Published online Dec 6, 2024. doi: 10.12998/wjcc.v12.i34.6721
Revised: September 2, 2024
Accepted: September 9, 2024
Published online: December 6, 2024
Processing time: 134 Days and 22.3 Hours
The combination of immune checkpoint inhibitors and chemotherapy has shown promising results for the treatment of advanced biliary tract cancer (BTC). Based on the results of the TOPAZ-1 trial, a gemcitabine and cisplatin plus durvalumab (GCD) regimen was recently approved as first-line therapy for patients with advanced BTC. However, post-GCD conversion surgery has not been previously studied. Herein, we describe a case of advanced intrahepatic cholangiocarcinoma (ICC) successfully treated with radical surgery after GCD.
A 65-year-old female diagnosed with advanced ICC with periductal infiltration into the hepatic hilum underwent eight cycles of GCD, followed by durvalumab maintenance treatment, with mild adverse events. Partial response was obtained. Subsequently, a conversion surgery with extended left hepatectomy and bile duct resection was performed. The resection margins were negative, and the patho
We describe the case of a patient who received successful conversion surgery after GCD treatment for advanced ICC.
Core Tip: We report a case of an advanced intrahepatic cholangiocarcinoma (ICC) successfully treated with radical surgery after gemcitabine and cisplatin plus durvalumab. The present case was pathologically classified as a small duct type. This rare case highlights the potential role of conversion surgery in the multimodal treatment strategies for advanced ICC.
- Citation: Igata Y, Kudo M, Kojima M, Kami S, Aoki K, Satake T, Kobayashi T, Sugimoto M, Kobayashi S, Konishi M, Gotohda N. Conversion surgery after gemcitabine and cisplatin plus durvalumab for advanced intrahepatic cholangiocarcinoma: A case report. World J Clin Cases 2024; 12(34): 6721-6727
- URL: https://www.wjgnet.com/2307-8960/full/v12/i34/6721.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i34.6721
Following the TOPAZ-1 trial in 2022, gemcitabine and cisplatin plus durvalumab (GCD) was approved as first-line regimen for advanced biliary tract cancer (BTC) in Japan. This trial showed that GCD significantly prolonged overall and progression-free survival in patients with advanced BTC compared to gemcitabine and cisplatin alone[1]. However, conversion surgery after GCD has not been reported. Herein, we describe a case of advanced intrahepatic cholangiocarcinoma (ICC) successfully treated with radical surgery after eight cycles of GCD and maintenance durvalumab treatment.
Blood test abnormality on routine examination.
A 65-year-old woman presented with elevated gamma-glutamyl transpeptidase levels during routine blood tests.
The patient had a history of hypertension and dyslipidemia.
The patient did not have a history of viral hepatitis. Her family was free from any cancerous conditions.
The physical examination was unremarkable.
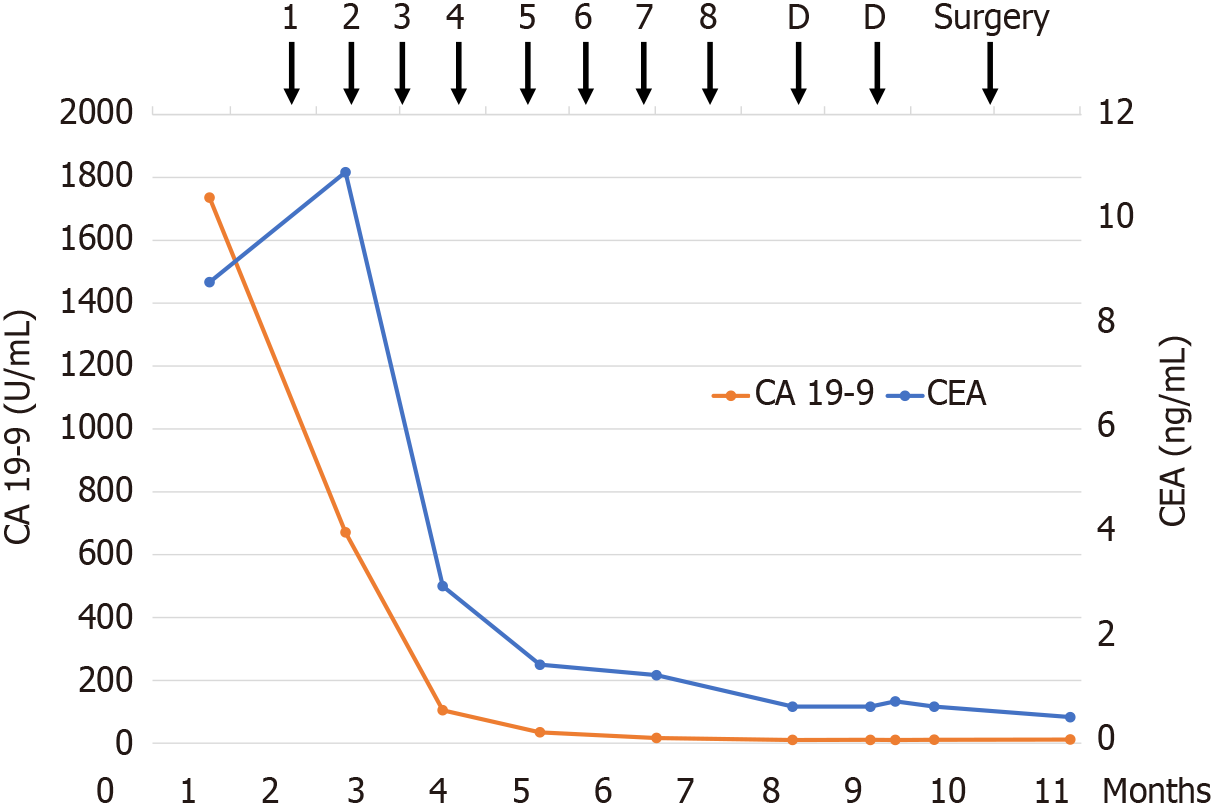
Laboratory results showed elevated carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) levels, at 8.8 ng/mL and 1736.0 U/mL, respectively. We performed a cancer genomic profiling test that showed stable microsate
Abdominal ultrasonography revealed the presence of a liver tumor. Suspicion of ICC in the left lobe of the liver was raised, based on imaging studies, and the patient was referred to our hospital. The clinical stage was cT4N0M0 IIIB based on the 8th edition of the Union for International Cancer Control staging system[2].
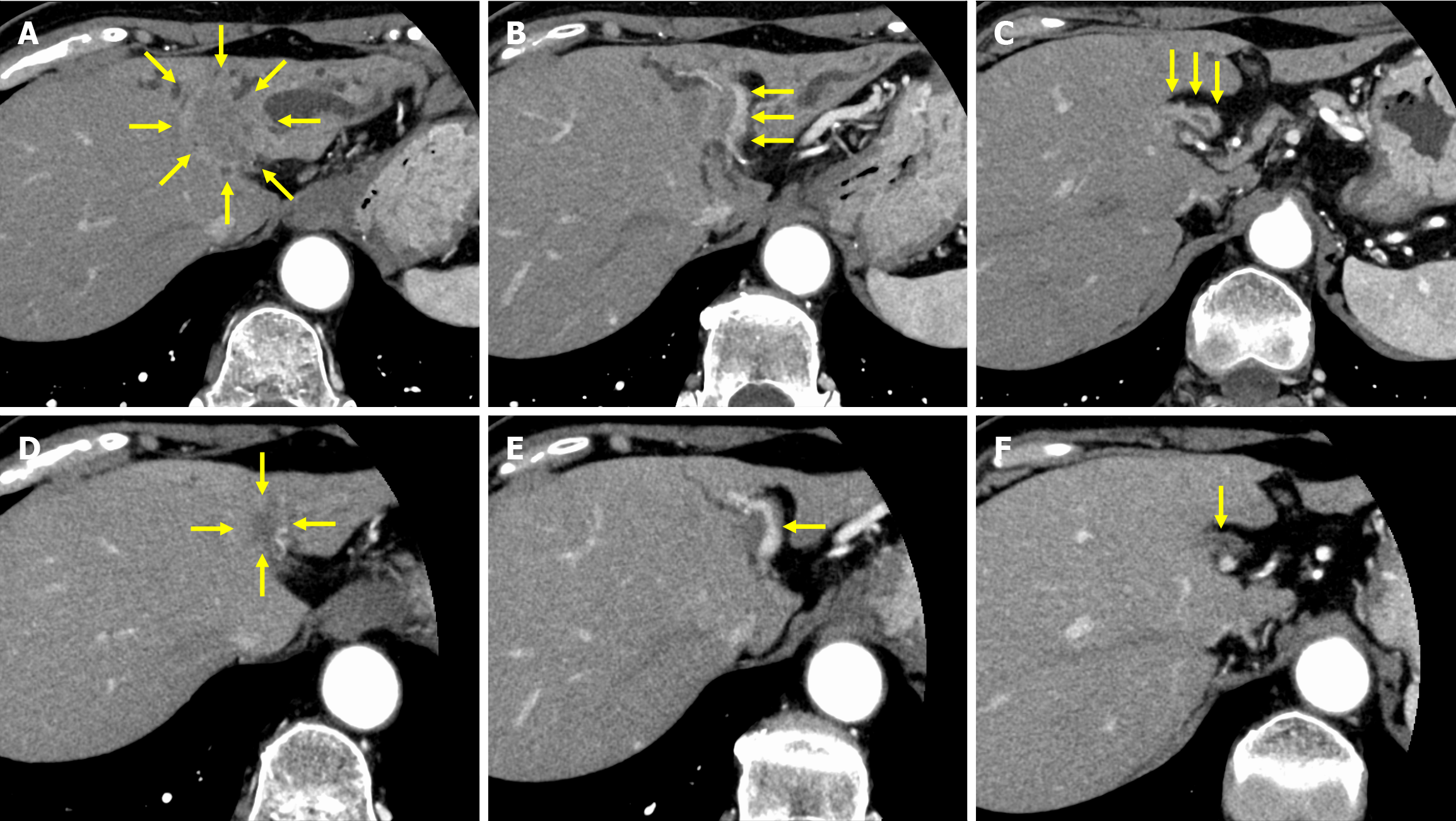
Dynamic computed tomography (CT) revealed dilated intrahepatic bile ducts in the lateral and medial segments and a 22-mm irregular, ill-defined tumor at the obstruction site of the left hepatic duct, with suspected direct invasion of the extrahepatic bile duct. Bile duct wall enhancement continuous from the tumor extended contralaterally to the anterior and posterior sectoral ducts and distally to the confluence of the cystic duct. Tumor invasion into the left hepatic artery and portal vein was suspected, suggesting periductal infiltration to the hepatic hilum. These radiographic findings were compatible with mass-forming and periductal-infiltrating ICC (Figure 1A–C). No liver metastases were detected by contrast-enhanced magnetic resonance imaging. Endoscopic ultrasonography-guided biopsy was performed on the left hepatic duct, and pathological examination revealed adenocarcinoma.
A multidisciplinary team meeting was held and the patient was diagnosed with advanced ICC.
Systemic chemotherapy and immunotherapy with GCD was initiated.
Gemcitabine (1000 mg/m2) and cisplatin (25 mg/m2) were administered on days 1 and 8 of each 21-d cycle. Durvalumab was administered at a dose of 1500 mg every 21 days and adverse events were assessed and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0[3].
After four cycles of treatment, CA 19-9 levels were normalized to 35.0 U/mL. After nine treatment cycles, contrast-enhanced CT showed substantial tumor shrinkage, while the bile duct wall enhancement had significantly decreased (Figure 1D–F). The tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors and it was a partial response[4]. As the level of the tumor marker, CA 19-9, was within the reference range, the patient underwent resection after decision taken in a multidisciplinary team meeting. Regarding treatment-related adverse events, grade 3 neutropenia was observed along with nausea (grade 1), fatigue (grade 1), diarrhea (grade 1), and anemia (grade 2). All adverse events were well tolerated. Preoperative liver function testing showed a Child–Pugh score of 5 points (class A), an indocyanine green retention rate at 15 minutes of 9.6%. Therefore, left hepatectomy and caudate lobectomy with bile duct resection were performed (Figure 2).
An upper midline incision was made. After the operability assessment, the hepatoduodenal ligament was dissected with common bile duct division at the superior border of the pancreatic head. Frozen sections of the common bile duct were sent for rapid biopsy to confirm the negative margins. A lymphadenectomy of the portal triad region was performed. The left and middle hepatic arteries and the left portal vein were divided separately. The left liver was mobilized by dividing the falciform and triangular ligaments. Subsequently, the caudate lobe was mobilized from the inferior vena cava by ligating the short hepatic veins. The ductus venosus was ligated into the root of the hepatic vein. The left hepatic vein was divided using a surgical stapling device. Liver parenchymal transection along the middle hepatic vein was performed using an intermittent Pringle maneuver, and the right hepatic duct was divided with a scalpel for complete resection of the left liver and caudate lobe. After confirmation of a negative right hepatic duct margin on examination of a frozen section, Roux-en-Y hepaticojejunostomy was performed. The operative time was 256 minutes, and the estimated blood loss was 50 mL.
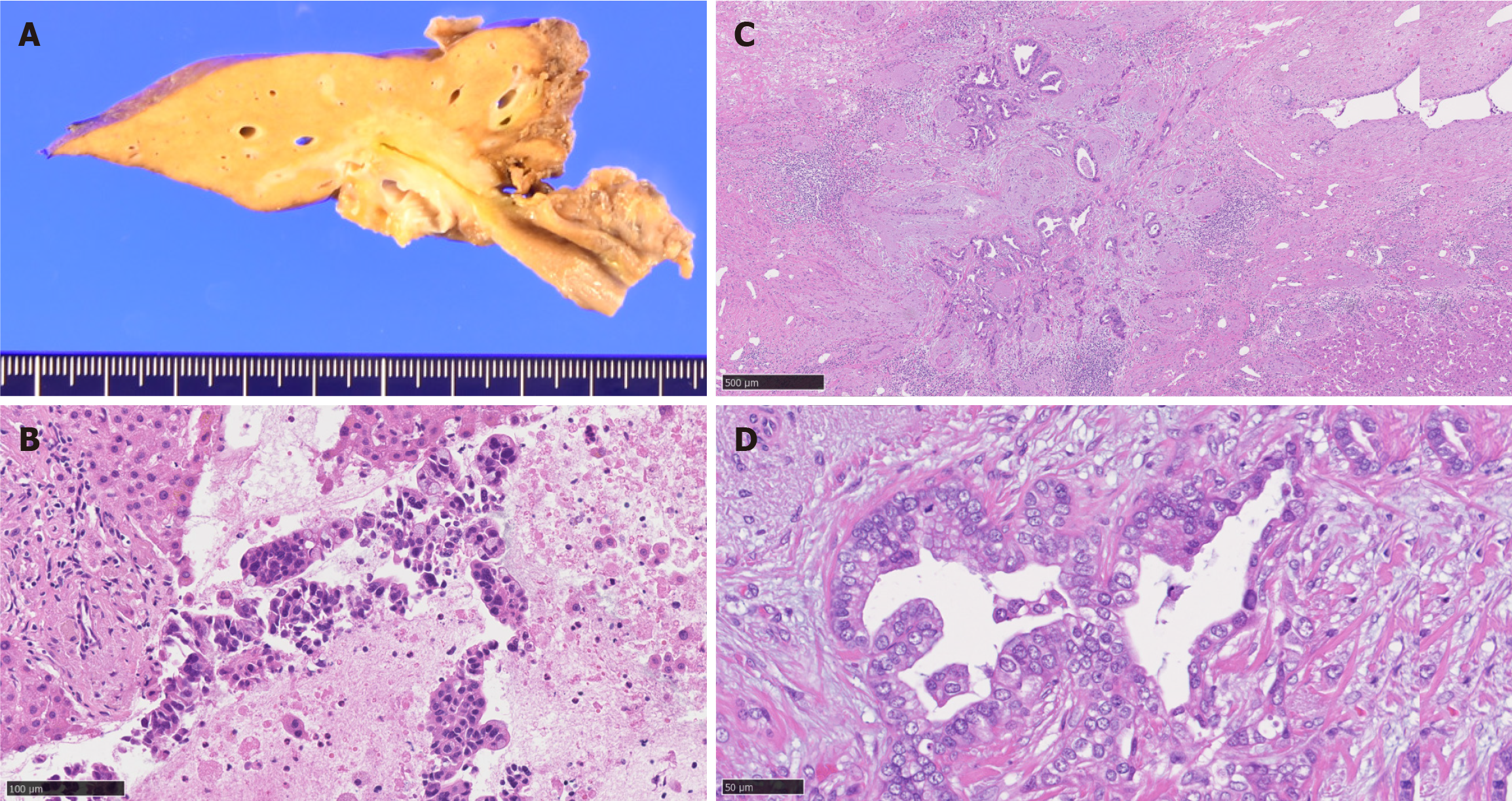
Pathological examination confirmed an 18-mm tumor comprising a moderately differentiated adenocarcinoma without lymph node metastasis. The tumor exhibited smaller ductular-like structures lined by small nonmucinous cuboidal cells, leading to the sub-classification of “small duct type” according to the latest World Health Organization (WHO) criteria[5]. The resection margins were negative (Figure 3). The postoperative course was uneventful and the patient was discharged 13 days postoperatively. Adjuvant S-1 chemotherapy was scheduled; however, the patient was readmitted for the treatment of a hepatic abscess 1 month postoperatively. The abscess was treated and at 8 months postoperatively the patient remained disease-free. Since no cases describing administration of chemotherapy after the conversion surgery are available, adjuvant treatment was not recommended and the patient was observed during follow-up.
To the best of our knowledge, this is the first case report describing a patient with advanced ICC who underwent conversion surgery with radical liver resection after GCD chemo-immunotherapy. The surgical margins were clear, and the ICC was pathologically classified as small duct type[5].
Evidence for conversion surgery in advanced ICC is lacking owing to the aggressive disease biology and relatively low response rates to current standard treatment regimens. The KHBO1401 trial compared a triplet regimen of gemcitabine, cisplatin, and S-1 chemotherapy with a doublet regimen of gemcitabine and cisplatin. Nakamura et al[6] reported a conversion rate of 3.3%, with a 1-year overall survival rate of 87.5% in the conversion surgery group vs 56.0% in the control group. Authors concluded that conversion surgery could be a potential treatment option for unresectable and recurrent BTC[6]. The results of a single-arm phase-2 trial using gemcitabine, cisplatin, and nab-paclitaxel for advanced BTC showed that 21% (8/38) of patients with ICC ultimately underwent resection[7]. The findings of a phase-3 trial comparing gemcitabine, cisplatin, and nab-paclitaxel with gemcitabine and cisplatin alone did not show significant improvement in overall survival (median 14 months vs 12.7 months, respectively); however, a tendency toward improved overall response rates was reported for the experimental treatment arm (34% vs 25%, respectively, P = 0.11)[8]. According to literature review on conversion surgery for BTC, R0 resection rates are estimated at 30.8%–100%, with a median disease-free survival at 14.4–26 months, and median overall survival at 10.8–50.1 months; these figures support conversion surgery as a potentially efficient treatment strategy[9]. Therefore, conversion surgery could improve survival rates of selected patients with BTC who respond to systemic therapy.
The addition of immunotherapy to conventional chemotherapy confers with improved outcomes for patients with advanced BTC. Real-world data analysis of GCD for advanced BTC revealed an overall response rate of 34.5%, consistent with results from the TOPAZ-I trial[10]. The efficacy of combined immunotherapy and chemotherapy has also been demonstrated in the KEY-NOTE-966 phase-3 study that tested gemcitabine and cisplatin plus pembrolizumab in patients with BTC[11]. An interim analysis from the TOPAZ-1 trial showed that the addition of durvalumab to gemcitabine and cisplatin did not have a detrimental effect on patient-reported outcomes, supporting GCD as an effective and tolerable treatment regimen[12].
In the TOPAZ-I trial, the number of patients in the GCD arm with a sustained response decreased from 88.9% at 3 months to 59.3% at 6 months. Although cases of a durable response due to the addition of immune checkpoint inhibitors have been reported, efforts are needed to focus on opportunities for conversion surgery[1]. Further research is required to determine the appropriate timing for conversion surgery.
According to the WHO, ICC can be pathologically classified into small and large duct types[5]. The present case was classified as a small duct type. The response to palliative chemotherapy is more favorable in small than in large duct ICC[13]. Furthermore, the immune microenvironment of a tumor is categorized as cold or hot, depending on the degree of CD8+ T-cell infiltration; hot tumors are associated with a better response to immune checkpoint inhibitors[14]. The relationship between histological classification and the immune microenvironment has been described, while small duct ICC is associated with a hot immune phenotype, which is consistent with the good response observed in our patient[15]. To date, three case reports on conversion surgery after combination of pembrolizumab and chemotherapy have been published, although the histological duct type classification was not described[16-18]. We did not investigate the immune microenvironment of this tumor, and lack of this information constitutes a limitation of this report. Further studies are required to elucidate the tumor microenvironment in patients with BTC.
GCD chemoimmunotherapy aided in downstaging advanced small duct type ICC, while conversion surgery was successful in the case presented. Further trials and reports are warranted to elucidate the efficacy and validity of multimodal treatment strategies, including conversion surgery, for patients with advanced ICC.
The authors are especially grateful to the patient and her family.
| 1. | Oh DY, Ruth He A, Qin S, Chen LT, Okusaka T, Vogel A, Kim JW, Suksombooncharoen T, Ah Lee M, Kitano M, Burris H, Bouattour M, Tanasanvimon S, McNamara MG, Zaucha R, Avallone A, Tan B, Cundom J, Lee CK, Takahashi H, Ikeda M, Chen JS, Wang J, Makowsky M, Rokutanda N, He P, Kurland JF, Cohen G, Valle JW. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022;1:EVIDoa2200015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 524] [Article Influence: 174.7] [Reference Citation Analysis (1)] |
| 2. | Brierley JD, Gospodarowicz MK, Wittekind C. Skin Tumours. TNM Online. 2017;. [DOI] [Full Text] |
| 3. | National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. CTEP 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 [Accessed March 29, 2024]. |
| 4. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21548] [Article Influence: 1346.8] [Reference Citation Analysis (1)] |
| 5. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2409] [Article Influence: 481.8] [Reference Citation Analysis (3)] |
| 6. | Nakamura I, Hatano E, Baba H, Kamei K, Wada H, Shimizu J, Kanai M, Yoshimura K, Nagano H, Ioka T. Impact of conversion surgery after chemotherapy in patients with initially unresectable and recurrent biliary tract cancer. Ann Gastroenterol Surg. 2023;7:1009-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, Raghav KPS, Iwasaki M, Masci P, Ramanathan RK, Ahn DH, Bekaii-Saab TS, Borad MJ. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 341] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 8. | Shroff RT, Guthrie KA, Scott AJ, Borad MJ, Goff LW, Matin K, Mahipal A, Kalyan A, Javle MM, Aghajanian C, Tan BR, Cheema PS, Patel AK, Iyer RV, Kelley RK, Thumar JR, El-khoueiry AB, Chiorean EG, Hochster HS, Philip PA. SWOG 1815: A phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. J Clin Oncol. 2023;41:LBA490-LBA490. [DOI] [Full Text] |
| 9. | Oh MY, Kim H, Choi YJ, Byun Y, Han Y, Kang JS, Sohn H, Lee JM, Kwon W, Jang JY. Conversion surgery for initially unresectable extrahepatic biliary tract cancer. Ann Hepatobiliary Pancreat Surg. 2021;25:349-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Rimini M, Fornaro L, Lonardi S, Niger M, Lavacchi D, Pressiani T, Lucchetti J, Giordano G, Pretta A, Tamburini E, Pirrone C, Rapposelli IG, Diana A, Martinelli E, Garajová I, Simionato F, Schirripa M, Formica V, Vivaldi C, Caliman E, Rizzato MD, Zanuso V, Nichetti F, Angotti L, Landriscina M, Scartozzi M, Ramundo M, Pastorino A, Daniele B, Cornara N, Persano M, Gusmaroli E, Cerantola R, Salani F, Ratti F, Aldrighetti L, Cascinu S, Rimassa L, Antonuzzo L, Casadei-Gardini A. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer: An early exploratory analysis of real-world data. Liver Int. 2023;43:1803-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 29.5] [Reference Citation Analysis (1)] |
| 11. | Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, Verslype C, Bouattour M, Park JO, Barajas O, Pelzer U, Valle JW, Yu L, Malhotra U, Siegel AB, Edeline J, Vogel A; KEYNOTE-966 Investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401:1853-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 451] [Article Influence: 225.5] [Reference Citation Analysis (0)] |
| 12. | Burris HA 3rd, Okusaka T, Vogel A, Lee MA, Takahashi H, Breder V, Blanc JF, Li J, Bachini M, Żotkiewicz M, Abraham J, Patel N, Wang J, Ali M, Rokutanda N, Cohen G, Oh DY. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer (TOPAZ-1): patient-reported outcomes from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2024;25:626-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 40] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 13. | Yoon JG, Kim MH, Jang M, Kim H, Hwang HK, Kang CM, Lee WJ, Kang B, Lee CK, Lee MG, Chung HC, Choi HJ, Park YN. Molecular Characterization of Biliary Tract Cancer Predicts Chemotherapy and Programmed Death 1/Programmed Death-Ligand 1 Blockade Responses. Hepatology. 2021;74:1914-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 14. | De Guillebon E, Dardenne A, Saldmann A, Séguier S, Tran T, Paolini L, Lebbe C, Tartour E. Beyond the concept of cold and hot tumors for the development of novel predictive biomarkers and the rational design of immunotherapy combination. Int J Cancer. 2020;147:1509-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Kawamura E, Matsubara T, Kawada N. New Era of Immune-Based Therapy in Intrahepatic Cholangiocarcinoma. Cancers (Basel). 2023;15:3993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Li X, Jiang Z, Wu Y, Gong W, Liao X, Li X. Case report: Conversion therapy for advanced intrahepatic cholangiocarcinoma using PD-1 inhibitor plus S-1 and nab-paclitaxel. Front Oncol. 2022;12:935817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Zhang W, Luo C, Zhang ZY, Zhang BX, Chen XP. Conversion therapy for advanced intrahepatic cholangiocarcinoma with lenvatinib and pembrolizumab combined with gemcitabine plus cisplatin: A case report and literature review. Front Immunol. 2022;13:1079342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Zhang X, Chen X, Wang Z, Meng X, Hoffmann-Sommergruber K, Cavallari N, Wu Y, Gao J, Li X, Chen H. Goblet cell-associated antigen passage: A gatekeeper of the intestinal immune system. Immunology. 2023;170:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |