Published online Dec 26, 2024. doi: 10.12998/wjcc.v12.i36.6877
Revised: September 14, 2024
Accepted: September 25, 2024
Published online: December 26, 2024
Processing time: 78 Days and 15.8 Hours
This editorial explores the clinical implications of organizing pneumonia (OP) secondary to pulmonary tuberculosis, as presented in a recent case report. OP is a rare condition characterized by inflammation in the alveoli, which spreads to alveolar ducts and terminal bronchioles, usually after lung injuries caused by infections or other factors. OP is classified into cryptogenic (idiopathic) and secondary forms, the latter arising after infections, connective tissue diseases, tumors, or treatments like drugs and radiotherapy. Secondary OP may be triggered by infections caused by bacteria, viruses, fungi, mycobacteria, or parasites. Key diagnostic features include subacute onset of nonspecific respira
Core Tip: Diagnostic features of organizing pneumonia typically include subacute onset of nonspecific respiratory symptoms such as dry cough, pleuritic chest pain, and exertional dyspnea. Imaging with computed tomography scans typically reveals three patterns: (1) Bilateral subpleural consolidation; (2) Nodular consolidation; and (3) A reticular pattern.
- Citation: Limkul L, Tovichien P. Secondary organizing pneumonia after infection. World J Clin Cases 2024; 12(36): 6877-6882
- URL: https://www.wjgnet.com/2307-8960/full/v12/i36/6877.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i36.6877
Organizing pneumonia (OP) is characterized by inflammatory debris in the alveoli, which spreads to alveolar ducts and terminal bronchioles, forming intraluminal granulation tissue known as Masson bodies[1]. The incidence of OP is estimated to range between 1.97 cases per 100000 individuals and 7 cases per 100000 individuals[2]. OP is classified into two forms: (1) Cryptogenic OP (COP), which has no identifiable cause; and (2) Secondary OP (SOP), which is linked to known triggers such as infections, connective tissue diseases, inflammatory bowel diseases, hematologic cancers, certain medications, radiation, or post-transplantation complications[3-5]. This article focuses specifically on secondary OP, especially those cases that arise after infections.
The clinical symptoms of OP are often vague and non-specific, which frequently leads to delays in diagnosis. A high index of suspicion is crucial for diagnosing OP in patients with subacute respiratory symptoms. Persistent abnormal radiographic patterns, such as parenchymal consolidation, nodules, or reticulation, following known or unknown pulmonary insults, can further guide diagnosis. Since OP is diagnosed by excluding other possible causes, this article will cover the clinical approach, radiographic findings, and further investigations to ensure timely diagnosis and effective treatment.
OP develops following a range of injuries to alveolar epithelial cells, including infections. In response to these injuries, the immune system targets pathogens in damaged regions of the alveolar epithelium. T lymphocytes and neutrophils become activated, releasing inflammatory cytokines. These cytokines trigger fibroblast activation, forming granulation tissue, which forms structures known as Masson bodies, resembling intra-alveolar buds[3,6]. In its early stages, this tissue formation process is reversible. Early diagnosis and timely treatment can prevent the development of irreversible pulmonary fibrosis, a condition that permanently impairs gas exchange.
SOP after infection, acute respiratory distress syndrome (ARDS), and hypersensitivity pneumonitis (HP) are all characterized by inflammatory lung responses but differ significantly in their underlying pathogenesis. SOP typically follows a lung infection, leading to fibroblast activation and granulation tissue formation in the alveoli, where the resulting fibrosis is often reversible with treatment. ARDS, often triggered by severe injuries such as sepsis or pneumonia, is characterized by diffuse alveolar damage, acute inflammation, and a cytokine storm that can potentially lead to fibrosis in later stages. HP is an immune-mediated disorder caused by repeated inhalation of environmental antigens, leading to granuloma formation and chronic inflammation, which carries a risk of irreversible fibrosis. While macrophages and neutrophils play central roles in SOP and ARDS, HP is primarily driven by T-cell-mediated immunity, resulting in distinct patho
SOP develops following alveolar epithelial cell injuries caused by identifiable etiologies. SOP has multiple causes, including infections, connective tissue diseases, inflammatory bowel diseases, hematologic malignancies, certain drugs, radiation, and post-transplantation complications[3]. Among these, infections are the most common cause of SOP. Common bacterial pathogens associated with SOP include Chlamydia pneumoniae, Mycoplasma pneumoniae, Streptococcus pneumoniae, Legionella pneumophila, and Pseudomonas aeruginosa[7,10]. SOP frequently arises in cases of non-resolving pneumonia, even when the infectious agent is successfully treated with antibiotics.[7]
Many viruses have been reported to cause OP, including Herpes virus, human immunodeficiency virus (HIV), adenovirus, cytomegalovirus, influenza, parainfluenza, and SARS-CoV-2 viruses[7,10]. OP has also been noted as a complication in patients with coronavirus disease 2019, especially in those with persistent abnormal chest imaging[3,11,12]. Parasites such as Plasmodium vivax and fungal infections like Cryptococcus neoformans, Penicillium janthinellum, and Pneumocystis jirovecii can also cause OP[3,7,10]. Although tuberculosis is a rare cause of OP, as noted by Liu et al[13], other case reports have found evidence of post-tuberculosis infection leading to OP[3,5,6,14].
Although OP typically presents with a subacute onset, meaning symptoms develop gradually over weeks, it lacks specific clinical features. This absence of distinct features often delays diagnosis by 6 weeks to 10 weeks[3,7,15]. Common symptoms at the time of presentation include a dry cough, flu-like symptoms, pleuritic chest pain, and exertional dyspnea. Additionally, fever, fatigue, and weight loss are frequently reported. Hemoptysis is rare, as is the rapid pro
The physical examination may reveal hypoxemia, crackles, and bronchial breath sounds on lung auscultation[3]. Clubbing is almost always absent, and physical findings may be normal in up to 25% of patients[10,15].
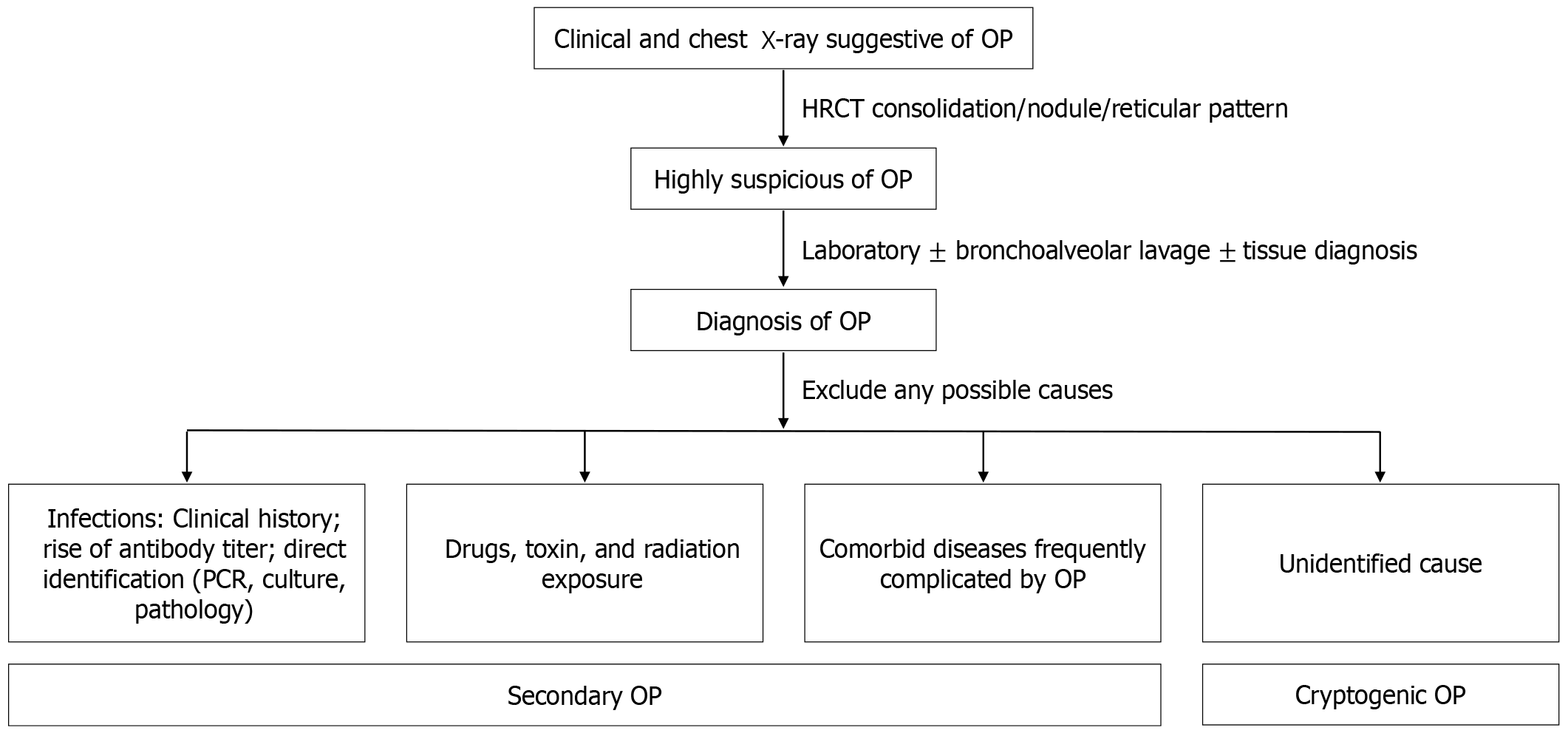
SOP and COP share similar symptoms and imaging findings, regardless of the underlying cause, including infections. Nevertheless, a study suggested that certain differences exist: Fever and pleural effusion are more common in SOP, while COP patients tend to experience longer symptom duration and show higher lymphocyte counts in bronchoalveolar lavage (BAL) fluid[5]. However, despite these observed differences, COP diagnosis is still mainly achieved by excluding other potential causes. In SOP following an infection, there should be evidence of a previous lung infection, such as an increase in antibody titers specific to a pathogen or direct identification of the pathogen in a respiratory sample. Figure 1 summarizes the diagnostic process and identification of OP causes.
Abnormal chest imaging patterns suggestive of OP include migratory parenchymal consolidation and persistent or new focal parenchymal opacities unresponsive to antibiotics. OP has various radiographic appearances on chest computed tomography (CT), but no specific pattern differentiates secondary from cryptogenic OP. Some reports propose stan
Parenchymal consolidation is the most common radiographic finding in OP, occurring in nearly three-quarters of cases[15]. The consolidation tends to be migratory, patchy, and asymmetrically distributed along peribronchovascular and subpleural areas[16]. It often appears in all lung zones, typically bilaterally, with a peripheral and lower-lung predo
Nodules may range from micronodules (< 4 mm) to larger nodules (up to 1 cm), typically with irregular or spiculated margins and air bronchograms[15].
A curvilinear opacity often follows ground-glass or consolidative opacity, extending to the pleura and surrounded by aerated lung[15,16]. The reversed halo sign, also known as the "atoll sign" is defined by ground-glass attenuation surrounded by consolidation, which may sometimes display a linear morphology. This pattern occurs due to central inflammation of the alveolar septa, with peripheral granulation tissue forming in the airspaces[17]. The reversed halo sign has also been observed in other conditions, such as infections (e.g., tuberculosis and mucormycosis), pulmonary infarction, and vasculitis[16].
Additionally, the patient in the case report by Liu et al[13] had typical imaging findings of multifocal ground-glass opacity, predominantly in the bilateral subpleural regions.
Since OP is a diagnosis of exclusion, bronchoscopy with BAL is highly valuable for ruling out other causes, such as ongoing infections, malignancy, or inflammatory disorders[3]. BAL usually reveals a mixed cellularity pattern, with increased lymphocytes, neutrophils, and eosinophils[3,7,15]. Additionally, BAL shows that lymphocytes are activated, often presenting with a decreased CD4/CD8 ratio[10]. A case report by Liu et al[13] suggests that BAL is critical in diagnosing SOP, particularly after an infection. BAL can help exclude treatment failure from a previous infection, whether due to ongoing infection with the same pathogen, co-infection with another pathogen, or drug-resistant organisms. These causes may lead to persistent or progressive abnormalities on imaging. In cases where SOP is suspected after an infection, BAL is helpful for patients where prior infections cannot be confirmed via sputum collection, nasopharyngeal swabs for respiratory viruses, or serology testing for specific pathogens. Since corticosteroids are the mainstay treatment for OP, infectious causes should be ruled out before initiating treatment.
A mild to moderate restrictive defect is the most common pulmonary function abnormality observed in OP. Airflow obstruction may also be present, especially in patients with a history of smoking. Additionally, the diffusion capacity of carbon monoxide is often reduced in proportion to the severity of the restrictive defect[10].
Tissue diagnosis may be necessary for certain patients, especially those with clinical or radiographic worsening during observation or despite empiric therapy. It is also crucial for patients with a higher suspicion of high-risk conditions such as malignancy, infection, or vasculitis. In such cases, tissue sampling can be done through transbronchial biopsy, CT-guided core needle biopsy, or surgical biopsy, depending on institutional resources[7,15]. OP is typically characterized by organizing fibrosis, with intraluminal polypoid plugs of loose connective tissue in the alveolar spaces, ducts, and distal airways, known as Masson bodies[3,15]. Three histologic patterns are observed in OP. The first is cicatricial OP, characterized by intraluminal polypoid plugs with dense fibrotic collagen. The second is acute fibrinous OP, and the third is granulomatous OP, which features epithelioid cell granuloma or multinucleated giant cells, often suggesting an infectious cause[15].
Laboratory investigations are often essential in diagnosing OP. A complete blood count may reveal leukocytosis and neutrophilia in up to 50% of cases[3,18]. In addition, inflammatory markers like erythrocyte sedimentation rate and c-reactive protein levels are typically elevated, making them useful for follow-up. These markers also help predict the patient’s response to treatment and the likelihood of relapse[15].
Corticosteroid therapy is the primary treatment for OP, often resulting in rapid clinical improvement and the resolution of opacities on chest imaging[10]. The typical dosage of corticosteroids ranges from 0.5 mg/kg/day to 1.5 mg/kg/day, with gradual tapering over 6 months to 12 months[15]. However, in cases of SOP following infection, corticosteroids may worsen or cause a relapse of the infection. Therefore, active infection should be ruled out and treated first. In cases where infection cannot be excluded, empirical antimicrobial agents can be used alongside corticosteroids. For instance, in the case report by Liu et al[13], BAL was performed to rule out active infection, and both antituberculosis therapy and corticosteroids were administered since corticosteroid therapy alone carries the risk of tuberculosis dissemination.
OP usually responds well to corticosteroid treatment, although some patients may require additional immunosuppressive agents. Studies show no significant difference in outcomes between patients with SOP after infection and those with COP[5,15,19]. However, relapse occurs in 13% to 58% of cases, often during steroid tapering, particularly when the dose is reduced too quickly[10]. In milder cases, macrolides may be useful for managing symptoms or as a bridging treatment when transitioning off corticosteroids[20]. If tapering corticosteroids results in treatment failure or relapse, additional immunosuppressive therapies such as cyclophosphamide, azathioprine, mycophenolate, or rituximab may be required[15].
OP presents a diagnostic challenge due to its non-specific respiratory symptoms, which can resemble other common respiratory conditions. A high degree of clinical suspicion is crucial when respiratory symptoms develop gradually over weeks, accompanied by progressive or persistent abnormal opacities on radiographic imaging. This remains important, even in patients with a history of known or unknown lung injuries. Early clinical suspicion allows physicians to initiate prompt investigations, leading to a quicker diagnosis and more effective treatment.
OP is a rare condition characterized by lung inflammation and the deposition of inflammatory cell debris in the alveoli and bronchioles. SOP can develop after various types of lung injury, with infection being one of the most common causes. The diagnosis of OP is based on recognizing subacute, non-specific respiratory symptoms alongside abnormal radiographic findings. These findings may include parenchymal consolidation with a characteristic migratory, bilateral subpleural distribution, parenchymal nodules, or a reticular pattern. Bronchoscopy with BAL helps rule out other potential causes, particularly ongoing infections after an initial insult. This step ensures that corticosteroid therapy, the primary treatment for OP, is initiated appropriately.
| 1. | Baque-Juston M, Pellegrin A, Leroy S, Marquette CH, Padovani B. Organizing pneumonia: what is it? A conceptual approach and pictorial review. Diagn Interv Imaging. 2014;95:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Gudmundsson G, Sveinsson O, Isaksson HJ, Jonsson S, Frodadottir H, Aspelund T. Epidemiology of organising pneumonia in Iceland. Thorax. 2006;61:805-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Ketchersid K. A review of organizing pneumonia. JAAPA. 2023;36:16-19. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Krupar R, Kümpers C, Haenel A, Perner S, Stellmacher F. [Cryptogenic organizing pneumonia versus secondary organizing pneumonia]. Pathologe. 2021;42:55-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Choi KJ, Yoo EH, Kim KC, Kim EJ. Comparison of clinical features and prognosis in patients with cryptogenic and secondary organizing pneumonia. BMC Pulm Med. 2021;21:336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Huang LL, Wang C, Liu Y, Gu XY, Wang WX, Chen W, Hu CM. Resolution of an insidious and migratory Mycobacterium tuberculosis-associated secondary organizing pneumonia: a case report and literature review. BMC Infect Dis. 2023;23:372. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Cordier JF. Organising pneumonia. Thorax. 2000;55:318-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 195] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3976] [Cited by in RCA: 3851] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 9. | Selman M, Pardo A, King TE Jr. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186:314-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 308] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 10. | Cordier JF. Cryptogenic organising pneumonia. Eur Respir J. 2006;28:422-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 315] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 11. | Golbets E, Kaplan A, Shafat T, Yagel Y, Jotkowitz A, Awesat J, Barski L. Secondary organizing pneumonia after recovery of mild COVID-19 infection. J Med Virol. 2022;94:417-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Tonon CR, Tanni SE, Rocha J, Godoy I, Polegato BF, Pereira FWL, Martins D, Prudente RA, Franco ET, Brizola F, Baldi BG, Okoshi MP. Organizing pneumonia and COVID-19. Am J Med Sci. 2023;366:458-463. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Liu M, Dong X, Ding Z, Wang Q, Li D. Organizing pneumonia secondary to pulmonary tuberculosis: A case report. World J Clin Cases. 2024;12:5974-5982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Kim EJ, Kim KC. Pulmonary tuberculosis presenting secondary organizing pneumonia with organized polypoid granulation tissue: case series and review of the literature. BMC Pulm Med. 2020;20:252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Cherian SV, Patel D, Machnicki S, Naidich D, Stover D, Travis WD, Brown KK, Naidich JJ, Mahajan A, Esposito M, Mina B, Lakticova V, Cohen SL, Muller NL, Schulner J, Shah R, Raoof S. Algorithmic Approach to the Diagnosis of Organizing Pneumonia: A Correlation of Clinical, Radiologic, and Pathologic Features. Chest. 2022;162:156-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 16. | Arenas-Jiménez JJ, García-Garrigós E, Ureña Vacas A, Sirera Matilla M, Feliu Rey E. Organizing pneumonia. Radiologia (Engl Ed). 2022;64 Suppl 3:240-249. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Voloudaki AE, Bouros DE, Froudarakis ME, Datseris GE, Apostolaki EG, Gourtsoyiannis NC. Crescentic and ring-shaped opacities. CT features in two cases of bronchiolitis obliterans organizing pneumonia (BOOP). Acta Radiol. 1996;37:889-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Cordier JF. Cryptogenic organizing pneumonitis. Bronchiolitis obliterans organizing pneumonia. Clin Chest Med. 1993;14:677-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Drakopanagiotakis F, Paschalaki K, Abu-Hijleh M, Aswad B, Karagianidis N, Kastanakis E, Braman SS, Polychronopoulos V. Cryptogenic and secondary organizing pneumonia: clinical presentation, radiographic findings, treatment response, and prognosis. Chest. 2011;139:893-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Epler GR. Bronchiolitis obliterans organizing pneumonia, 25 years: a variety of causes, but what are the treatment options? Expert Rev Respir Med. 2011;5:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |