Published online Dec 16, 2024. doi: 10.12998/wjcc.v12.i35.6840
Revised: September 11, 2024
Accepted: October 10, 2024
Published online: December 16, 2024
Processing time: 74 Days and 18 Hours
Cat scratch disease (CSD) is the most common human infection caused by Barto
The child was ultimately diagnosed with CSD. The primary manifestations included nocturnal fever, enlargement of lymph nodes in the neck, axilla and groin, and suspected brucellosis; however, both brucellosis tests conducted during the course of the illness yielded negative results. Bone marrow cytology indicated stimulated proliferation. Lymph node biopsy indicated hyperplasia of lymphoid tissue in the cervical lymph nodes (right), with combined immunohistochemical findings indicating reactive hyperplasia. Immunohistochemical analysis revealed CD20 B (+), CD3 T (+), BCL-6 (+), and BCL-2 (-). CD21 FDC networks were present and Ki67 expression in the germinal center was ~80%. Blood next-generation sequencing indicated B. henselae sequence number was 3. Serological test results demonstrated positive antibody response to B. henselae IgG (+), B. henselae IgM (+), Bartonella quintana (B. quintana) IgG (-) and B. quintana IgM (-), and the final diagnosis was CSD.
In patients presenting with fever at night and swollen lymph nodes of unknown origin, CSD should be considered.
Core Tip: We report a case of cat scratch disease (CSD) characterized by nocturnal fever as the primary manifestation, accompanied by cervical and inguinal lymphadenopathy. In patients presenting with fever of unknown origin as the predominant symptom, a comprehensive physical examination and thorough medical history are essential to minimize the risk of missed or incorrect diagnosis. CSD is typically self-limiting and often does not require treatment, and symptomatic management and regular follow-up suffice for patients with milder symptoms. However, special attention should be given to immunodeficient and immunocompromised individuals who may require timely antimicrobial therapy.
- Citation: Yin QL, Liu YQ, Zhang HM, Zhang YL, Qi SM, Wen JQ, Zhang WH. Cat scratch disease in children with nocturnal fever: A case report. World J Clin Cases 2024; 12(35): 6840-6847
- URL: https://www.wjgnet.com/2307-8960/full/v12/i35/6840.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i35.6840
Cat scratch disease (CSD) is a zoonotic infectious disease. The main host is mammals, and it is transmitted by hematophagous insects such as phlebotomine sandflies, human body lice and cat fleas, or introduced into humans through biting and clawing by cats[1,2]. Most typical CSD is characterized by self-limited regional lymph node enlargement or lymphadenitis, usually associated with systemic symptoms, and 10% of patients show involvement of other organs, indicating atypical CSD[3,4]. We here report a child diagnosed with CSD whose main presentation was nocturnal fever, and discuss its clinical significance.
A 4-year-old girl of Han nationality was admitted to the Pediatric Respiratory Department of Xianyang Rainbow Hospital, China on December 30, 2023. The girl had intermittent fever for 14 d, cough for 6 d, with the neck, axillary and inguinal lymph node enlargement.
Her fever occurred 14 days ago without an apparent cause, reaching a peak temperature of 40.0 ℃. The parents admi
There was no history of past illness.
There was no personal and family history.
Temperature: 36.8 ℃, pulse: 94 beats/minute, respiratory rate: 24 breaths/minute, blood pressure: 92/54 mmHg, body weight 17 kg. Alertness and cooperation were good. No rash or bleeding spots were observed throughout the body. Soybean-sized enlarged lymph nodes were palpable in the neck, axilla and groin, which were soft, nontender and had good mobility. The remaining superficial lymph nodes were not palpable. The conjunctivas were not hyperemic. The lips were not cyanotic, bayberry tongue was positive, the oral mucosa was smooth, pharyngeal hyperemia was obvious, the tonsils were enlarged II°, and no purulent secretions were observed. The neck was soft and without resistance. On auscultation, heart and lung sounds were normal, abdominal examination was normal and nervous system examination was normal.
Initial blood tests showed the following (December 29, 2023): Hemoglobin 114 g/L; white blood cell (WBC) count: 10.19 × 109/L; platelet count: 213 × 109/L; serum amyloid A (SAA): 156.3 mg/L; C-reactive protein (CRP) 156.3 mg/L; erythrocyte sedimentation rate (ESR): 40 mm/hour; interleukin (IL)-6: 24.05 pg/mL; Mycobacterium tuberculosis antibody (-); ferritin: 92.19 ng/mL; Epstein-Barr virus (EBV)-IgG (-) and EBV-IgM (-); brucellosis agglutination test: < 1: 25 (-).
Chest computed tomography (CT) revealed bronchiolitis in both lungs and small nodular lesions in the upper lobe of the left lung.
The patient was finally diagnosed with CSD.
On days 1 and 2, the patient was given symptomatic and supportive treatment. Blood tests showed: Hemoglobin: 99 g/L; WBC count: 4.50 × 109/L; platelet count: 274 × 109/L; ESR: 52 mm/hour; high-sensitivity (hs)-CRP: 56.70 mg/L; CRP: 50.90 mg/L; and SAA: 251.6 mg/L. Subsets of lymphocytes, thyroid function, immunoglobulin, IL-6, antistreptolysin, rheumatoid factor, electrolytes, procalcitonin, myocardial enzymes, tumor markers, tuberculous IgG antibody and epidemic hemorrhagic fever virus antibody were all negative. Ultrasound showed bilaterally enlarged cervical lymph nodes, splenomegaly, right renal cyst, but no obvious heart and coronary artery anomalies.
On days 3 and 4, she continued to experience recurrent fever, reaching a peak temperature of 39.4 ℃, accompanied by paroxysmal coughing. Nucleic acid detection for six respiratory viruses showed parainfluenza I RNA (+). IL-6 10.72 level was pg/mL and IFN-γ 96.02 pg/mL. Mycoplasma pneumoniae DNA, EBV DNA, Bordetella pertussis DNA, enterovirus, Chlamydia pneumoniae antibody, and novel coronavirus nucleic acid were all negative. CT revealed no discernible abnor
On days 5 and 7, she still had fever, and the highest temperature was 39.8 ℃. The fever was reduced to normal levels through administration of oral antipyretics and alleviation of paroxysmal coughing. Blood next-generation sequencing (NGS) revealed that sequence number of Bartonella henselae (B. henselae) was 3. Autoantibody, anti-neutrophil cytoplasmic antibody, T-Spot and sputum culture were negative. Further enquiry about the patient's medical history revealed that she kept dogs and cats at home. Her mother complained that the patient was scratched by a cat 3-4 months ago. CSD was suspected and trimethoprim/sulfamethoxazole (TMP/SMX) was administered.
On days 8 and 9, she presented with nocturnal fever, while remaining afebrile during the day. Furthermore, the duration between fever episodes increased compared to previous occurrences.
On days 10 to 12, she still had nocturnal fever. Blood cell morphology smear showed that red blood cells were a consistent size, with a pale area of light staining. WBCs were predominantly granulocytes, with some toxic granules. Platelets were scattered in the visible. In order to exclude hematological disorders and necrotizing lymphadenitis, with the consent of the families, bone marrow puncture and biopsy analysis were performed.
On days 13 to 16 the nocturnal fever persisted, with B. henselae IgG (+) and IgM (+). Reports of bone marrow cytology and lymph node biopsy pathology are presented in the next section. She was diagnosed with CSD, and the treatment regimen was adjusted from TMP/SMZ to doxycycline.
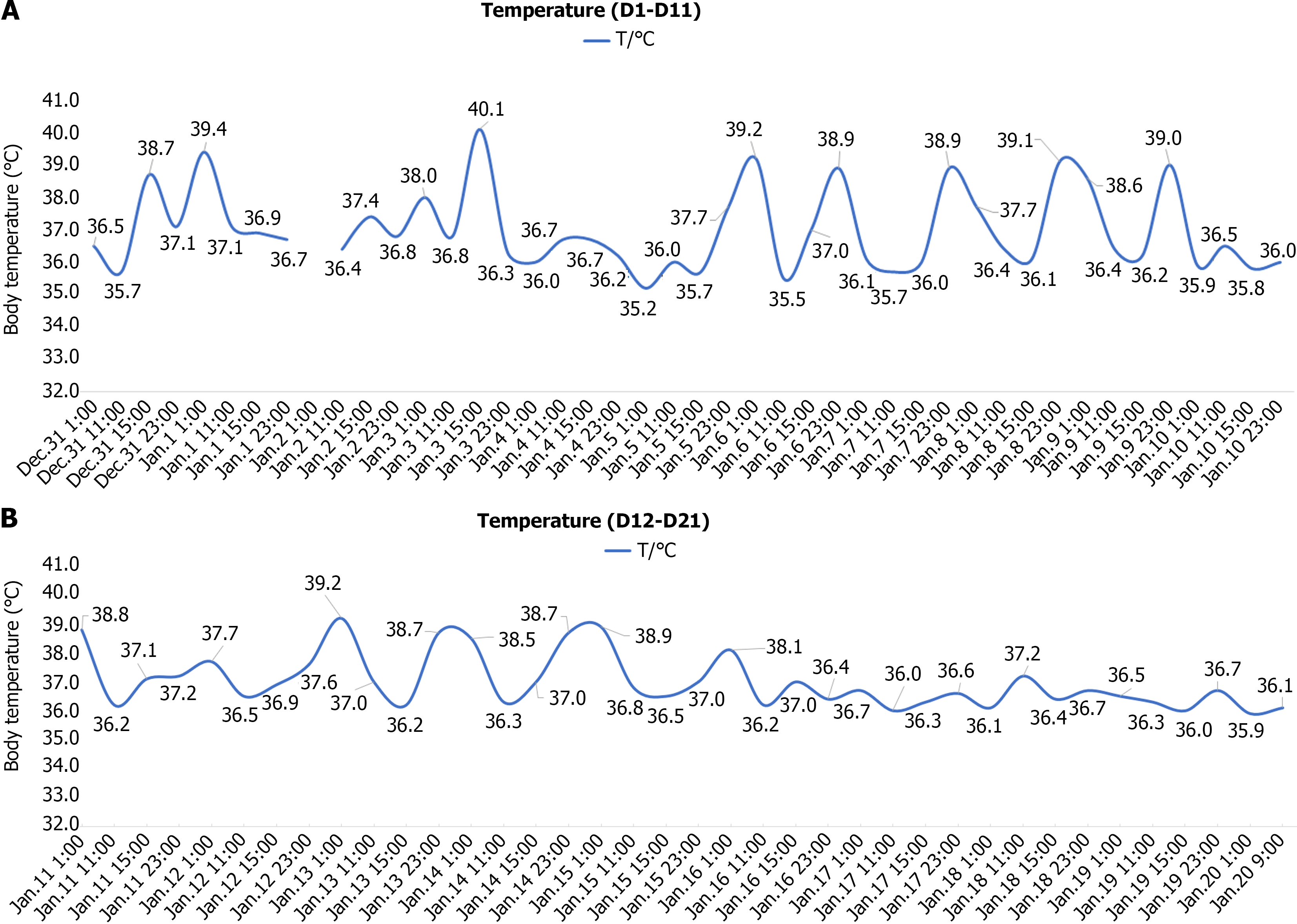
On days 17 to 20, her temperature remained within the normal range, and the specific serological test for Brucella was negative. Subsequent re-examination of blood, CRP, hs-CRP, ESR and SAA was normal. Therefore, she was discharged and doxycycline was maintained for 1 wk. Her temperature records are shown in Figure 1.
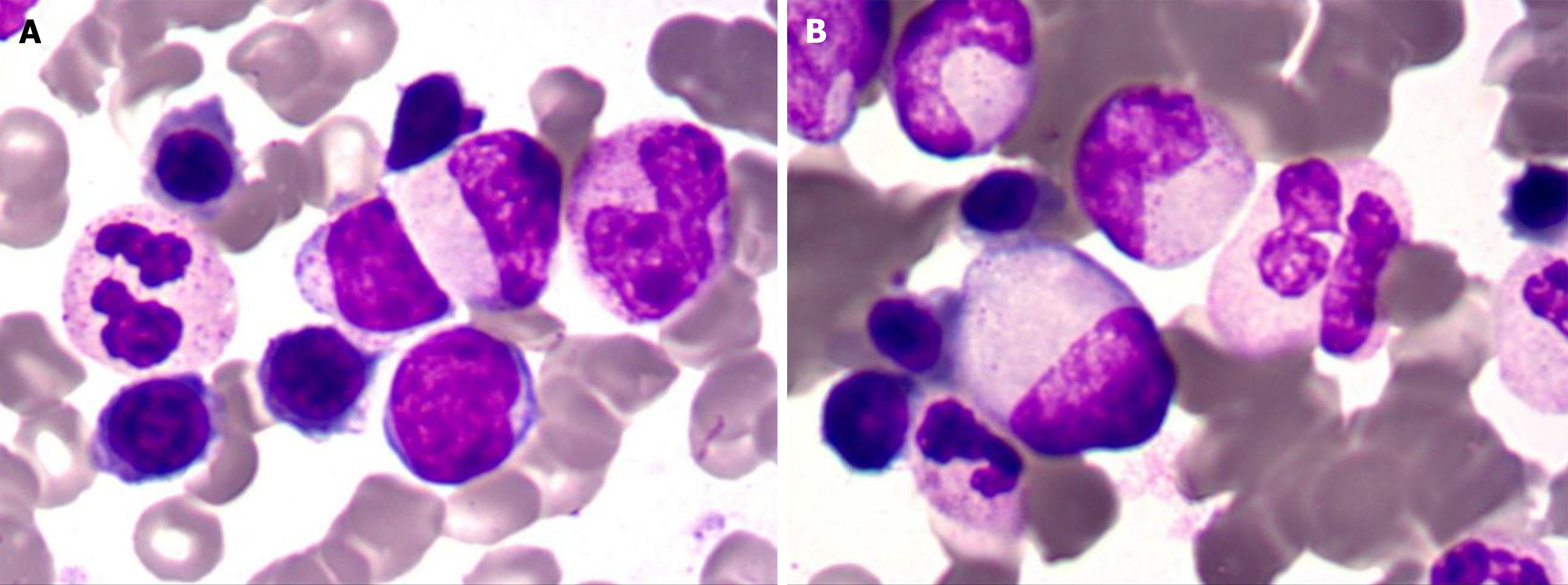
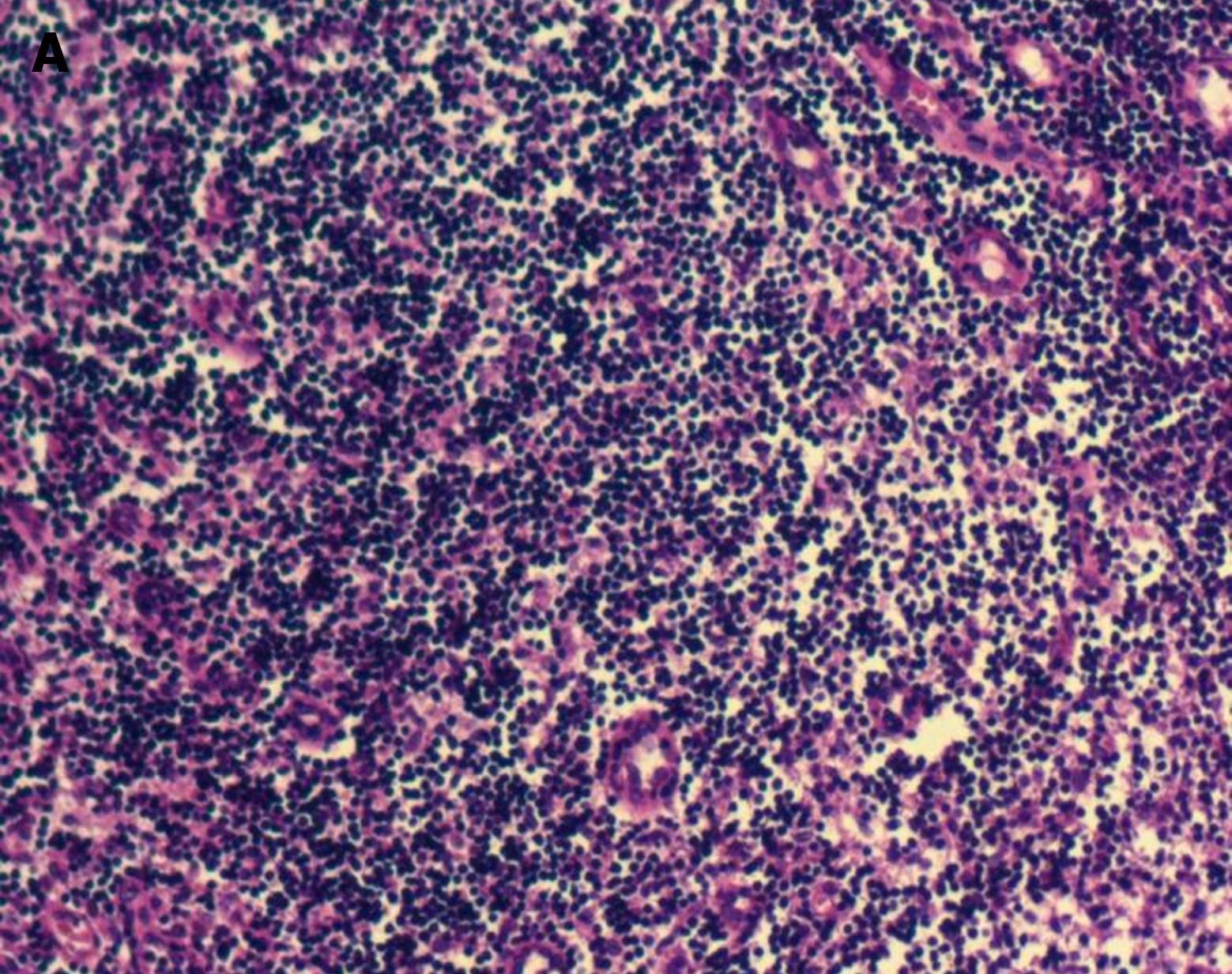
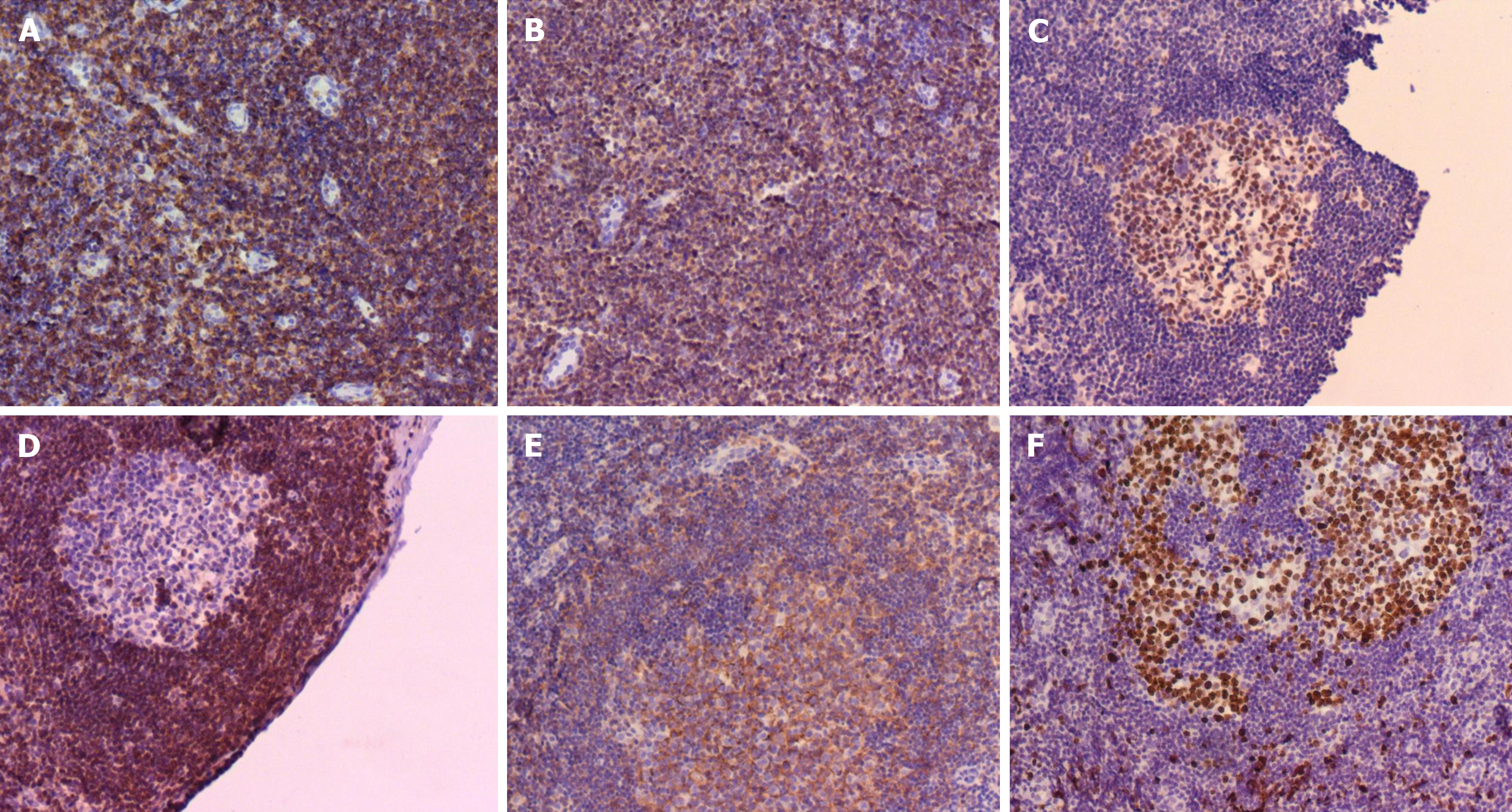
Bone marrow cytology showed proliferation (Figure 2). Lymph node biopsy showed hyperplasia of lymphoid tissue in the cervical lymph nodes (right; Figure 3). Lymph node biopsy immunohistochemical analysis showed CD20 B (+), CD3 T (+), BCL-6 (+) and BCL-2 (-). CD21 FDC networks were present (+) and Ki67 expression in the germinal center was approximately 80%. All the findings indicated reactive hyperplasia (Figure 4).
After doxycycline treatment, the body temperature returned to normal and all clinical symptoms resolved. She was discharged on day 20. Follow-up examination of routine blood tests, CRP, ESR, and hepatic and renal function revealed no significant abnormalities 1 and 2 months later. Her temperature returned to the normal range.
Fever of unknown origin (FUO) refers to a body temperature of ≥ 38.3 ℃ on at least two occasions, persisting for a duration of ≥ 3 three weeks, or having multiple febrile episodes over this time period. There must be no obvious diagnosis despite comprehensive medical history collection and examination, as well as appropriate tests including routine blood tests, inflammatory markers, autoimmune screening, cultures, chest radiography and abdominal imaging[5]. There are > 200 potential causes of FUO which can be broadly categorized into infectious diseases, neoplastic diseases, noninfectious inflammatory diseases, and other conditions. The patient presented with FUO lasting for 28 d prior to establishment of a definitive diagnosis[6].
The primary manifestation of this case was nocturnal fever, accompanied by enlargement of cervical, axillary and inguinal lymph nodes. The fever type did not meet the characteristics of continuous, remittent, intermittent, recurrent or undulant fever commonly observed in brucellosis[7]. Brucellosis typically presents with symptoms such as fever, fatigue, night sweats and joint pain. The characteristic pattern is nocturnal fever and sweating that subsides in the morning. Clinical symptoms and physical examination are usually nonspecific; however, lymphadenopathy along with hepatomegaly or splenomegaly may be present. Brucellosis is a zoonotic disease caused by Gram-negative bacteria from the genus Brucella. It is mainly transmitted through consumption of raw milk and milk products or contact with infected animals or their feces. Its clinical symptoms often overlap with other febrile diseases[8]. Our patient was admitted to the hospital with FUO as the chief complaint, accompanied by neck, axillary and inguinal lymph node enlargement. The possibility of brucellosis was considered high because of the history of cats and dogs at home, the course of the disease with persistent fever for several weeks, sweating, enlarged lymph nodes, and fever characterized by nocturnal fever. Abdominal ultrasound revealed splenomegaly which suggested brucellosis; however, the agglutination tests for brucellosis were negative twice, and therefore were not considered for the time being. After further history taking it was discovered that a cat had scratched the patient 3-4 months ago. Combined with the child's clinical symptoms, CSD was considered, so relevant serological tests and metagenomic NGS (mNGS) were done. Blood NGS revealed that sequence number of B. henselae was 3, and positive IgM/IgG for B. henselae, which led to the final diagnosis of CSD[9-11].
CSD is a self-limiting disease caused by B. henselae. It is the most common cause of regional lymphadenitis in children and adolescents during late summer and autumn. The pathogenesis of CSD is still unclear. Some researchers believe that Bartonella induces a proinflammatory response by upregulating expression of cell adhesion molecules on endothelial cells, leading to local infection[12]. In the United States, the incidence of CSD is highest in children aged 5–9 years, with 12500 patients diagnosed with CSD each year; 500 of whom require hospitalization[13]. The clinical spectrum of CSD ranges from asymptomatic infection to multiorgan involvement. Approximately 90% of patients present with typical CSD, characterized by self-limiting regional lymphadenopathy or lymphadenitis. Around 30% of patients experience systemic symptoms, including fever, chills, night sweats, headache and abdominal pain. Atypical CSD occurs in approximately 10% of cases and is associated with tissue and organ involvement beyond the lymph nodes such as the eyes, nervous system, heart, liver, spleen, skin or musculoskeletal system (e.g., erythema nodosum and neuroretinitis). These manifestations are more commonly observed in immunocompromised individuals who have a higher risk for complications[14].
The diagnostic criteria for CSD include the following: (1) Exposure to cats or fleas, with or without scratches or local inoculation lesions (such as skin papules, eye granulomas, and mucous membranes); (2) Laboratory/radiology findings: negative purified protein derivative or serology for other infectious causes of adenopathy; sterile pus aspirated from lymph nodes, polymerase chain reaction assay positive; B. henselae, B. quintana or Afipia felis: Highest sensitivity. CT scan: liver/spleen abscesses; (3) Positive ELISA or indirect fluorescent antibody test and serological titer > 1:64 for B. henselae are indicative. A fourfold increase in titer between acute and convalescent samples is also suggestive; and (4) Biopsy of lymph nodes, skin, liver, bone, or eye granuloma showing granulomatous inflammation compatible with CSD; positive Warthin–Starry silver stain. If three of the four criteria are met, a diagnosis of CSD can be made. In atypical cases, four criteria must be met[15]. CSD should be considered when a patient presents with persistent or chronic lymph node enlargement (≥ 10 mm) for > 3 weeks. However, due to the prolonged incubation period of CSD, patients often fail to provide an epidemiological history during their doctor visits. There is no gold-standard method for the diagnosis of CSD and the commonly used rates of misdiagnosis and underdiagnosis are usually high. Current examination techniques for diagnosis of CSD include serological testing, PCR, and tissue biopsy. Of these, serological tests are the most commonly used, such as immunofluorescent antibody test and enzyme immunoassay, which are considered highly suggestive of a recent infection when the IgG value is > 1:256; the production of IgM is usually transient. Bartonella culture yields rare positive results and requires stringent conditions, while antigen and antibody detection are not routinely performed in most hospitals. However, serological sensitivity is also limited and there is significant cross reactivity with other microorganisms. PCR is a specific method for detecting Bartonella infections, and although PCR detection at the lesion site is both specific and sensitive, it necessitates invasive collection of tissue or pus samples from the affected area, which may lead to infection spread and low patient acceptance, and its diagnostic value is limited in patients with low levels of bacteremia. Tissue biopsy is an invasive examination and not the first choice for doctors, and cytology and pathology findings do not have specificity in diagnosing CSD. Although mNGS offers higher sensitivity compared to traditional culture methods and enables rapid identification of pathogens, its analysis and interpretation can be complicated by sample contamination, reagent processing issues, or microbial contaminants present within the laboratory environment. mNGS is expensive and not typically chosen as a first-line option by clinicians for CSD diagnosis. Therefore, laboratory diagnosis of CSD remains challenging[16-20]. For the early identification and diagnosis of CSD, clinicians must, firstly, ask patients in detail whether they have a history of cat exposure; secondly, choose the appropriate investigations in combination with the patient's medical history and clinical symptoms; and lastly, regardless of the time of year, CSD needs to be taken into account in the case of lymph node enlargement with unexplained fever.
The need for antimicrobial treatment of CSD depends on the clinical manifestations and immune function of the patient. For patients with normal immune function or mild disease, antibiotic treatment is generally not necessary and self-healing occurs within 2–4 months. Timely and standardized antimicrobial therapy should be used for patients with low immunity, immunodeficiency or severe illness[21]. B. henselae is a small, curved, aerobic Gram-negative bacillus that stains with silver. In bacillary angiomatosis, lobular proliferation of small blood vessels occurs with the presence of bacilli in adjacent connective tissue and blood vessels. New combinations of antibiotics with bactericidal effects and enhanced cellular lipid barrier penetration should be considered for the treatment of bartonellosis. Macrolides and tetracyclines are recommended as first-line therapy due to their superior intracellular activity. The results of an in vitro susceptibility study in 2006 demonstrated that Bartonella is susceptible to many antibiotics. However, considering Bartonella's characteristics as an intracellular bacterium, it is advisable to utilize intracellular bactericides such as macrolides, tetracyclines and rifampicin for anti-infective treatment[15,22]. The Infectious Diseases Society of America[23] recommends azithromycin for CSD treatment. When azithromycin cannot be used, TMP/SMZ serves as a reasonable alternative. Doxycycline has shown efficacy in severe neuroretinitis, endocarditis or chronic bacteremia caused by Bartonella[24]. In the management of patients with CSD, we need timely and regular follow-up visits to closely observe the changes in the patient's condition, especially for patients with immunodeficiency.
Our patient presented with nocturnal fever and lymphadenopathy as the primary clinical manifestations. The administration of azithromycin and TMP/SMZ were not effective and the patient recovered after receiving oral doxycycline. This highlights the importance for clinicians to remain vigilant for CSD when encountering patients with nocturnal fever.
We thank the Metagenomics Next-generation Sequencing Technology Research Center of Anlong Gene for its technological support.
| 1. | Chomel BB. Cat-scratch disease. Rev Sci Tech. 2000;19:136-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Pecora F, Abate L, Scavone S, Petrucci I, Costa F, Caminiti C, Argentiero A, Esposito S. Management of Infectious Lymphadenitis in Children. Children (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Nawrocki CC, Max RJ, Marzec NS, Nelson CA. Atypical Manifestations of Cat-Scratch Disease, United States, 2005-2014. Emerg Infect Dis. 2020;26:1438-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | Deregibus MI, Bagnara EI, Buchovsky A. Cat-scratch disease: Experience in a tertiary care children's hospital. Arch Argent Pediatr. 2023;121:e202202592. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Hu B, Chen TM, Liu SP, Hu HL, Guo LY, Chen HY, Li SY, Liu G. Fever of unknown origin (FUO) in children: a single-centre experience from Beijing, China. BMJ Open. 2022;12:e049840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 6. | Santana LFE, Rodrigues MS, Silva MPA, Brito RJVC, Nicacio JM, Duarte RMSC, Gomes OV. Fever of unknown origin - a literature review. Rev Assoc Med Bras (1992). 2019;65:1109-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Qureshi KA, Parvez A, Fahmy NA, Abdel Hady BH, Kumar S, Ganguly A, Atiya A, Elhassan GO, Alfadly SO, Parkkila S, Aspatwar A. Brucellosis: epidemiology, pathogenesis, diagnosis and treatment-a comprehensive review. Ann Med. 2023;55:2295398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 8. | Wu ZG, Song ZY, Wang WX, Xi WN, Jin D, Ai MX, Wu YC, Lan Y, Song SF, Zhang GC, Yao XB, Gao Z, Liu CY, Sun K, Yu DS, Xie BG, Sun SL. Human brucellosis and fever of unknown origin. BMC Infect Dis. 2022;22:868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Zhou T, Zheng Y, Zhang H, Liu Y. A case report of diagnosis of cat-scratch disease using metagenomic next-generation sequencing. Front Cell Infect Microbiol. 2023;13:1322651. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Li P, Qian Z, Tao Y. Application of metagenomic next-generation sequencing in the diagnosis of Bartonella neuroretinitis: a case report and literature review. J Ophthalmic Inflamm Infect. 2024;14:17. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Allizond V, Costa C, Sidoti F, Scutera S, Bianco G, Sparti R, Banche G, Dalmasso P, Cuffini AM, Cavallo R, Musso T. Serological and molecular detection of Bartonella henselae in specimens from patients with suspected cat scratch disease in Italy: A comparative study. PLoS One. 2019;14:e0211945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Chomel BB, Kasten RW. Bartonellosis, an increasingly recognized zoonosis. J Appl Microbiol. 2010;109:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 13. | Nelson CA, Saha S, Mead PS. Cat-Scratch Disease in the United States, 2005-2013. Emerg Infect Dis. 2016;22:1741-1746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 14. | Maiques-Tobias E, Tomatis-Souverbielle C, Watson J, Ramilo O, Mejias A. [Disseminated cat scratch disease: The wide variety of clinical presentations]. An Pediatr (Engl Ed). 2019;90:393-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Margileth AM. Recent Advances in Diagnosis and Treatment of Cat Scratch Disease. Curr Infect Dis Rep. 2000;2:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Hempel A, Manzoor F, Petrescu D. Hemophagocytic lymphohistiocytosis secondary to unrecognized Bartonella henselae infection: a case report. Trop Dis Travel Med Vaccines. 2023;9:14. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Koutantou M, Kambas K, Makka S, Fournier PE, Raoult D, Angelakis E. Limitations of Serological Diagnosis of Typical Cat Scratch Disease and Recommendations for the Diagnostic Procedure. Can J Infect Dis Med Microbiol. 2023;2023:4222511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Sander A, Berner R, Ruess M. Serodiagnosis of cat scratch disease: response to Bartonella henselae in children and a review of diagnostic methods. Eur J Clin Microbiol Infect Dis. 2001;20:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Vaca DJ, Dobler G, Fischer SF, Keller C, Konrad M, von Loewenich FD, Orenga S, Sapre SU, van Belkum A, Kempf VAJ. Contemporary diagnostics for medically relevant fastidious microorganisms belonging to the genera Anaplasma,Bartonella,Coxiella,OrientiaandRickettsia. FEMS Microbiol Rev. 2022;46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 20. | Li M, Yan K, Jia P, Wei E, Wang H. Metagenomic next-generation sequencing may assist diagnosis of cat-scratch disease. Front Cell Infect Microbiol. 2022;12:946849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 21. | Biancardi AL, Curi AL. Cat-scratch disease. Ocul Immunol Inflamm. 2014;22:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Conrad DA. Treatment of cat-scratch disease. Curr Opin Pediatr. 2001;13:56-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Shorbatli LA, Koranyi KI, Nahata MC. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |