonell KE, Preheim BA, Diedrich A, Muldowney JAS 3rd, Peltier AC, Robertson D, Biaggioni I, Shibao CA. Initiation of droxidopa during hospital admission for management of refractory neurogenic orthostatic hypotension in severely ill patients. J Clin Hypertens (Greenwich). 2019;21:1308-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Costa-Pinto R, Yong ZT, Yanase F, Young C, Brown A, Udy A, Young PJ, Eastwood G, Bellomo R. A pilot, feasibility, randomised controlled trial of midodrine as adjunctive vasopressor for low-dose vasopressor-dependent hypotension in intensive care patients: The MAVERIC study. J Crit Care. 2022;67:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Dowd J, Bailey D, Moussa K, Nair S, Doyle R, Culpepper-Morgan JA. Ischemic colitis associated with pseudoephedrine: four cases. Am J Gastroenterol. 1999;94:2430-2434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Klestov A, Kubler P, Meulet J. Recurrent ischaemic colitis associated with pseudoephedrine use. Intern Med J. 2001;31:195-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Sica DA, Gehr TW. Desmopressin : safety considerations in patients with chronic renal disease. Drug Saf. 2006;29:553-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 68. | Opgenorth D, Baig N, Fiest K, Karvellas C, Kutsogiannis J, Lau V, Macintyre E, Senaratne J, Slemko J, Sligl W, Wang X, Bagshaw SM, Rewa OG. LIBERATE: a study protocol for midodrine for the early liberation from vasopressor support in the intensive care unit (LIBERATE): protocol for a randomized controlled trial. Trials. 2022;23:194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 69. | Nadeem R. Midodrine Helps Early Discharge of Patients From the ICU, Though Results in Higher Rate of Natural Death. Crit Care Med. 2020;48:e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 70. | Costa-Pinto R, Jones DA, Udy AA, Warrillow SJ, Bellomo R. Midodrine use in critically ill patients: a narrative review. Crit Care Resusc. 2022;24:298-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Rubinstein S, Haimov M, Ross MJ. Midodrine-induced vascular ischemia in a hemodialysis patient: a case report and literature review. Ren Fail. 2008;30:808-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA. 1997;277:1046-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 268] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 73. | Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, Fedorowski A, Furlan R, Kenny RA, Martín A, Probst V, Reed MJ, Rice CP, Sutton R, Ungar A, van Dijk JG; ESC Scientific Document Group. Practical Instructions for the 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39:e43-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 74. | Wieruszewski PM, Khanna AK. Vasopressor Choice and Timing in Vasodilatory Shock. Crit Care. 2022;26:76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 75. | Smith BS, Yogaratnam D, Levasseur-Franklin KE, Forni A, Fong J. Introduction to drug pharmacokinetics in the critically ill patient. Chest. 2012;141:1327-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |