Published online Dec 16, 2024. doi: 10.12998/wjcc.v12.i35.6782
Revised: August 26, 2024
Accepted: September 9, 2024
Published online: December 16, 2024
Processing time: 85 Days and 8.8 Hours
In recent years, cancer immunotherapy has introduced novel treatments, such as monoclonal antibodies, which have facilitated targeted therapies against tumor cells. Programmed death-1 (PD-1) is an immune checkpoint expressed in T cells that regulates the immune system’s activity to prevent over-activation and tissue damage caused by inflammation. However, PD-1 is also expressed in tumor cells and functions as an immune evasion mechanism, making it a therapeutic target to enhance the immune response and eliminate tumor cells. Consequently, immune checkpoint inhibitors (ICIs) have emerged as an option for certain tumor types. Nevertheless, blocking immune checkpoints can lead to immune-related adverse events (irAEs), such as psoriasis and cytokine release syndrome (CRS), as exemp
Core Tip: The introduction of cancer immunotherapies, particularly the utilization of monoclonal antibodies that inhibit immune checkpoints, has yielded significant benefits, including enhanced survival rates and a diminished likelihood of adverse effects. However, immune-related adverse events can manifest in certain patients, presenting mild symptoms such as fever, fatigue, headache, rash, arthralgia, and myalgia. In more severe cases, circulatory shock or multiorgan failure can occur, which can be mortal. This editorial examines the possible immunologic mechanisms underlying cytokine release syndrome and the exacerbation of psoriasis in patients receiving anti-programmed death-1 monoclonal antibodies.
- Citation: Maldonado-García JL, Fragozo A, Pavón L. Cytokine release syndrome induced by anti-programmed death-1 treatment in a psoriasis patient: A dark side of immune checkpoint inhibitors. World J Clin Cases 2024; 12(35): 6782-6790
- URL: https://www.wjgnet.com/2307-8960/full/v12/i35/6782.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i35.6782
Currently, there is revolutionary progress in cancer therapies, introducing new treatments to eliminate tumor cells, as the "magic bullets" proposed by Paul Ehrlich in the 20th century[1,2]. These treatments are based on biotechnological advances that made it possible to generate immunotherapies using highly-specific monoclonal antibodies or adoptive cell therapy treatments to prolong survival and significantly improving the quality of life of patients[3,4].
Cancer immunotherapy has many advantages such as greater precision and efficacy in eliminating tumor cells, restoration of immune system that contributes to tumor cells killing, and a higher long-term survival rate and lower incidence of adverse effects than conventional treatments[5]. Despite the potential of cancer immunotherapy, we must consider important aspects. Not all patients are suitable candidates for these treatments, and the high costs involved are a significant concern. Furthermore, immunotherapy may lead to immune-mediated complications and unspecific responses and even trigger autoimmune diseases or systemic inflammation, potentially resulting in patient fatalities[5,6].
The case reported by Zhou et al[7] about a patient with advanced gastric cancer who received sintilimab, a monoclonal antibody against programmed death 1 (PD-1), a membrane protein expressed on T lymphocytes, is an example of an immune-mediated complication is t. The patient developed severe rashes accompanied by cytokine release syndrome (CRS). In addition, the patient had a medical history of infantile paralysis, hypertension, diabetes mellitus, and plaque psoriasis. As antitumor therapy, the patient received a regimen of oxaliplatin, capecitabine, and sintilimab. Despite this, the patient developed an exacerbated low-grade skin rash accompanied by fever, with a body temperature of 38 °C. Finally, CRS and psoriasis were diagnosed. This editorial article discusses the immunological mechanisms behind the exacerbation of psoriasis secondary to administration of immune checkpoint inhibitors (ICIs) such as sintilimab. Although immune-mediated side effects to the use of ICI are rare, first-contact physicians and non-oncology specialists who provide clinical follow-up to patients must consider them to provide early interventions upon suspicion of these complications.
The substantial advances in cancer therapies have prolonged patients’ survival and significantly improved their life quality. Novel cancer immunotherapies include adoptive cell therapy (ACT) and ICI[3,4]. ACT involves the transfer of modified or unmodified T cells to eradicate cancer cells. T cell modification is initially performed by extracting T cells from the patient, followed by genetic modification to express specific receptors on their surface[8]. Receptors that can be modified include chimeric antigen receptors (CAR), designed to recognize and bind to specific proteins on the surface of cancer cells. CAR-T receptors contain an antigen recognition domain and an intracellular signaling domain able to activate efficiently and co-stimulate lymphocytes and T cell receptors to recognize specific antigens presented by cancer cells via major histocompatibility complex (MHC)[9]. Meanwhile, ACT without receptor modification consists only of extracting T cells, performing an ex-vivo expansion, and finally infusing the expanded T cells back into the patient to eliminate cancer cells[10].
The mechanism of action of ICIs is based on stimulating the T-cell-mediated response to destroy tumor cells. These treatments stimulate immune responses to cancer cells by blocking immune checkpoints[8]. Immune checkpoints function as an on/off switch of the immune system and maintain the homeostatic balance between suppression and activation to prevent an overactivation of the immune system[11]. As examples of immune checkpoints, we could mention PD-1, programmed death-ligand 1 (PD-L1), cytotoxic T lymphocyte-associated protein 4 (CTLA-4), B7 homolog 3 protein, B7 homolog 4 protein, leukocyte immunoglobulin-like receptor B1, leukocyte immunoglobulin-like receptor B2, lymphocyte activation gene 3, T-cell immunoglobulin and mucin containing protein-3, CD47, CD137, and CD70[11,12]. Immune checkpoint markers are highly expressed in cancer cells and are critical in tumor cells’ immune evasion mechanisms. Furthermore, immune checkpoints favor the maintenance of tumor cell malignancy, promoting self-renewal, epithelial-mesenchymal transition, metastasis, drug resistance, anti-apoptosis, angiogenesis, or improvement of energy metabolism[12,13]. Currently, available ICIs are monoclonal antibodies directed against CTLA-4, PD-1, or PD-L1. CTLA-4 acts as an initial brake on T-cell activation, while PD-1 and PD-L1 prevent excessive T-cell activation and chronic inflammation. ICIs favor increased T-cell activation after blocking PD-1 or CTLA-4 pathways, causing a more effective antitumor response[14-16].
Overactivation of T cells by ICIs can trigger a series of toxic effects known as immune-related adverse events (irAEs)[17]. This can occur in any organ, although they commonly affect barrier organs (i.e., the skin, gastrointestinal tract, lungs, and the liver) due to their direct exposure, and role in metabolism and elimination, as well as the inherent sensitivity to the harmful effects of treatments[17-19]. In this way, patients with autoimmune diseases, such as rheumatoid arthritis or Crohn’s disease, have a higher risk of developing some irAEs secondary to the use of ICI due to the inflammatory envi
The incidence of IrAEs in patients receiving ICIs varies depending on the treatment regimen[25]. For instance, administering anti-CTLA-4 antibodies (ipilimumab) is linked to a 60% risk of developing IrAEs of any severity, with only 10%-30% of cases resulting in severe manifestations[26]. Notably, the occurrence and severity of immune-mediated adverse effects secondary to using anti-CTLA-4 antibodies show a dose-dependent pattern[26,27]. In contrast, the use of anti-PD-1 antibodies is associated with a lower risk of developing IrAEs, with an estimated incidence of approximately 10% among patients. Of these, 5%-20% may experience severe IrAEs[25,28]. The combination of immune checkpoint inhibitors, such as the combination of an anti-CTLA-4 antibody with an anti-PD-1 antibody, increases the severity and incidence of IrAEs by approximately 30%. Moreover, these adverse effects appear at earlier stages of combined treatment compared to monotherapy[25,29].
Cytokine release syndrome (CRS), defined as a systemic inflammatory response due to the release of inflammatory mediators such as cytokines, chemokines, oxygen radicals, complement factors, and coagulation[30,31], was described in the early 1990s when the murine monoclonal antibody directed against the human T-cell receptor CD3 complex, also called Muromonab-CD3 or OKT3, was introduced as an immunosuppressive treatment during solid organ trans
Although the pathophysiology of CRS is not fully understood, the activation of several cell populations, including activated myeloid cells like monocytes/macrophages, dendritic cells, and/or activated lymphocytes such as natural killer cells, T cells, and B cells; and/or non-immune cells, i.e., endothelial cells has been suggested as the main proposal. Moreover, there is a characteristic increase in serum tumor necrosis factor alpha (TNF-α) and interferon-γ during the first 1-2 hours, followed by increases in circulating interleukin (IL)-6 and IL-10, and in some cases, IL-2 and IL-8[30,32,40]. CRS has a broad spectrum of symptoms, varying from mild symptoms, including fever, fatigue, headache, rash, arthralgia, and myalgia[41,42]. Severe CRS cases are characterized by hypotension and high fever. They may progress to a systemic inflammatory response complicated by circulatory shock, vascular leakage, disseminated intravascular coagulation, and multiorgan failure[30].
Psoriasis is a chronic inflammatory disease primarily affecting the skin and joints, characterized by erythema, thickening, and skin scaling[43]. Psoriasis is caused by a complex interplay between genetic factors, external and internal triggers, and immunological factors[44]. The genetic factors of psoriasis involve nine genomic regions (PSORS 1-9) where HLA-C*06: 02 (PSORS 1) is the allele most closely related to disease susceptibility and severity[45]. Immunologically, psoriasis pathophysiology involves a complex interplay between innate and adaptive immune response mechanisms. It has been proposed that psoriasis has a mixed pattern of autoimmune and autoinflammatory disease[46,47], both of which will be discussed below.
External or internal factors can trigger psoriasis. External factors include infections by bacteria such as Staphylococcus aureus and Streptococcus pyogenes, viruses including human papillomavirus or retroviruses, fungi like Malassezia and Candida albicans[48]; skin injuries (cuts and burns)[49]; obesity, smoking or excessive alcohol consumption[50,51]. On the other hand, internal factors include dysbiosis in the skin and gut microbiome[48,52], stress[51], dyslipidemia[53] and, in women, a dysregulation of progesterone and estrogen can exacerbate symptoms[54-56].
External triggers such as skin trauma, dysbiosis, or smoking cause keratinocytes to release antimicrobial peptides such as cathelicidin (LL37)[57], S100 family proteins like S100A7 (psoriacin), S100A8 (calgranulin A), and S100A9 (Calgranulin B)[58] and β-defensins[59]. These antimicrobial peptides bind to the DNA or RNA of damaged cells[60], forming complexes, such as the DNA-LL37, which activates plasmacytoid dendritic cells (pDC) through toll-like receptors (TLR) 7, which triggers the production of type I interferons (IFN), like IFN-α and -β complex[61]. In addition, the RNA-LL37 complex activates myeloid dendritic cells through TLR8, promoting the release of TNF-α, IL-23, and IL-12[60,62]. After recognizing the DNA-LL-37 complex, mature DCs upregulate CCR7 expression and migrate to draining lymph nodes through a process finely regulated by chemokines and their receptors. In lymph nodes, DCs present antigens to naive T cells through the MHC, providing co-stimulatory signals and activating T cell proliferation and differentiation. Simultaneously, IL-23 secreted by activated DCs favors the differentiation of T helper 17 (Th17) cells, known to produce IL-17A/F, IL-22, and TNF-α. Furthermore, IL-12 secreted by DCs induces the differentiation of T helper 1 (Th1) cells, which produce IFN-γ, IL-2, and TNF-α[63-65]. The autoreactive T cells migrate to the epidermis, where they will continue to produce Th1 and Th17 cytokines, which cause the proliferation and activation of keratinocytes, forming the characteristic thickening of the epidermis and the appearance of psoriatic plaques[66].
As previously mentioned, psoriasis includes features of an autoimmune disease, such as antigen presentation via MHC and activation of Th1 and Th17 lymphocytes that favor the activation of phagocytes, such as macrophages or neutrophils. Likewise, psoriasis has features of autoinflammatory disease, such as persistent activation of alarmins and damage signals that activate TLRs, chemotaxis, and activation of monocytes. It is important to emphasize that the activity of Th1 and Th17 lymphocytes feedback to the function of innate cells such as macrophages and neutrophils and vice versa[46,47,67].
In addition to the etiopathogenic mechanisms previously described for psoriasis, other factors that might contribute to the development of the disease include the use of certain drugs such as beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, lithium, anti-malaria drugs, interferons, terbinafine, bupropion, immunosuppressants, and antineoplastics such as nivolumab (monoclonal antibody anti-PD-1) and imatinib[68,69].
Some patients with anti-TNF-α therapy (infliximab, etanercept, adalimumab, and certolizumab) displayed a subtype of psoriasis named paradoxical psoriasis[70,71]. However, the pathophysiology of drug-induced psoriasis remains unclear. Nonetheless, the disease has clinical differences depending on the drug that triggers it. For instance, an increase of IFN-α and pDC in lesions has been observed in paradoxical psoriasis caused by anti-TNF-α therapies; other findings include eczematiform spongiotic pattern, psoriasis-like dermatitis (with infiltration of intraepidermal or subcorneal neutrophils); and lichenoid reaction with focal interface dermatitis[70,71]. In contrast, anti-PD-1-induced psoriasis has histopathological features that are similar to chronic psoriasis, in which a predominance of adaptive immunity is observed, with abundant CD3+, CD8+ T cells and CD11c+ dendritic cells infiltrating the skin lesions, as well as an increase in IL-23, IL-6, TNF-α, IFN-γ, and IL-17[72].
Inhibition of the PD-1 immunomodulatory pathway can result in hyperactivation of Th1 and Th17 lymphocytes[73]. In physiological situations, PD-1 activation inhibits T and B cell signaling pathways, reducing cytokine production and cell proliferation and promoting apoptosis. This mechanism contributes to regulating the immune response, preventing overactivation of the immune system[74]. A remarkable example of this effect is the case presented by Zhou et al[7], in which a patient with advanced gastric cancer and chronic plaque psoriasis developed a severe CRS after treatment with sintilimab, a PD-1 inhibitor.
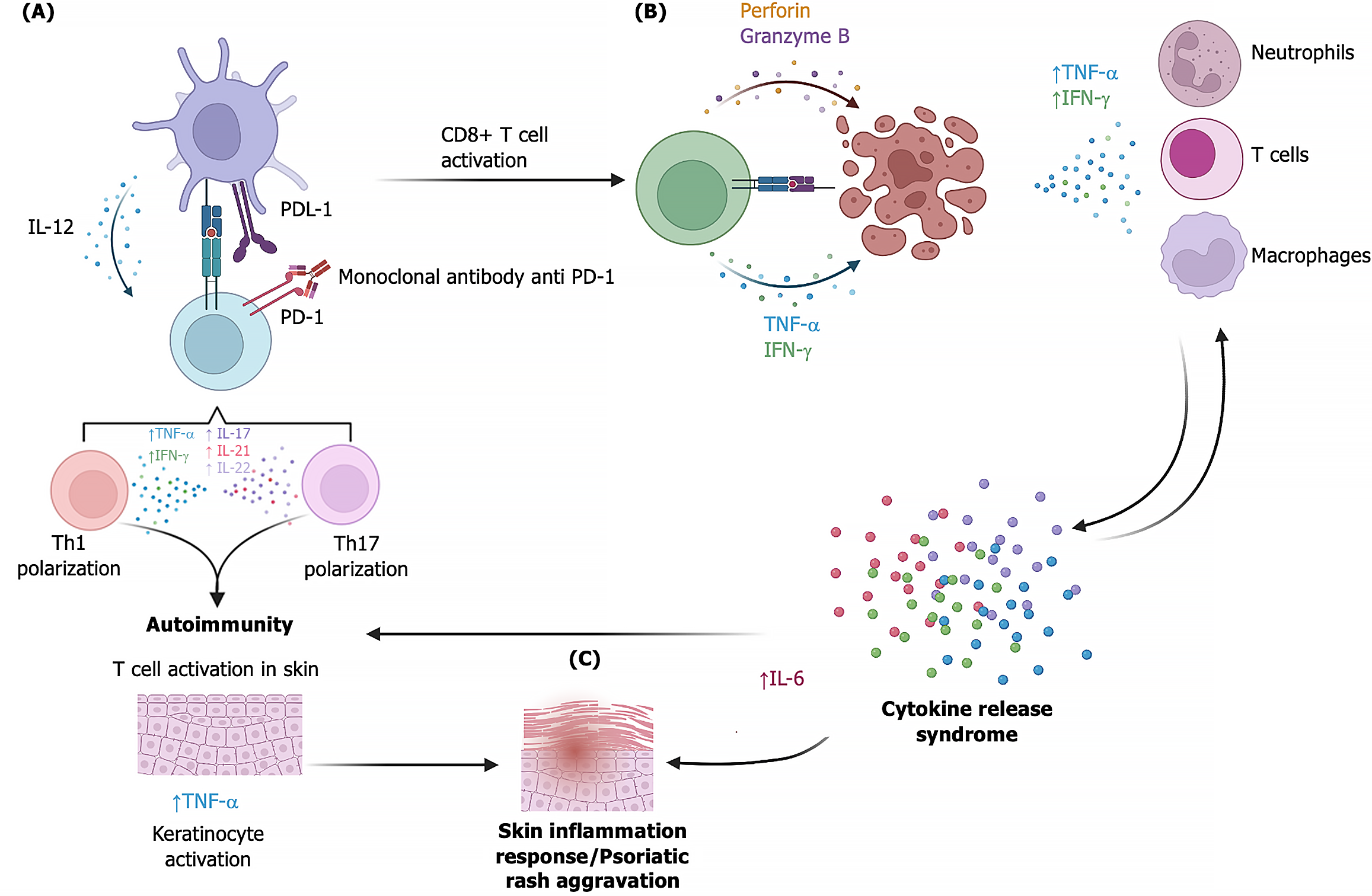
PD-1-inhibitor-induced psoriasis is presumably caused by the activation of various cell populations such as neutrophils, dendritic cells, Th1 and Th7 cells, and Treg cells[75]. In addition, dendritic cells release cytokines such as IFN-γ, IL-1, IL-17, and IL-22, which are associated with the development of de novo psoriasis or exacerbation of psoriasis[61,64,65]. The use of anti-PD-1 monoclonal antibodies can increase the half-life of neutrophils, as well as the increase of Th1 and Th17 cells, leading to a rise in the production of proinflammatory cytokines such as IL-2, IL-6, IL-12, IL-17, IL-22, IFN-γ, among others[75]. Also, over-activation of effector T cells can trigger CRS due to increased IFN-γ, TNF-α, and IL-6[30,76]. The evidence suggests that CRS and Th1/Th17 cell overactivation and IL-6 release are responsible for developing anti-PD-1 induced psoriasis (Figure 1)[75,77,78]. Data obtained from animal models of anti-PD-1-induced psoriasis indicate that IL-6 elevation in plasma plays a crucial role in developing skin lesions and promotes CD8 T-cell infiltration into the epidermis[77]. Furthermore, CD8+ T cells were observed to enhance IFN-γ production resulting in keratinocyte activation[77]. Macrophages also participate in the activation and proliferation of keratinocytes through the release of TNF-α, macrophage migration inhibitory factor, and IL-20, and facilitate the angiogenesis observed in psoriasis by releasing vascular endothelial growth factor, transforming growth factor-β, platelet-derived growth factor, and TNF-α[22,75].
Several studies have evaluated the baseline levels and changes in cytokines in patients with different types of cancer treated with ICI. In patients treated with anti-PD1, no significant differences were observed in TNF-α levels before and after treatment; on the contrary, elevated IL-6 Levels are associated with worse outcomes in terms of treatment response and an increased risk of irAE. However, an increase in IL-1β levels when using an anti-PD1 has been related to a better response, as has IFN-γ[79]. These studies suggest that increased levels of several cytokines, such as IL-1β, IL-6, IFN-γ, and TNF-α after ICI treatment are associated with an increased response rate or an increased predisposition to irAE, which would lead to discontinuation or suppression of Anti-PD1 treatment[79,80]. These results emphasize the importance of monitoring the levels of these cytokines during treatment to identify patients who might benefit from closer follow-up and make a timely therapeutic adjustment. In addition, these findings may help develop more effective strategies to prevent and treat adverse effects in the future.
According to previous reports, most cases of psoriasis induced by anti-PD-1 therapies present as exacerbations of psoriasis, and in some cases, de novo psoriasis lesions appear. The average time of symptom onset has been reported to be 10 weeks. In addition, no correlation has been reported between the severity of the antitumor response and psoriasis symptoms[72,73,75,81].
Anti-PD-1-induced psoriasis is classified into Grade I, Grade II, and Grade III according to the Common Criteria for Adverse Event Evaluation (CTCAE v5.0) classification, which considers the following parameters: Psoriasis area and severity index; body surface area; and investigator's global assessment[82]. Most of these patients develop grades I or II, with only a few reaching grade III. An algorithm has been proposed for the management of these patients. Initially, it is recommended to perform a directed interrogation for a history of psoriasis. If the patient has a history of psoriasis, it is recommended to monitor closely for irAE[73,82,83]. For the treatment of psoriasis, it is recommended that patients with grade 1 receive topical treatment, such as corticosteroids and vitamin D analogs, maintaining their current dose of ICI; patients with grade II should be treated with systemic therapies, such as phototherapy and retinoids, in addition to the measures of grade I treatment, with continuation or adjustment of the dose of ICI. Subsequently, the evolution should be evaluated two weeks later. Patients with Grade III psoriasis should be treated with higher doses of traditional systemic therapy based on grade II management. If treatment fails or the patient shows deterioration, the use of biological drugs such as TNF-α antagonists or IL-17A/IL-23 antagonists (Guselkumab or Secukinumab), can be considered, in addition to which the ICI regimen should be adjusted[83,84].
Although the incidence of anti-PD-1 induced psoriasis is relatively low, it is essential to consider that the use of ICI is not exempt from developing adverse events, in addition to the fact that the clinical follow-up given to these patients should be multidisciplinary to carry out effective interventions and minimize the damage caused by irAE in patients[22,73,81]
Cancer immunotherapy has made significant progress, and novel treatments have emerged to bolster the immune system’s ability to eliminate tumor cells. Immune checkpoints, such as PD-1 or CTLA-4, are crucial in reducing the risk of excessive inflammation. Still, they are also expressed by tumor cells and facilitate evasion of the immune system. Consequently, they have been identified as therapeutic targets for cancer treatment. However, blocking immune checkpoints can lead to irAEs secondary to exacerbated inflammation, which may manifest as skin symptoms like psoriasis. The potential mechanisms underlying anti-PD-1-induced psoriasis, as described by Zhou et al[7] clinical case, involve an immune imbalance resulting from enhanced CD4+ and CD8+ T cell function due to PD-1 blockade. Increased CD4+ T cell activity promotes differentiation of Th1/Th17 cells, while improved CD8+ T cell activity favors antitumor activity that may trigger cytokine release syndrome. Both events contribute to keratinocyte activation through IL-6 and TNF-α, leading to the development of psoriasis lesions. Although these adverse events occur in a minority of patients, they serve as a clear reminder of the imperative for caution and meticulous attention to detail in the clinical evaluation of patients before the administration of immunotherapy with ICIs.
| 1. | Zipfel PF, Skerka C. From magic bullets to modern therapeutics: Paul Ehrlich, the German immunobiologist and physician coined the term 'complement'. Mol Immunol. 2022;150:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 2. | Strebhardt K, Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 858] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 3. | Papież MA, Krzyściak W. Biological Therapies in the Treatment of Cancer-Update and New Directions. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 4. | Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC, Kitui SK, Manyazewal T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021;9:20503121211034366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 692] [Cited by in RCA: 672] [Article Influence: 168.0] [Reference Citation Analysis (0)] |
| 5. | Tan S, Li D, Zhu X. Cancer immunotherapy: Pros, cons and beyond. Biomed Pharmacother. 2020;124:109821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 420] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 6. | Yin Q, Wu L, Han L, Zheng X, Tong R, Li L, Bai L, Bian Y. Immune-related adverse events of immune checkpoint inhibitors: a review. Front Immunol. 2023;14:1167975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 164] [Reference Citation Analysis (0)] |
| 7. | Zhou MH, Ye MF, Zhang ZX, Tao F, Zhang Y. Cytokine release syndrome triggered by programmed death 1 blockade (sintilimab) therapy in a psoriasis patient: A case report. World J Clin Cases. 2024;12:3555-3560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Sahu M, Suryawanshi H. Immunotherapy: The future of cancer treatment. J Oral Maxillofac Pathol. 2021;25:371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 9. | Cappell KM, Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 2023;20:359-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 723] [Cited by in RCA: 614] [Article Influence: 307.0] [Reference Citation Analysis (0)] |
| 10. | Laskowski T, Rezvani K. Adoptive cell therapy: Living drugs against cancer. J Exp Med. 2020;217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Zheng J. Functions of Immune Checkpoint Molecules Beyond Immune Evasion. Adv Exp Med Biol. 2020;1248:201-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 12. | Guo Z, Zhang R, Yang AG, Zheng G. Diversity of immune checkpoints in cancer immunotherapy. Front Immunol. 2023;14:1121285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 13. | Gaikwad S, Agrawal MY, Kaushik I, Ramachandran S, Srivastava SK. Immune checkpoint proteins: Signaling mechanisms and molecular interactions in cancer immunotherapy. Semin Cancer Biol. 2022;86:137-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 14. | Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39:98-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1427] [Cited by in RCA: 1709] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 15. | Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front Oncol. 2018;8:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 586] [Cited by in RCA: 974] [Article Influence: 139.1] [Reference Citation Analysis (0)] |
| 16. | Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, Ladwa R, O'Byrne K, Kulasinghe A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol. 2022;29:3044-3060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 667] [Article Influence: 222.3] [Reference Citation Analysis (0)] |
| 17. | Les I, Martínez M, Pérez-Francisco I, Cabero M, Teijeira L, Arrazubi V, Torrego N, Campillo-Calatayud A, Elejalde I, Kochan G, Escors D. Predictive Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Events. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 18. | Choi J, Lee SY. Clinical Characteristics and Treatment of Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Immune Netw. 2020;20:e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 19. | Wang SJ, Dougan SK, Dougan M. Immune mechanisms of toxicity from checkpoint inhibitors. Trends Cancer. 2023;9:543-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 100] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 20. | Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 602] [Article Influence: 200.7] [Reference Citation Analysis (0)] |
| 21. | Okiyama N, Tanaka R. Immune-related adverse events in various organs caused by immune checkpoint inhibitors. Allergol Int. 2022;71:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 22. | Popa LG, Giurcaneanu C, Portelli MG, Mihai MM, Beiu C, Orzan OA, Ion A, Anghel TH. Perspectives on Psoriasiform Adverse Events from Immune Checkpoint Inhibitors: Lessons Learned from Our Practice. Medicina (Kaunas). 2024;60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Zhang X, Fu Z, Yan C. Cytokine release syndrome induced by pembrolizumab: A case report. Medicine (Baltimore). 2022;101:e31998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Tay SH, Toh MMX, Thian YL, Vellayappan BA, Fairhurst AM, Chan YH, Aminkeng F, Bharwani LD, Huang Y, Mak A, Wong ASC. Cytokine Release Syndrome in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Case Series of 25 Patients and Review of the Literature. Front Immunol. 2022;13:807050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 25. | Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, Kuntzer T, Michielin O, Peters S, Coukos G, Spertini F, Thompson JA, Obeid M. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 1451] [Article Influence: 241.8] [Reference Citation Analysis (0)] |
| 26. | Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, Lebbé C, Ferraresi V, Smylie M, Weber JS, Maio M, Bastholt L, Mortier L, Thomas L, Tahir S, Hauschild A, Hassel JC, Hodi FS, Taitt C, de Pril V, de Schaetzen G, Suciu S, Testori A. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med. 2016;375:1845-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 964] [Cited by in RCA: 1024] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 27. | Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1108] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 28. | Gelao L, Criscitiello C, Esposito A, Goldhirsch A, Curigliano G. Immune checkpoint blockade in cancer treatment: a double-edged sword cross-targeting the host as an "innocent bystander". Toxins (Basel). 2014;6:914-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2210] [Cited by in RCA: 2221] [Article Influence: 222.1] [Reference Citation Analysis (0)] |
| 30. | García Roche A, Díaz Lagares C, Élez E, Ferrer Roca R. Cytokine release syndrome. Reviewing a new entity in the intensive care unit. Med Intensiva (Engl Ed). 2019;43:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Cosenza M, Sacchi S, Pozzi S. Cytokine Release Syndrome Associated with T-Cell-Based Therapies for Hematological Malignancies: Pathophysiology, Clinical Presentation, and Treatment. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 32. | Shah D, Soper B, Shopland L. Cytokine release syndrome and cancer immunotherapies - historical challenges and promising futures. Front Immunol. 2023;14:1190379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 33. | Xing X, Hu X. Risk factors of cytokine release syndrome: stress, catecholamines, and beyond. Trends Immunol. 2023;44:93-100. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Morris G, Bortolasci CC, Puri BK, Marx W, O'Neil A, Athan E, Walder K, Berk M, Olive L, Carvalho AF, Maes M. The cytokine storms of COVID-19, H1N1 influenza, CRS and MAS compared. Can one sized treatment fit all? Cytokine. 2021;144:155593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 35. | Mansouri V, Yazdanpanah N, Rezaei N. The immunologic aspects of cytokine release syndrome and graft versus host disease following CAR T cell therapy. Int Rev Immunol. 2022;41:649-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Hsiao M, Akhtari M, Wang LY. Investigating the Relationship between Cytokine Release Syndrome, Graft-Versus-Host Disease, and One-Year Mortality after Haploidentical Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Tr. 2019;25:S216. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Ceschi A, Noseda R, Palin K, Verhamme K. Immune Checkpoint Inhibitor-Related Cytokine Release Syndrome: Analysis of WHO Global Pharmacovigilance Database. Front Pharmacol. 2020;11:557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 38. | Van De Vyver AJ, Marrer-Berger E, Wang K, Lehr T, Walz AC. Cytokine Release Syndrome By T-cell-Redirecting Therapies: Can We Predict and Modulate Patient Risk? Clin Cancer Res. 2021;27:6083-6094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Frey N, Porter D. Cytokine Release Syndrome with Chimeric Antigen Receptor T Cell Therapy. Biol Blood Marrow Transplant. 2019;25:e123-e127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 313] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 40. | Yildizhan E, Kaynar L. Cytokine release syndrome. J Oncol Sci. 2018;4:134-141. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Gödel P, Shimabukuro-Vornhagen A, von Bergwelt-Baildon M. Understanding cytokine release syndrome. Intensive Care Med. 2018;44:371-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B, von Bergwelt-Baildon MS. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 727] [Cited by in RCA: 1115] [Article Influence: 159.3] [Reference Citation Analysis (0)] |
| 43. | Armstrong AW, Patel M, Li C, Garg V, Mandava MR, Wu JJ. Real-world switching patterns and associated characteristics in patients with psoriasis treated with biologics in the United States. J Dermatolog Treat. 2023;34:2200870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 44. | Xu X, Zhang HY. The Immunogenetics of Psoriasis and Implications for Drug Repositioning. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Man AM, Orăsan MS, Hoteiuc OA, Olănescu-Vaida-Voevod MC, Mocan T. Inflammation and Psoriasis: A Comprehensive Review. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 46. | Liang Y, Sarkar MK, Tsoi LC, Gudjonsson JE. Psoriasis: a mixed autoimmune and autoinflammatory disease. Curr Opin Immunol. 2017;49:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 47. | Kölliker Frers R, Otero-Losada M, Kobiec T, Herrera MI, Udovin L, Kusnier CF, Capani F. Interleukin-1 Links Autoimmune and Autoinflammatory Pathophysiology in Mixed-Pattern Psoriasis. Mediators Inflamm. 2021;2021:2503378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Chen L, Li J, Zhu W, Kuang Y, Liu T, Zhang W, Chen X, Peng C. Skin and Gut Microbiome in Psoriasis: Gaining Insight Into the Pathophysiology of It and Finding Novel Therapeutic Strategies. Front Microbiol. 2020;11:589726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 49. | Sieminska I, Pieniawska M, Grzywa TM. The Immunology of Psoriasis-Current Concepts in Pathogenesis. Clin Rev Allergy Immunol. 2024;66:164-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 64] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 50. | Constantin MM, Bucur S, Mutu CC, Poenaru E, Olteanu R, Ionescu RA, Nicolescu AC, Furtunescu F, Constantin T. The Impact of Smoking on Psoriasis Patients with Biological Therapies in a Bucharest Hospital. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Kamiya K, Kishimoto M, Sugai J, Komine M, Ohtsuki M. Risk Factors for the Development of Psoriasis. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 353] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 52. | Olejniczak-Staruch I, Ciążyńska M, Sobolewska-Sztychny D, Narbutt J, Skibińska M, Lesiak A. Alterations of the Skin and Gut Microbiome in Psoriasis and Psoriatic Arthritis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 53. | Mirghani H, Altemani AT, Altemani ST, Alhatlani JAA, Alsulaimani NMI, AlHuraish DSA, Al Mudhi AHA, Ghabban WJR, Alanazi AH, Alamrani BA. The Cross Talk Between Psoriasis, Obesity, and Dyslipidemia: A Meta-Analysis. Cureus. 2023;15:e49253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 54. | Gratton R, Del Vecchio C, Zupin L, Crovella S. Unraveling the Role of Sex Hormones on Keratinocyte Functions in Human Inflammatory Skin Diseases. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 55. | Liu S, He M, Jiang J, Duan X, Chai B, Zhang J, Tao Q, Chen H. Triggers for the onset and recurrence of psoriasis: a review and update. Cell Commun Signal. 2024;22:108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 56. | Sobolev V, Soboleva A, Denisova E, Denieva M, Dvoryankova E, Suleymanov E, Zhukova OV, Potekaev N, Korsunskaya I, Mezentsev A. Differential Expression of Estrogen-Responsive Genes in Women with Psoriasis. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Yamazaki F. Psoriasis: Comorbidities. J Dermatol. 2021;48:732-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 58. | Liang H, Li J, Zhang K. Pathogenic role of S100 proteins in psoriasis. Front Immunol. 2023;14:1191645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 59. | Kolbinger F, Loesche C, Valentin MA, Jiang X, Cheng Y, Jarvis P, Peters T, Calonder C, Bruin G, Polus F, Aigner B, Lee DM, Bodenlenz M, Sinner F, Pieber TR, Patel DD. β-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139:923-932.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 60. | Grän F, Kerstan A, Serfling E, Goebeler M, Muhammad K. Current Developments in the Immunology of Psoriasis. Yale J Biol Med. 2020;93:97-110. [PubMed] |
| 61. | Armstrong AW, Read C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA. 2020;323:1945-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 1326] [Article Influence: 265.2] [Reference Citation Analysis (0)] |
| 62. | Tokuyama M, Mabuchi T. New Treatment Addressing the Pathogenesis of Psoriasis. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 63. | Antal D, Alimohammadi S, Bai P, Szöllősi AG, Szántó M. Antigen-Presenting Cells in Psoriasis. Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 64. | Boutet MA, Nerviani A, Gallo Afflitto G, Pitzalis C. Role of the IL-23/IL-17 Axis in Psoriasis and Psoriatic Arthritis: The Clinical Importance of Its Divergence in Skin and Joints. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 65. | Conrad C, Gilliet M. Psoriasis: from Pathogenesis to Targeted Therapies. Clin Rev Allergy Immunol. 2018;54:102-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 66. | Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1207] [Article Influence: 109.7] [Reference Citation Analysis (0)] |
| 67. | Schön MP. Adaptive and Innate Immunity in Psoriasis and Other Inflammatory Disorders. Front Immunol. 2019;10:1764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 68. | Milavec-Puretić V, Mance M, Ceović R, Lipozenčić J. Drug induced psoriasis. Acta Dermatovenerol Croat. 2011;19:39-42. [PubMed] [DOI] [Full Text] |
| 69. | Balak DM, Hajdarbegovic E. Drug-induced psoriasis: clinical perspectives. Psoriasis (Auckl). 2017;7:87-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 70. | Lu J, Lu Y. Paradoxical psoriasis: The flip side of idiopathic psoriasis or an autocephalous reversible drug reaction? J Transl Autoimmun. 2023;7:100211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 71. | Vasconcellos JB, Pereira DD, Vargas TJ, Levy RA, Pinheiro GD, Cursi ÍB. Paradoxical psoriasis after the use of anti-TNF in a patient with rheumatoid arthritis. An Bras Dermatol. 2016;91:137-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Morelli M, Carbone ML, Scaglione GL, Scarponi C, Di Francesco V, Pallotta S, De Galitiis F, Rahimi S, Madonna S, Failla CM, Albanesi C. Identification of immunological patterns characterizing immune-related psoriasis reactions in oncological patients in therapy with anti-PD-1 checkpoint inhibitors. Front Immunol. 2024;15:1346687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 73. | Sánchez-Martínez E, Sáez-Belló M, Ochenduszko S, Mateu-Puchades A. Psoriasis and Anti-PD-1 and Anti-PD-L1 Immunotherapy: Three Cases, a Review of the Literature, and a Proposed Management Algorithm. Actas Dermosifiliogr. 2022;113:427-431. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 74. | Kuszczak B, Wróbel T, Wicherska-Pawłowska K, Rybka J. The Role of BCL-2 and PD-1/PD-L1 Pathway in Pathogenesis of Myelodysplastic Syndromes. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 75. | Wan Z, Huang J, Ou X, Lou S, Wan J, Shen Z. Psoriasis de novo or exacerbation by PD-1 checkpoint inhibitors. An Bras Dermatol. 2024;99:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 76. | Zhang Y, Wen X, OuYang Y, Hu Y, Fang X, Zhang J, Yuan Y. Severe cytokine release syndrome induced by immune checkpoint inhibitors in cancer patients - A case report and review of the literature. Heliyon. 2024;10:e24380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 77. | Tanaka R, Ichimura Y, Kubota N, Saito A, Nakamura Y, Ishitsuka Y, Watanabe R, Fujisawa Y, Kanzaki M, Mizuno S, Takahashi S, Fujimoto M, Okiyama N. Activation of CD8 T cells accelerates anti-PD-1 antibody-induced psoriasis-like dermatitis through IL-6. Commun Biol. 2020;3:571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 78. | Alves NRM, Kurizky PS, da Mota LMH, de Albuquerque CP, Esper JT, Campos ASC, Reis VP, Ferro HM, Gil-Jaramillo N, Brito-de-Sousa JP, Leal LCL, Nóbrega OT, Araújo CN, Santos Júnior ACMD, Martins GA, Martins Filho OA, Gomes CM. Elevated serum IL-6 levels predict treatment interruption in patients with moderate to severe psoriasis: a 6-year real-world cohort study. An Bras Dermatol. 2024;99:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Wang M, Zhai X, Li J, Guan J, Xu S, Li Y, Zhu H. The Role of Cytokines in Predicting the Response and Adverse Events Related to Immune Checkpoint Inhibitors. Front Immunol. 2021;12:670391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 80. | Singh R, Koppu S, Perche PO, Feldman SR. The Cytokine Mediated Molecular Pathophysiology of Psoriasis and Its Clinical Implications. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 81. | Voudouri D, Nikolaou V, Laschos K, Charpidou A, Soupos N, Triantafyllopoulou I, Panoutsopoulou I, Aravantinos G, Syrigos K, Stratigos A. Anti-PD1/PDL1 induced psoriasis. Curr Probl Cancer. 2017;41:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 82. | Yan BX, Chen XY, Ye LR, Chen JQ, Zheng M, Man XY. Cutaneous and Systemic Psoriasis: Classifications and Classification for the Distinction. Front Med (Lausanne). 2021;8:649408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 83. | Apalla Z, Nikolaou V, Fattore D, Fabbrocini G, Freites-Martinez A, Sollena P, Lacouture M, Kraehenbuehl L, Stratigos A, Peris K, Lazaridou E, Richert B, Vigarios E, Riganti J, Baroudjian B, Filoni A, Dodiuk-Gad R, Lebbé C, Sibaud V. European recommendations for management of immune checkpoint inhibitors-derived dermatologic adverse events. The EADV task force 'Dermatology for cancer patients' position statement. J Eur Acad Dermatol Venereol. 2022;36:332-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 84. | Rodríguez‐jiménez P, Ibarguren AM, Butrón‐bris B, Martos‐cabrera L, Llamas‐velasco M. Can we treat anti‐PD1 induced psoriasis in patients with active cancer with biologic therapy? Report of three cases. JEADV Clin Pract. 2023;2:379-381. [DOI] [Full Text] |