Published online Nov 26, 2024. doi: 10.12998/wjcc.v12.i33.6620
Revised: September 5, 2024
Accepted: September 11, 2024
Published online: November 26, 2024
Processing time: 130 Days and 7.2 Hours
Mucocutaneous separation (MCS) is a common postoperative complication in enterostomy patients, potentially leading to significant morbidity. Early identification of risk factors is crucial for preventing this condition. However, predictive models for MCS remain underdeveloped.
To construct a risk prediction model for MCS in enterostomy patients and assess its clinical predictive accuracy.
A total of 492 patients who underwent enterostomy from January 2019 to March 2023 were included in the study. Patients were divided into two groups, the MCS group (n = 110), and the non-MCS (n = 382) based on the occurrence of MCS within the first 3 weeks after surgery. Univariate and multivariate analyses were used to identify the independent predictive factors of MCS and the model constructed. Receiver operating characteristic curve analysis was used to assess the model’s performance.
The postoperative MCS incidence rate was 22.4%. Suture dislodgement (P < 0.0001), serum albumin level (P < 0.0001), body mass index (BMI) (P = 0.0006), hemoglobin level (P = 0.0409), intestinal rapture (P = 0.0043), incision infection (P < 0.0001), neoadjuvant therapy (P = 0.0432), stoma site (P = 0.0028) and elevated intra-abdominal pressure (P = 0.0395) were potential predictive factors of MCS. Suture dislodgement [P < 0.0001, OR: 28.0075 95%CI: (11.0901-82.1751)], serum albumin level (P = 0.0008, OR: 0.3504, 95%CI: [0.1902-0.6485]), BMI [P = 0.0045, OR: 2.1361, 95%CI: (1.2660-3.6235)], hemoglobin level [P = 0.0269, OR: 0.5164, 95%CI: (0.2881-0.9324)], intestinal rapture [P = 0.0351, OR: 3.0694, 95%CI: (1.0482-8.5558)], incision infection [P = 0.0179, OR: 0.2885, 95%CI: (0.0950-0.7624)] and neoadjuvant therapy [P = 0.0112, OR: 1.9769, 95%CI: (1.1718-3.3690)] were independent predictive factors and included in the model. The model had an area under the curve of 0.827 and good clinical utility on decision curve analysis.
The mucocutaneous separation prediction model constructed in this study has good predictive performance and can provide a reference for early warning of mucocutaneous separation in enterostomy patients.
Core Tip: In this study a risk prediction model for mucocutaneous separation in enterostomy patients was developed, identifying key predictive factors such as suture dislodgement, serum albumin levels, and body mass index. The model demonstrated strong predictive accuracy with an area under the curve of 0.827, offering a valuable tool for early intervention and improved patient outcomes in clinical practice.
- Citation: Liu Y, Li H, Wu JJ, Ye JH. Risk factors and risk prediction model for mucocutaneous separation in enterostomy patients: A single center experience. World J Clin Cases 2024; 12(33): 6620-6628
- URL: https://www.wjgnet.com/2307-8960/full/v12/i33/6620.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i33.6620
Enterostomy is one of the most commonly performed surgeries by gastrointestinal surgeons. It involves temporary or permanent diversion of the patient’s intestinal tract to an artificial opening (stoma) in the abdominal wall as a means of excreting intestinal contents[1]. According to statistics, hundreds of thousands of people worldwide undergo enterostomy surgery each year due to conditions such as inflammation, trauma, or intestinal cancer, with the number of patients undergoing ostomy surgery in China exceeding one hundred thousand annually, and the total number currently exceeding one million[2]. However, enterostomy also imposes varying degrees of physiological and psychological burdens on patients, severely affecting their quality of life and imposing significant economic burdens on families and society[3,4]. Leakage of intestinal contents after enterostomy surgery can lead to serious complications, with reported complication rates ranging from 10% to 50%[5,6]. Mucocutaneous separation (MCS) of enterostomy is one of the common early complications following enterostomy surgery, with its occurrence frequency accounting for 4% to 24% of entero
MCS, the separation of the edge of the intestinal stoma mucosa from the sutured area of the abdominal wall skin, commonly occurs within 1 to 3 weeks after surgery. Incomplete tissue healing at the suture site results in an open wound between the mucosa and the skin[7]. The overall incidence rate of MCS outside China ranges from 3.7% to 9.7%[8,9], while in China, it is reported to be 16.33%[10]. MCS often leads to a series of adverse reactions of varying degrees, including acute peritonitis, incision infection, and stoma retraction. Additionally, scarring produced after the healing of MCS can cause stoma stenosis or retraction[11]. When MCS occurs, the reduced adhesion effect at the separation site impairs the healing process, not only slowing down wound healing but also increasing damage to the skin around the stoma. The occurrence of MCS after enterostomy prolongs the patient’s hospital stay, causing physical and psychological distress to patients while dramatically increasing the difficulty, satisfaction, and workload of nursing care. Therefore, early detection and management of MCS post-enterostomy are of paramount importance for patients, their families, and healthcare providers.
Previous studies on MCS vary in terms of included variables and sample sizes, with most being retrospective studies and controversial in their conclusions. To date, there have been no reports on the establishment of a risk assessment model for MCS. This study aims to explore risk factors for MCS, establish a risk prediction assessment model, and assess the accuracy of the model.
A prospective cohort was established, including patients who underwent enterostomy at our center from January 2019 to March 2023. Participants were included based on the following inclusion criteria: Patients determined to need enterostomy after admission; Aged 18 years and above; Patients without mental disorders, capable of normal communication and interaction; Able to maintain regular contact for follow-up. Participants were excluded based on the following exclusion criteria: Had prior abdominal surgery; Occurrence of severe complications such as pulmonary embolism, myocardial infarction, or stroke after surgery; critically ill or deceased patients; Were lost to follow-up. This study was approved by the ethics committee of our hospital. All participants provided informed consent.
An expert team consisting of ten experienced gastroenterologists was set up to evaluate participants’ clinical presentation to determine whether MCS had occurred or not. All members of the team were blinded by the research objectives and details.
Determination of MCS was based on expert consensus[12] and relevant guidelines[13]. The criteria for defining MCS, its clinical manifestations, and predisposing factors were summarized in the assessment criteria for MCS. Predisposing factors were defined as poor peristomal tissue healing resulting in an open wound between the skin and mucosa. A normal stoma exhibits glossiness, redness, and slight convexity, with tight adhesion between the peristomal mucosa and skin. The surrounding skin color is normal and intact. Clinical symptoms of MCS include varying degrees of separation depth ranging from 1 to 1.4 cm within the subcutaneous abdominal wall layers. White or yellowish odorless fluid may exude from the separation site.
Statistical analysis was conducted using R version 4.1.1. Continuous data are presented as mean ± SD and were compared between groups using the independent sample t-test. Categorical data are reported as absolute numbers and percentages. Categorical variables were compared using the χ2 test. Univariable logistic regression was used to identify potential predictive factors of MCS and multivariate logistic regression was then used to identify independent predictors of MCS. The independent predictors were then used to construct a binomial logistic predictive model. Receiver operating characteristic (ROC) curve analysis was also used to evaluate the model’s performance. Decision curve analysis (DCA) was used to assess the utility of the model. The model was expressed as a nomogram. P values < 0.05 were considered statistically significant.
A total of 548 participants were initially included, however, 56 were excluded due to being critically ill (n = 6), deceased
| Variable | Non-MCS (n = 382) | MCS (n = 110) | P value |
| Sex | 0.4106 | ||
| Male | 241 (63.1) | 64 (58.2) | |
| Female | 141 (36.9) | 46 (41.8) | |
| Age (years) | |||
| < 60 | 115 (30.1) | 35 (31.8) | 0.8209 |
| ≥ 60 | 267 (69.9) | 75 (68.25) | |
| Intestinal rapture | 14 (3.7) | 12 (10.95) | 0.0059 |
| Diabetic | 89 (23.3) | 26 (23.6) | 1.0000 |
| Immunotherapy | 153 (40.0) | 45 (40.9) | 0.9592 |
| Neoadjuvant therapy | 153 (40.0) | 56 (50.9) | 0.0548 |
| Suture dislodgement | 42 (11.0) | 63 (57.3) | < 0.0001 |
| Incision infection | 49 (12.8) | 42 (38.2) | < 0.0001 |
| BMI (kg/m2) | |||
| < 24 | 257 (67.3) | 54 (49.1) | 0.0007 |
| ≥ 24 | 125 (32.7) | 56 (50.9) | |
| Stoma site | |||
| Ileum | 228 (59.7) | 83 (75.4) | 0.0036 |
| Colon | 154 (40.3) | 27 (24.6) | |
| Elevated intra-abdominal pressure | 183 (47.9) | 65 (59.1) | 0.0501 |
| Hemoglobin level | |||
| ≤ 90 | 82 (21.5) | 34 (30.9) | 0.0501 |
| > 90 | 300 (78.5) | 76 (69.1) | |
| Albumin level | |||
| ≤ 28 | 54 (14.1) | 34 (30.9) | < 0.0001 |
| > 28 | 328 (85.9) | 76 (69.1) |
Based on the univariate logistic regression analysis, suture dislodgement (P < 0.0001), serum albumin level (P < 0.0001), BMI (P = 0.0006), hemoglobin level (P = 0.0409), intestinal rapture (P = 0.0043), incision infection (P < 0.0001), neoadjuvant therapy (P = 0.0432), stoma site (P = 0.0028) and elevated intra-abdominal pressure (P = 0.0395) were determined to be potential predictive factors of MCS. These were included in the multivariate logistic regression analysis. Following multivariate logistic regression analysis, suture dislodgement [P < 0.0001, OR: 28.0075 95%CI: (11.0901-82.1751)], serum albumin level [P = 0.0008, OR: 0.3504, 95%CI: (0.1902-0.6485)], BMI [P = 0.0045, OR: 2.1361, 95%CI: (1.2660-3.6235)], hemoglobin level [P = 0.0269, OR: 0.5164, 95%CI: (0.2881-0.9324)], intestinal rapture [P = 0.0351, OR: 3.0694, 95%CI: (1.0482-8.5558)], incision infection [P = 0.0179, OR: 0.2885, 95%CI: (0.0950-0.7624)] and neoadjuvant therapy [P = 0.0112, OR: 1.9769, 95%CI: (1.1718-3.3690)] were determined to be independent predictive factors of MCS. The results of univariate and multivariate logistic regression analysis are presented in Table 2.
| Variable | Univariate, P value | Multivariate, P value | OR | 95%CI | |
| Lower | Upper | ||||
| Suture dislodgement | < 0.0001 | < 0.0001 | 28.0075 | 11.0901 | 82.1751 |
| Serum albumin | < 0.0001 | 0.0008 | 0.3504 | 0.1902 | 0.6485 |
| BMI | 0.0006 | 0.0045 | 2.1361 | 1.2660 | 3.6235 |
| Hemoglobin | 0.0409 | 0.0269 | 0.5164 | 0.2881 | 0.9324 |
| Intestinal rapture | 0.0043 | 0.0351 | 3.0694 | 1.0482 | 8.5558 |
| Incision infection | < 0.0001 | 0.0179 | 0.2885 | 0.0950 | 0.7624 |
| Neoadjuvant therapy | 0.0432 | 0.0112 | 1.9769 | 1.1718 | 3.3690 |
| Stoma site | 0.0028 | 0.1996 | 0.6908 | 0.3881 | 1.2071 |
| Elevated intra-abdominal pressure | 0.0395 | 0.1194 | 1.5162 | 0.2881 | 0.9324 |
| Diabetic | 0.9412 | - | - | - | - |
| Age | 0.7309 | - | - | - | - |
| Immunotherapy | 0.8717 | - | - | - | - |
| Sex | 0.3506 | - | - | - | - |
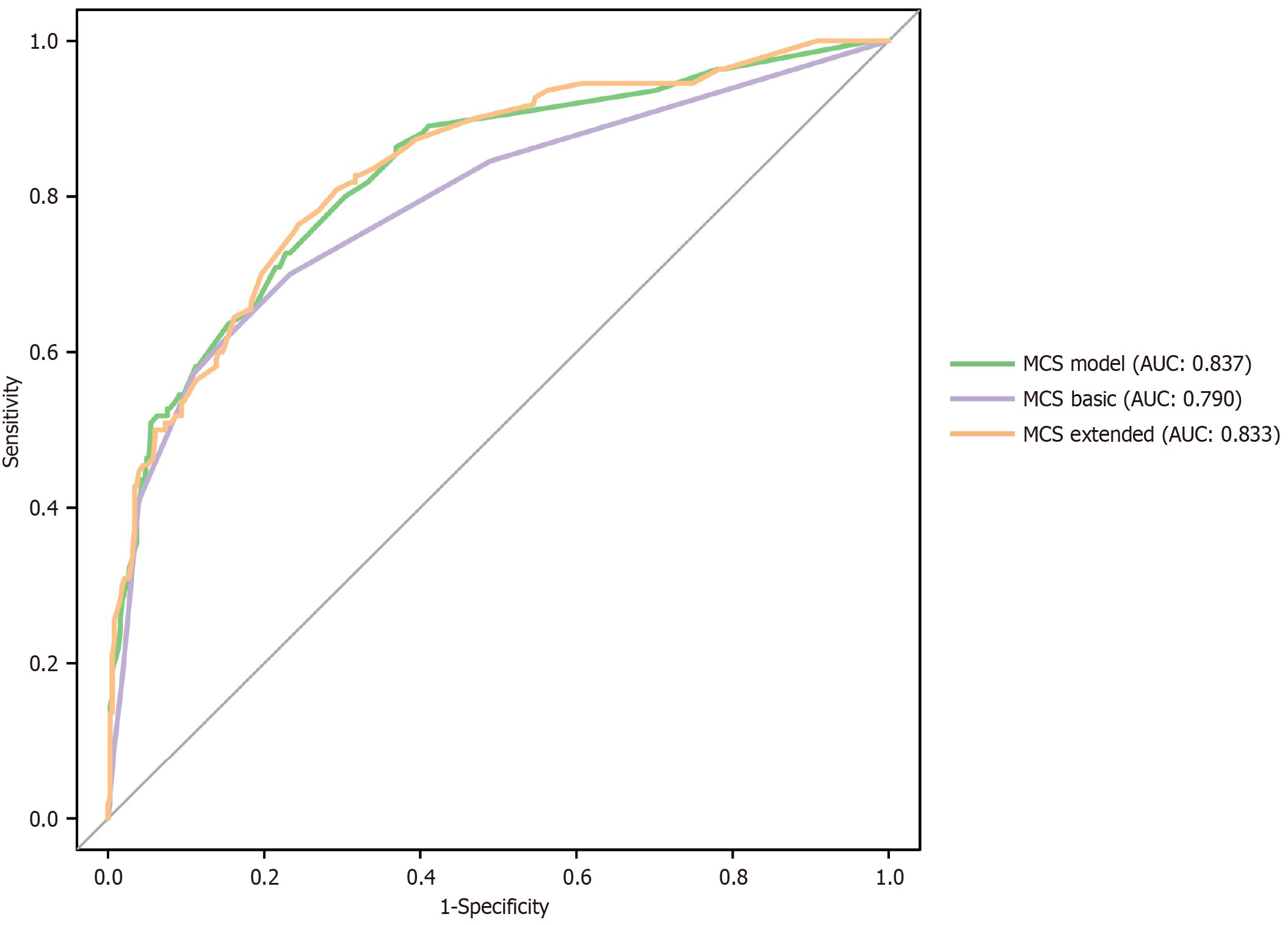
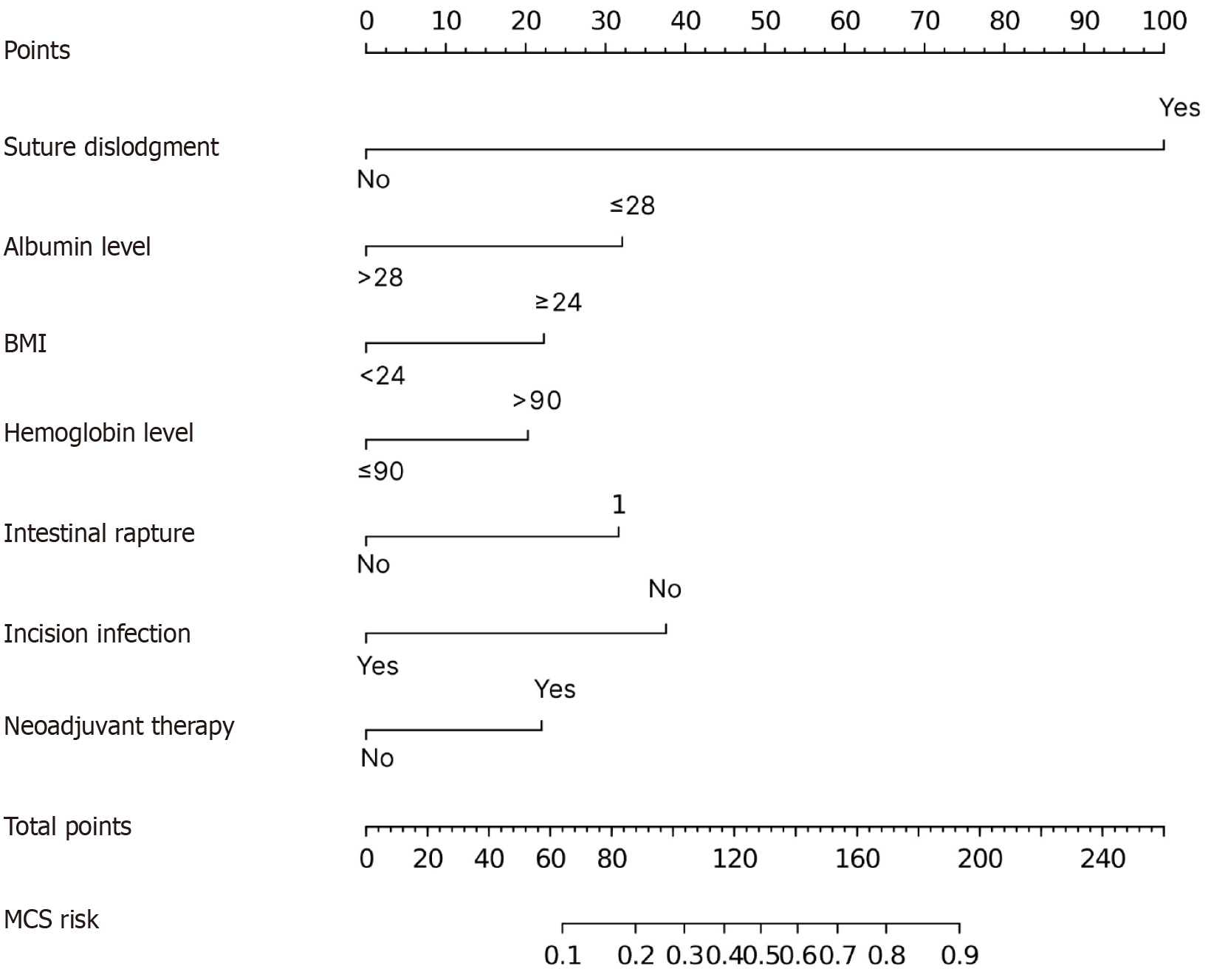
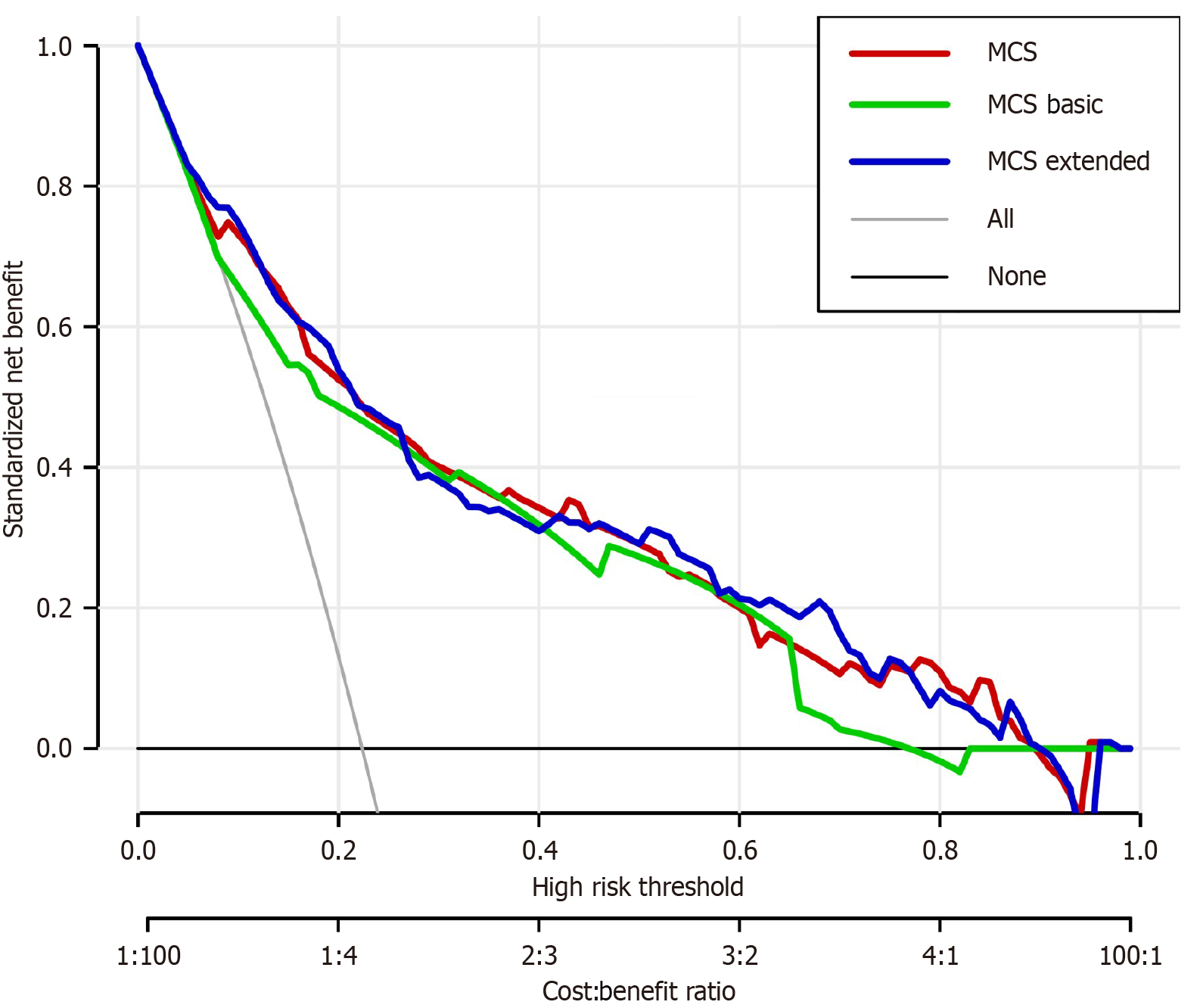
Based on the factors identified above, we constructed first a logistic model including only the independent predictive factors (suture dislodgement, serum albumin level, BMI, hemoglobin level, intestinal rapture, incision infection and neoadjuvant therapy) to predict MCS (MSC model). The nomogram had an area under the curve (AUC) of 0.827. For comparison, we also constructed a simplified predictive model including only suture dislodgement, albumin level and BMI (MCS Basic) and an extended model that included all the predictive factors identified from univariate analysis (MCS Extended). These two models had an AUC: 0.790 and AUC: 0.833, respectively. There was no significant difference in performance between the MCS model and the MCS extended models (P = 0.266). Both the MCS model and MCS Extended were significantly better than MCS Basic (P = 0.005 and P = 0.004, respectively). ROC curves for the models are shown in Figure 1. The MCS model was expressed as a nomogram (Figure 2). Nomograms of MCS Extended and models are shown in Supplementary Figures 1 and 2. Clinical utility of the models was compared using DCA. Overall, the MCS model had the best clinical utility compared to the other two models. However, the clinical utility at risk threshold between 0.4 and 0.8 was better in the extended model. Below the risk threshold of 0.4, MSC model and MSC Extended were comparable. Above the 0.8 risk threshold the MCS model was better. DCA analysis results are shown in Figure 3.
Enterostomy surgery is commonly used in the treatment of inflammatory bowel dis- ease, intestinal rupture, intestinal obstruction, rectal and anal tumors, and other diseases. Recent studies have shown that high Duke stage, preoperative neoadjuvant chemoradiotherapy, diabetes, inflammatory bowel disease, immunosuppressants, steroids, BMI, albumin levels, and regular follow-up visits by patients are high-risk factors for complications in enterostomy skin. Previous studies have shown that compared to colostomy, patients with ileostomy have higher daily stool output, which is often pasty or watery, carrying various digestive enzymes, making it easy to damage the skin[14].
The results of this study indicate that the risk of MCS in patients with ileostomy is significantly higher than in patients with colostomy. Therefore, for patients with ileostomy who have a large amount of stool output and rapid dissolution of the adhesive base, an enhanced adhesive base should be used, and the ostomy support rod should be removed promptly within one month after surgery. Surgical reasons causing ischemic necrosis of the intestinal mucosa, intraoperative use of electrosurgical devices, and obesity leading to incisional fat liquefaction can all cause skin and mucosal separation[15]. Peristomal fat liquefaction, peristomal abscesses, and infection at the site of enterostomy skin and mucosal sutures significantly increase the probability of MCS[16]. The results of this study show that partial necrosis of the enterostomy intestinal mucosa, detachment of enterostomy mucosal sutures, subcutaneous fluid infection around the enterostomy, liquefaction of local tissue around the enterostomy, and incision infection all increase the risk of MCS. It is recommended that when patients have symptoms of infection, secretions from the wound should be collected for bacterial culture, and antibiotic treatment should be administered according to the doctor’s orders. With good intestinal function, solid food intake should be increased to prevent fecal contamination of the wound. Physicians should remove ineffective sutures at the site of MCS, and clean and remove necrotic tissue from the wound.
When the enterostomy roll edge leaks or becomes pale, it should be replaced promptly to prevent fecal leakage and delay wound healing. When serum albumin levels are < 35 g/L, the risk of postoperative site infection will increase by 2.5-fold[17]. Another study found that preoperative low albumin levels are independent risk factors for deep skin and mucosal separation[18]. Multiple studies have shown that patients in a malnourished state have low immune function and should receive enteral or parenteral nutrition support before and after surgery to improve immune function and effectively prevent skin and mucosal separation[19,20]. The results of this study indicate that when serum albumin levels are low, indicating malnutrition, the probability of MCS occurrence significantly increases. Poor nutritional status in patients is a high-risk factor for MCS occurrence. It is recommended to strengthen patient health education, provide dietary guidance to patients, and enhance patient awareness of nutritional supplementation and dietary balance.
This study was based on a prospective design, employing uniform criteria to identify the occurrence of MCS, and to avoid subjective bias, outcome assessment was conducted blindly. Additionally, predictive variables were rigorously selected by an expert team following relevant guidelines[21]. Through extensive literature review, expert consultations, and preliminary investigations, predictive factor variables were selected. Single-factor analysis and multi-factor analysis were conducted to obtain the strongest combination of variables for joint prediction. Furthermore, the measurement methods for the included predictive variables were simple, data acquisition was relatively convenient, and the model exhibited good reproducibility and operability. In addition to these findings, the potential of Negative Pressure Wound Therapy (NPWT) as a treatment for complicated cases of MCS is noteworthy. A recent case series by Ding et al[22] demonstrated the effectiveness of NPWT in managing moderate to severe MCS following ileal conduit urinary diversion. Their study showed that NPWT not only prevented infection but also facilitated the healing process in patients with significant MCS. This suggests that NPWT could be considered a therapeutic option in severe cases of MCS, particularly where conventional treatments may be insufficient. Future studies should explore the broader applicability of NPWT in MCS management following enterostomy surgery.
While this study offers valuable insights into the risk factors and predictive model for MCS in enterostomy patients, several limitations should be acknowledged. Firstly, as a single-center study, the findings may not be generalizable to other populations or settings, where different surgical techniques, postoperative care protocols, and patient demographics may influence the incidence and risk factors of MCS. Additionally, although the study employed a prospective design and rigorous criteria for identifying MCS, the potential for selection bias remains, as patients who were critically ill, deceased, or lost to follow-up were excluded from the analysis. Moreover, the reliance on expert assessment for determining MCS may introduce some level of subjectivity, despite the blinding procedures used. Lastly, while the model demonstrated good predictive accuracy, external validation in diverse clinical settings is necessary to confirm its broader applicability. Future studies should also explore the inclusion of additional variables, such as genetic predispositions and long-term patient outcomes, to enhance the robustness of the model.
MCS often occurs within 1 to 3 weeks after enterostomy surgery. If MCS is not promptly addressed, it can lead to complications such as irritant dermatitis, stoma retraction, stoma stenosis, and even stoma reconstruction, which can significantly increase the psychological and economic burden on patients and their families, causing immense suffering. The MCS risk prediction model developed in this study demonstrates good predictive performance, providing a scientific basis for early warning and precise prevention of MCS complications. It can guide the development of MCS prevention and intervention strategies. However, this study has certain limitations. It only underwent temporal validation, and the number of modeling samples needs to be further increased. The external validity of this model needs to be verified in different hospitals.
| 1. | Hesp WL, Lubbers EJ, de Boer HH, Hendriks T. Enterostomy as an adjunct to treatment of intra-abdominal sepsis. Br J Surg. 1988;75:693-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med. 2015;66:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 3. | Cheng C, Chan NY, Chio JH, Chan P, Chan AO, Hui WM. Being active or flexible? Role of control coping on quality of life among patients with gastrointestinal cancer. Psychooncology. 2012;21:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Recalla S, English K, Nazarali R, Mayo S, Miller D, Gray M. Ostomy care and management: a systematic review. J Wound Ostomy Continence Nurs. 2013;40:489-500; quiz E1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Chun LJ, Haigh PI, Tam MS, Abbas MA. Defunctioning loop ileostomy for pelvic anastomoses: predictors of morbidity and nonclosure. Dis Colon Rectum. 2012;55:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Tamura K, Matsuda K, Yokoyama S, Iwamoto H, Mizumoto Y, Murakami D, Nakamura Y, Yamaue H. Defunctioning loop ileostomy for rectal anastomoses: predictors of stoma outlet obstruction. Int J Colorectal Dis. 2019;34:1141-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Nastro P, Knowles CH, McGrath A, Heyman B, Porrett TR, Lunniss PJ. Complications of intestinal stomas. Br J Surg. 2010;97:1885-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Tsujinaka S, Tan KY, Miyakura Y, Fukano R, Oshima M, Konishi F, Rikiyama T. Current Management of Intestinal Stomas and Their Complications. J Anus Rectum Colon. 2020;4:25-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (2)] |
| 9. | Miyo M, Takemasa I, Ikeda M, Tujie M, Hasegawa J, Ohue M, Kato T, Mizushima T, Doki Y, Mori M. The influence of specific technical maneuvers utilized in the creation of diverting loop-ileostomies on stoma-related morbidity. Surg Today. 2017;47:940-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Ayik C, Bişgin T, Cenan D, Manoğlu B, Özden D, Sökmen S. Risk factors for early ostomy complications in emergency and elective colorectal surgery: A single-center retrospective cohort study. Scand J Surg. 2024;113:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Stelton S. CE: Stoma and Peristomal Skin Care: A Clinical Review. Am J Nurs. 2019;119:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Parini D, Bondurri A, Ferrara F, Rizzo G, Pata F, Veltri M, Forni C, Coccolini F, Biffl WL, Sartelli M, Kluger Y, Ansaloni L, Moore E, Catena F, Danelli P; Multidisciplinary Italian Study group for STOmas (MISSTO). Surgical management of ostomy complications: a MISSTO-WSES mapping review. World J Emerg Surg. 2023;18:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 13. | Wound, Ostomy and Continence Nurses Society; Guideline Development Task Force. WOCN Society Clinical Guideline: Management of the Adult Patient With a Fecal or Urinary Ostomy-An Executive Summary. J Wound Ostomy Continence Nurs. 2018;45:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Gray M, Black JM, Baharestani MM, Bliss DZ, Colwell JC, Goldberg M, Kennedy-Evans KL, Logan S, Ratliff CR. Moisture-associated skin damage: overview and pathophysiology. J Wound Ostomy Continence Nurs. 2011;38:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 15. | D'Ambrosio F, Pappalardo C, Scardigno A, Maida A, Ricciardi R, Calabrò GE. Peristomal Skin Complications in Ileostomy and Colostomy Patients: What We Need to Know from a Public Health Perspective. Int J Environ Res Public Health. 2022;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Colwell JC, McNichol L, Boarini J. North America Wound, Ostomy, and Continence and Enterostomal Therapy Nurses Current Ostomy Care Practice Related to Peristomal Skin Issues. J Wound Ostomy Continence Nurs. 2017;44:257-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Yuwen P, Chen W, Lv H, Feng C, Li Y, Zhang T, Hu P, Guo J, Tian Y, Liu L, Sun J, Zhang Y. Albumin and surgical site infection risk in orthopaedics: a meta-analysis. BMC Surg. 2017;17:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Li J, Liu X, Chen J. Risk Factors of Enterostomy Infection Caused by Bacterial Infection through Mathematical Modelling-Based Information Data Analysis. J Healthc Eng. 2021;2021:4634659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 19. | Liu XJ, Han J, Su X. Influence of continuous nursing on surgical site wound infection and postoperative complication for colorectal cancer patients with stoma: A meta-analysis. Int Wound J. 2024;21:e14480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 20. | Tsujinaka S, Suzuki H, Miura T, Sato Y, Murata H, Endo Y, Hoshi K, Sato Y, Shibata C. Diagnosis, Treatment, and Prevention of Ileostomy Complications: An Updated Review. Cureus. 2023;15:e34289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 21. | Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2833] [Cited by in RCA: 3305] [Article Influence: 330.5] [Reference Citation Analysis (0)] |
| 22. | Ding J, Zhu Y, Ge H, Chen H, Wang L, Xie S, Zhang S, Deng Y, Yang R, Guo H. Negative Pressure Wound Therapy for Patients With Complicated Mucocutaneous Separation Following Ileal Conduit Urinary Diversion: A Case Series. J Wound Ostomy Continence Nurs. 2023;50:420-426. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |