Published online Nov 16, 2024. doi: 10.12998/wjcc.v12.i32.6551
Revised: June 24, 2024
Accepted: August 28, 2024
Published online: November 16, 2024
Processing time: 231 Days and 4.2 Hours
Immunoglobulin G4-related disease (IgG4-RD) is a complex immune-mediated condition that causes fibrotic inflammation in several organs. A significant clinical feature of IgG4-RD is hypertrophic pachymeningitis, which manifests as inflammation of the dura mater in intracranial or spinal regions. Although IgG4-RD can affect multiple areas, the spine is a relatively rare site compared to the more frequent involvement of intracranial structures.
A 70-year-old male presented to our hospital with a two-day history of fever, altered mental status, and generalized weakness. The initial brain magnetic resonance imaging (MRI) revealed multiple small infarcts across various cerebral regions. On the second day after admission, a physical examination revealed motor weakness in both lower extremities and diminished sensation in the right lower extremity. Electromyographic evaluation revealed findings consistent with acute motor sensory neuropathy. Despite initial management with intravenous immunoglobulin for presumed Guillain-Barré syndrome, the patient exhibited progressive worsening of motor deficits. On the 45th day of hospitalization, an enhanced MRI of the entire spine, focusing specifically on the thoracic 9 to lumbar 1 vertebral level, raised the suspicion of IgG4-related spinal pachymeningitis. Subsequently, the patient was administered oral prednisolone and participated in a comprehensive rehabilitation program that included gait training and lower extremity strengthening exercises.
IgG4-related spinal pachymeningitis, diagnosed on MRI, was treated with corticosteroids and a structured rehabilitation regimen, leading to significant improvement.
Core Tip: This case of immunoglobulin G4-related spinal pachymeningitis with neurological symptoms provides valuable insights into diagnosis and treatment strategies in rehabilitative medicine. Detailed examination of this rare condition highlights the importance of considering immunoglobulin G4-related disease in patients with unexplained spinal and neurological symptoms. It emphasizes the need for comprehensive diagnostic approaches, including advanced imaging techniques and electromyography, to ensure accurate diagnosis. Additionally, the case underscores the potential for effective treatment through steroid therapy and rehabilitation, significantly improving patient outcomes. This information helps clinicians recognize and manage similar cases, enhancing patient care in rehabilitative settings.
- Citation: Chae TS, Kim DS, Kim GW, Won YH, Ko MH, Park SH, Seo JH. Immunoglobulin G4-related spinal pachymeningitis: A case report. World J Clin Cases 2024; 12(32): 6551-6558
- URL: https://www.wjgnet.com/2307-8960/full/v12/i32/6551.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i32.6551
Immunoglobulin (Ig) G4, the least abundant of the four IgG subclasses, comprises only about 1%-4% of the total IgG immunoglobulins in the human body[1]. IgG4-related disease (IgG4-RD) is an immune-mediated disorder characterized by progressive multi-organ fibrosis. This condition is characterized by the accumulation of IgG4 lymphocytes, leading to an extensive extracellular matrix characterized by a dense lymphoplasmacytic infiltrate, rich in IgG4+ plasma cells and CD4+ T cells[1,2]. This inflammation often results in tumor-like lesions in various organs[3]. Although eosinophils were observed within the immune cell infiltrates, they were not predominant[4]. The pancreas is frequently affected in IgG4-RD[5], along with other common sites such as the bile duct, salivary and lacrimal glands, kidneys, lungs, and thyroid gland. Neurological manifestations include peripheral and central nervous system involvement, often presenting as localized or diffuse thickening of the cranial or spinal structures[6-11]. Spinal lesions, although less common than intracranial lesions[12], can mimic conditions such as spinal metastasis or hemorrhage[13], leading to frequent misdiagnoses. This report describes a rare case of spinal IgG4-related pachymeningitis coexisting with Guillain-Barré syndrome and multifocal embolic infarction.
A 70-year-old male patient presented to our hospital with a 2-day history of fever, mental deterioration, and general weakness.
The patient’s symptoms started two days prior to admission.
Approximately ten years ago, the patient was diagnosed with mitral regurgitation and aortic stenosis. Consequently, he underwent aortic valve replacement surgery and has since been on anticoagulant therapy.
The patient had no relevant personal or family history.
Neurological examination upon admission indicated that the patient was disoriented, manifested mildly decreased motor power in all extremities, as evidenced by a manual motor test (MMT) grade of 4, and showed signs of meningeal irritation, including neck stiffness.
Laboratory studies showed an increased segmental neutrophil percentage and C-reactive protein levels (77.30% and 234.36 mg/L, respectively), and blood cultures were positive for Staphylococcus aureus. Cerebrospinal fluid examination revealed a white blood cell count of 66/mm3, predominantly neutrophils (79%), and elevated protein levels (117 mg/dL).
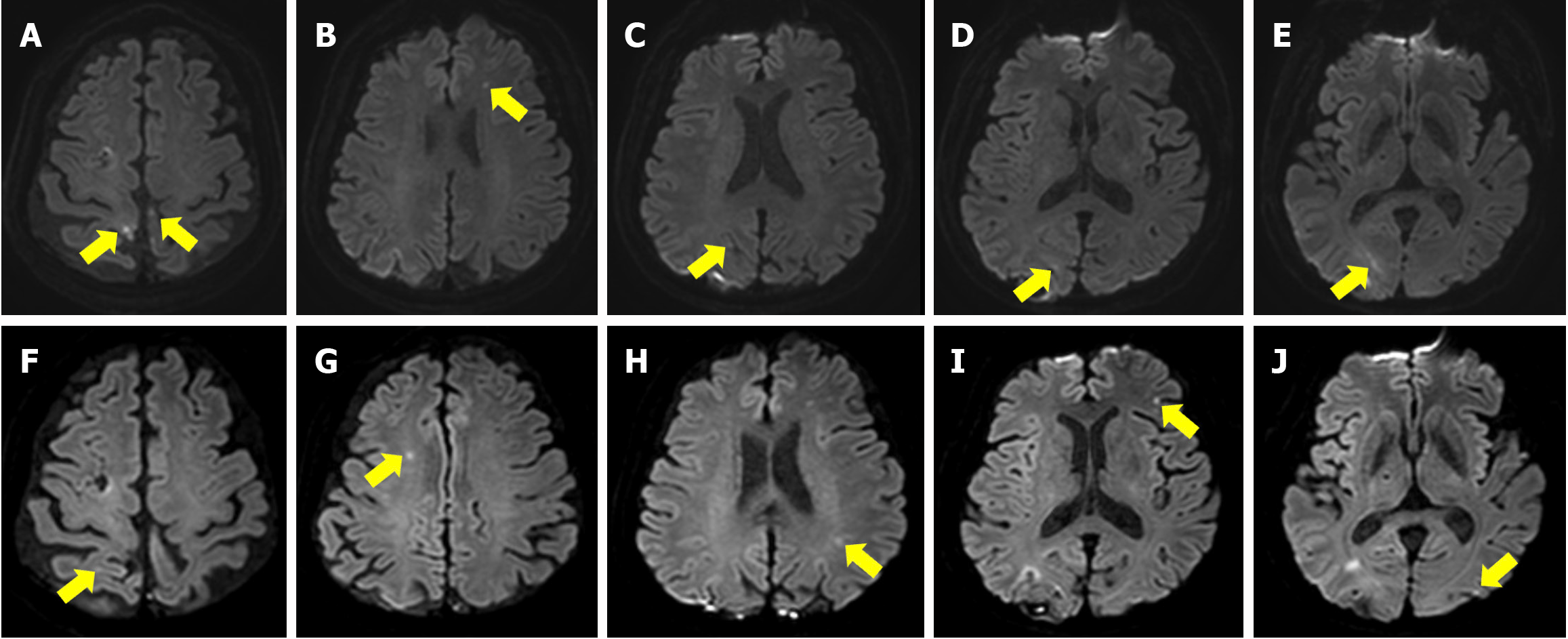
Brain magnetic resonance imaging (MRI) revealed small multifocal infarctions in the right occipital, frontal, and bilateral parietal lobes (Figure 1). Echocardiography revealed a well-functioning prosthetic mechanical aortic valve (AV) and mitral valve (MV) with an ejection fraction of 55%.
Based on these findings, bacterial encephalitis and multifocal infarction were suspected, and antibiotics, antiviral agents, and antiplatelet agents were administered. On hospitalization day (HD) 2, the patient developed bilateral lower extremity weakness (MMT grade 2) and decreased sensation in the right lower extremity. Electromyography performed the following day revealed acute motor-sensory neuropathy. Guillain-Barré syndrome was suspected, and the patient was treated with intravenous immunoglobulin for five days with monitoring of neurological and ventilation status. There was mild improvement in bilateral motor impairment from MMT grades 2 to 3 after treatment. On HD 7, the patient experienced a recurrence of the high fever and Osler’s nodes on both fingertips. Laboratory studies showed an elevated blood cell count (12210/μL) and decreased hemoglobin levels (9.2 g/dL). Follow-up echocardiography revealed vegetations on the MV and a prosthetic mechanical AV. The patient was treated with intravenous gentamicin. On HD 19, serial echocardiography revealed worsening vegetation on the MV and prosthetic mechanical AV. Additionally, motor impairment of all extremities worsened, particularly in the left lower limb (left knee flexor and extensor MMT grade 3; left ankle plantar flexor MMT grade 2; and left ankle dorsiflexor, toe flexor, and extensor MMT grade 1). Repeat brain MRI revealed new ischemic lesions in the right occipital, left parietal, and bilateral frontal lobes, suggestive of septic emboli (Figure 1). On HD 23, the patient underwent AV replacement and MV explanation with pericardial adhesiolysis. After surgery, the patient’s vital signs and laboratory results stabilized, although the lower extremity weakness showed no improvement.
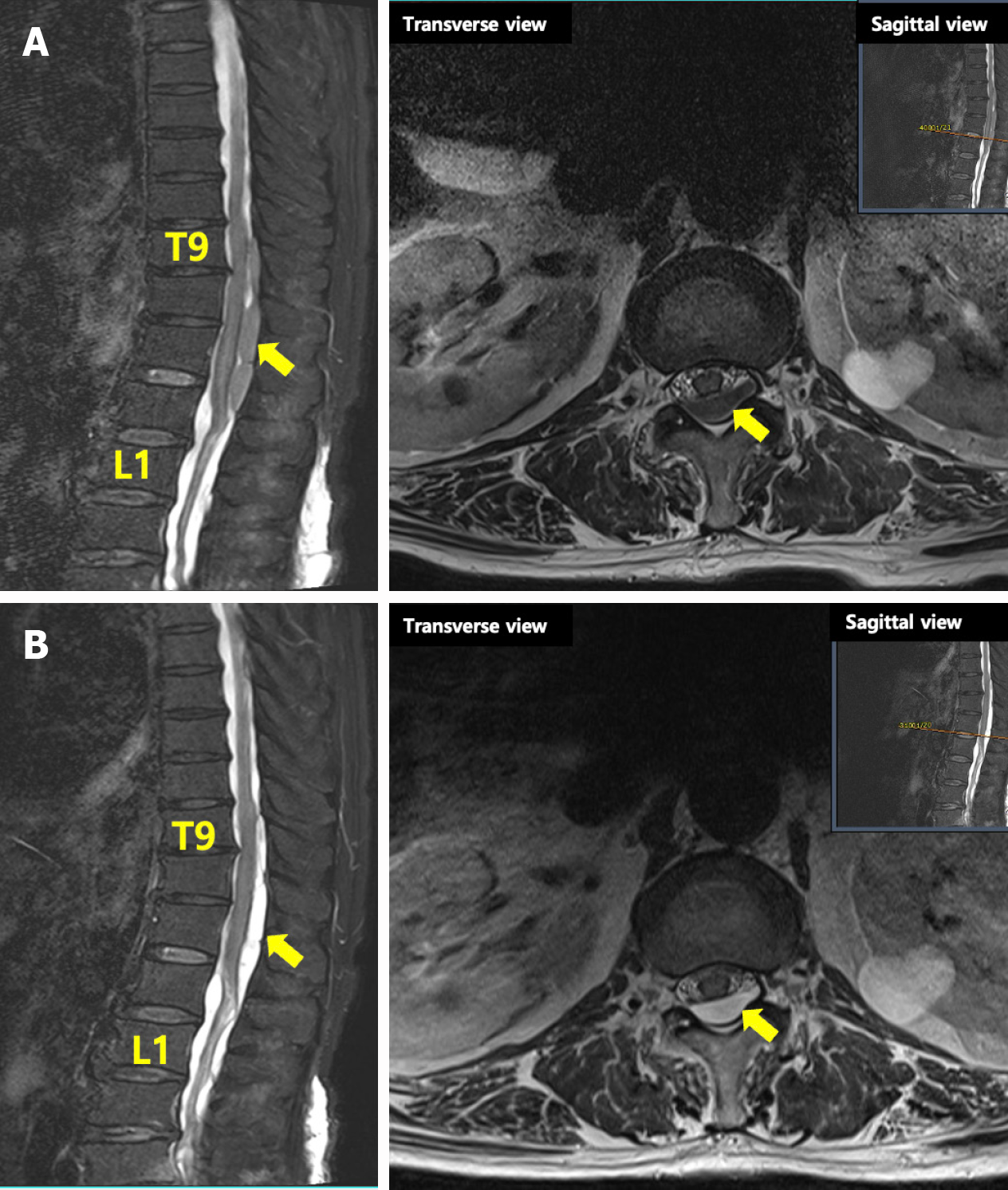
On HD 44, the patient was transferred to the Department of Physical Medicine and Rehabilitation. Neurological examination still revealed severe motor and sensory impairments in the lower extremities (left knee flexor and extensor MMT grade 2; left ankle plantar flexor MMT grade 1; and left ankle dorsiflexor, toe flexor, and extensor MMT grade 1), particularly on the left, despite relative sparing of the brain’s motor area. The range of motion of all joints was passively full, and the deep tendon reflexes were normal clinically with no pathology. In addition, the patient complained of difficulties with voiding and defecation. On HD 45, whole-spine MRI with enhancement was performed to evaluate other potential spinal diseases causing asymmetric lower-extremity muscle weakness. Imaging showed low-intensity T1 and mixed low- and high-intensity T2 signals from T9 to L1, suggesting IgG4-related spinal pachymeningitis (Figure 2A). The serum IgG4 level was elevated (318 mg/L). Follow-up electrophysiological studies revealed bilateral lumbosacral radiculopathy, abnormalities in the pudendal nerve and bulbocavernosus reflex, and generalized peripheral neuropathy.
Steroids are the most important treatment for IgG4-RD[14,15]. The patient was treated with oral prednisolone (30 mg/day, 0.5 mg/kg/day). Comprehensive rehabilitation included strength training of the lower extremities, gait training with a left ankle-foot orthosis, multisensory stimulation, functional electrical stimulation, and functional training to minimize caregiver assistance.
On HD 67, follow-up thoracic MRI showed improvement in the IgG4-related spinal pachymeningitis and spinal cord compression; thus, surgical treatment was not considered (Figure 2B). On HD 70, there was an improvement in the left lower extremity muscle strength, with MMT grades increasing from 1 to 2 in ankle dorsiflexion and 1st toe extension. After 18 days of oral steroid treatment and rehabilitation, the patient’s overall functional ability and MMT scores improved significantly. The patient continued to be monitored for approximately six months in an outpatient clinic and maintained the functional level observed at discharge.
IgG4-related spinal pachymeningitis, although less prevalent than intracranial lesions, is of significant clinical interest because of its frequent misdiagnosis as a spinal tumor, leading to inappropriate treatment strategies. In this discussion of IgG4-related spinal pachymeningitis, we compare our case with others reported in the literature, focusing on the underlying conditions, symptoms, diagnosis, and treatment outcomes (Table 1).
| Journal | Chief complaints | Underlying disease | Involved site | Treatment periods | Treatment | Ref. |
| Brain involved | ||||||
| J Neurocrit Care | Seizure, headache | Hypertension, asthma | Right frontal area | 10 days | Intra venous corticosteroid | Kim et al[22] |
| World J Clin Cases | Tingling, numbness, flickering movement in left lower extremity, headache | No specific findings | Interhemispheric fissure, left cerebral convexity | 7 days | Intra venous steroid 5 days | Lv et al[23] |
| BMC Surg | Seizure, headache | Hypertension, diabetes mellitus | Superior sagittal sinus | 14 days | Intra venous steroid, operative | Lin and Lai[24] |
| Immunol Res | Headache, bilateral lower extremity pain | No specific findings | Superior sagittal sinus | 10 days | 8 cycles of weekly rituximab and dexamethasone | Dong et al[9] |
| Intern Med | Headache, hearing impairment, frontal sinusitis | Lung cancer | Posterior fossa and bilateral posterior cerebral hemispheres | 14 days | Intra venous steroid, rituximab | Mori et al[25] |
| J Clin Neurol | Headache | Interstitial lung disease | Right hemisphere | 10 days | Intra venous steroid 5 days, rituximab 375 mg | Yim et al[26] |
| Spine involved | ||||||
| Front Neurol | Back pain, dysuresia, lower extremity weakness | No specific findings | Thoracic 1-6 | 14 days | Intra venous steroid 5 days | Zhang et al[16] |
| Saudi Med J | Neck pain, right side weakness | Cervical spinal disease | Thoracic 1 with cord compression | 14 days | Intra venous steroid 3 days, intra venous rituximab 2 weeks | Alrashdi[17] |
| World Neurosurg | Back pain, lower extremity dysesthesia, and weakness | Polycystic kidney disease, hyperlipidemia | Cervical 7-thoracic 5 epidural mass | 14 days | Intra venous steroid 40 mg 5 days, thoracic 4-5 laminectomy, rituximab 4 weeks, steroid 2 months | Slade et al[18] |
| Neurol India | Lower limb weakness and paresthesia | No specific findings | Thoracic 3-5 | 14 days | Laminectomy, Intra venous steroid, azathioprine | Karthigeyan et al[19] |
IgG4-related pachymeningitis, particularly with spinal involvement, often exhibits bilateral symptoms. Symptoms such as neck and back pain, paresthesia, sensory impairment, and motor weakness are indicative of spinal lesions, as demonstrated by the cases documented by Zhang et al[16], Alrashdi[17], Slade et al[18], and Karthigeyan et al[19]. These studies consistently highlight the diverse symptoms associated with spinal disorders, specifically pointing out the occurrence of weakness in both legs as a common manifestation. In this case, the patient’s symptoms were similar to those of spinal cord or nerve root compression but differed in the unilateral manifestation of symptoms, which is a less common presentation in IgG4-related spinal pachymeningitis. Although similar cases often involve bilateral or central dural involvement, our patient exhibited an atypical unilateral pattern.
Diagnosis of IgG4-RD is typically established through a comprehensive evaluation, including clinical symptoms, imaging studies, serological tests, and distinctive histopathological features. Key histopathological indicators are storiform fibrosis, obliterative phlebitis, and a dense lymphoplasmacytic infiltrate[10]. In serological assessments, a serum IgG4 level exceeding 135 mg/dL is considered noteworthy[5]. However, a concentration that is more than three times the upper limit of normal significantly strengthens the suspicion for IgG4-RD[20]. In this case, diagnosis was primarily based on elevated serum IgG4 levels and whole-spine MRI, consistent with diagnostic protocols.
Nerve conduction study and electromyography follow-up revealed a decrease in compound muscle action potential amplitude in nerve conduction study compared to previous tests, and abnormal spontaneous electromyography activity was observed in paralumbar and lower extremity muscles, suggesting the possibility of a spinal cord lesion or radiculopathy and providing additional diagnostic information. The spine MRI raised suspicions of IgG4-RD, leading to the initiation of treatment with oral steroids, which resulted in a favorable response, thereby facilitating both the diagnosis and treatment of the condition. The definitive diagnosis was established following the observation of a positive response to pharmacological treatment, which was informed and supported by spinal MRI findings.
Karthigeyan et al[19] and Winkel et al[21] reported cases where surgical intervention and biopsy were attempted before pharmacological treatment, based on findings in spine MRI of a plaque-like intensely enhancing epidural lesion that extended through the neural foramina into the paravertebral space. This approach is thought to increase the risk of post-surgical complications. One of the most crucial aspects in our patient was monitoring the drug response through spine MRI, where improvements post-treatment was confirmed in follow-up MRIs. This suggests that, similar to previous studies, it was possible to diagnose and treat without resorting to surgical intervention and biopsy.
Through this case reports, we propose that prompt diagnosis and early initiation of glucocorticoid therapy may circumvent the necessity for surgical decompression in the exceedingly rare instances of patients with IgG4-RD spinal tumors. In the treatment trajectory and outcomes, our patient exhibited a notably distinct response compared to previous cases of IgG4-related spinal pachymeningitis. Commonly, such cases, particularly with spinal or brain involvement, necessitate aggressive interventions, including intravenous steroids, immunosuppressants, and surgical procedures, as evidenced in literature[9,17-19,21-26]. However, our patient, despite showing severe spinal involvement and weakness, demonstrated a dramatic and positive response to a seemingly less intensive treatment regimen. This was characterized by an 18-day course of oral steroid therapy at 30 mg daily, coupled with focused rehabilitation efforts. The substantial functional improvement and symptom relief achieved in this case underscore the effectiveness of this approach. Significantly, the key factors contributing to the success of this treatment strategy in our patient appear to be early diagnosis and prompt initiation of therapy. The early intervention with oral steroids, combined with consistent rehabilitation, played a crucial role in the patient’s rapid and significant recovery. This suggests that for IgG4-related spinal pachymeningitis, timely diagnosis and treatment initiation, along with appropriate rehabilitation, might be as effective as more aggressive treatments.
Furthermore, the etiology of IgG4-related spinal pachymeningitis remains incompletely understood, despite reports demonstrating the effectiveness of specific treatments[27]. The ambiguity of the risk factors associated with this condition continues to be a challenge. In our patient’s case, a history of infective endocarditis and systemic sepsis suggested a potential immunological origin, a factor rarely observed in other reported cases. This unique aspect of the patient’s medical history may offer insights into the complexities surrounding IgG4-related spinal pachymeningitis. Significantly, our patient’s treatment was distinct in that it did not involve aggressive surgical interventions or the use of immunosuppressive agents[9,18,19], which are common in more severe cases. Instead, substantial improvement was achieved through a regimen of oral steroids and targeted rehabilitation, emphasizing the potential effectiveness of less aggressive treatment methods in managing IgG4-related spinal pachymeningitis in patients with specific underlying health conditions.
In conclusion, this case report of IgG4-related spinal pachymeningitis emphasizes the importance of a multidisciplinary approach to diagnosis and treatment. In this case, the distinct symptoms, diagnostic methods, and treatment outcomes highlight the variability in clinical presentations and approaches for managing this condition. Personalized treatment plans tailored to each patient’s unique history and clinical presentation are crucial for the effective management of this complex condition. Integrating medical treatment, radiological assessment, and rehabilitation is key, and ongoing research and interdisciplinary collaboration are essential to enhance the diagnostic accuracy and develop effective treatment strategies for this challenging condition.
It is clearly stated that a comprehensive multidisciplinary evaluation was undertaken for the patient, encompassing spine MRI, serum and cerebrospinal fluid analysis, nerve conduction studies, and electromyography, all aligned with the patient’s specific symptoms. This comprehensive diagnostic approach enables accurate diagnosis, followed by the establishment of a targeted treatment plan. Subsequent observation of the patient revealed notable therapeutic benefits of oral steroid therapy and rehabilitation.
We thanks for the grant from the Biomedical Research Institute of Jeonbuk National University Hospital, Jeonju, Korea.
| 1. | Maslinska M, Dmowska-Chalaba J, Jakubaszek M. The Role of IgG4 in Autoimmunity and Rheumatic Diseases. Front Immunol. 2021;12:787422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Stone JH, Khosroshahi A, Deshpande V, Chan JK, Heathcote JG, Aalberse R, Azumi A, Bloch DB, Brugge WR, Carruthers MN, Cheuk W, Cornell L, Castillo CF, Ferry JA, Forcione D, Klöppel G, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Masaki Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani D, Sato Y, Smyrk T, Stone JR, Takahira M, Umehara H, Webster G, Yamamoto M, Yi E, Yoshino T, Zamboni G, Zen Y, Chari S. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64:3061-3067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 488] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 3. | Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 849] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 4. | Perugino CA, Stone JH. IgG4-related disease: an update on pathophysiology and implications for clinical care. Nat Rev Rheumatol. 2020;16:702-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 5. | Sánchez-Oro R, Alonso-Muñoz EM, Martí Romero L. Review of IgG4-related disease. Gastroenterol Hepatol. 2019;42:638-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Rice CM, Spencer T, Bunea G, Scolding NJ, Sloan P, Nath U. Intracranial spread of IgG4-related disease via skull base foramina. Pract Neurol. 2016;16:240-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Bridges KJ, DeDeaux CH, Than KD. IgG4-related disease presenting as intradural extramedullary lesion: a case report and review of the literature. Br J Neurosurg. 2019;33:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | De Virgilio A, de Vincentiis M, Inghilleri M, Fabrini G, Conte M, Gallo A, Rizzo MI, Greco A. Idiopathic hypertrophic pachymeningitis: an autoimmune IgG4-related disease. Immunol Res. 2017;65:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Dong LL, Sheikh IS, Huang AH, Wu XH, Chen EG, Ying KJ. Immunoglobulin G4-related disease: case report and literature review. Immunol Res. 2021;69:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Wallace ZS, Carruthers MN, Khosroshahi A, Carruthers R, Shinagare S, Stemmer-Rachamimov A, Deshpande V, Stone JH. IgG4-related disease and hypertrophic pachymeningitis. Medicine (Baltimore). 2013;92:206-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Goulam-Houssein S, Grenville JL, Mastrocostas K, Munoz DG, Lin A, Bharatha A, Vlachou PA. IgG4-related intracranial disease. Neuroradiol J. 2019;32:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Lu Z, Tongxi L, Jie L, Yujuan J, Wei J, Xia L, Yumin Z, Xin L. IgG4-related spinal pachymeningitis. Clin Rheumatol. 2016;35:1549-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Okano A, Nakatomi H, Shibahara J, Tsuchiya T, Saito N. Intracranial Inflammatory Pseudotumors Associated with Immunoglobulin G4-Related Disease Mimicking Multiple Meningiomas: A Case Report and Review of the Literature. World Neurosurg. 2015;83:1181.e1-1181.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Khosroshahi A, Wallace ZS, Crowe JL, Akamizu T, Azumi A, Carruthers MN, Chari ST, Della-Torre E, Frulloni L, Goto H, Hart PA, Kamisawa T, Kawa S, Kawano M, Kim MH, Kodama Y, Kubota K, Lerch MM, Löhr M, Masaki Y, Matsui S, Mimori T, Nakamura S, Nakazawa T, Ohara H, Okazaki K, Ryu JH, Saeki T, Schleinitz N, Shimatsu A, Shimosegawa T, Takahashi H, Takahira M, Tanaka A, Topazian M, Umehara H, Webster GJ, Witzig TE, Yamamoto M, Zhang W, Chiba T, Stone JH; Second International Symposium on IgG4-Related Disease. International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease. Arthritis Rheumatol. 2015;67:1688-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 681] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 15. | Melenotte C, Seguier J, Ebbo M, Kaphan E, Bernit E, Saillier L, Audoin B, Feyeux D, Daniel L, Roche PH, Graillon T, Dufour H, Boutière C, Girard N, Closs-Prophette F, Guillaud C, Tieulié N, Regent A, Harlé JR, Hamidou M, Mekinian A, Grados A, Schleinitz N. Clinical presentation, treatment and outcome of IgG4-related pachymeningitis: From a national case registry and literature review. Semin Arthritis Rheum. 2019;49:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Zhang R, Gao J, Zhao T, Zhang B, Wang C, Wang C, Cui L, Chen J, Fang S. A Case With IgG4-Related Spinal Pachymeningitis Causing Spinal Cord Compression. Front Neurol. 2020;11:500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Alrashdi MN. Immunoglobulin G4-related spinal pachymeningitis. Saudi Med J. 2020;41:652-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Slade SJ, Bauer EM, Stone VV, Dave AJ. Spinal IgG4-Related Hypertrophic Pachymeningitis with Spinal Cord Compression: Case Report and Literature Review. World Neurosurg. 2019;130:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Karthigeyan M, Rajasekhar R, Salunke P, Gupta K. IgG4-related Pachymeningitis as a Cause of Spinal Epidural Compression: Can Intraoperative Frozen Sections Predict the Underlying Pathology? Neurol India. 2022;70:1223-1225. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Mahajan VS, Mattoo H, Deshpande V, Pillai SS, Stone JH. IgG4-related disease. Annu Rev Pathol. 2014;9:315-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 21. | Winkel M, Lawton CD, Sanusi OR, Horbinski CM, Dahdaleh NS, Smith ZA. Neuro-surgical considerations for treating IgG4-related disease with rare spinal epidural compression. Surg Neurol Int. 2018;9:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Kim JI, Song JT, Kwon HJ, Lee JY. A Case of IgG4 Related Pachymeningitis. J Neurocrit Care. 2016;9:162-165. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Lv K, Cao X, Geng DY, Zhang J. Imaging findings of immunoglobin G4-related hypophysitis: A case report. World J Clin Cases. 2022;10:9440-9446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Lin CK, Lai DM. IgG4-related intracranial hypertrophic pachymeningitis with skull hyperostosis: a case report. BMC Surg. 2013;13:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Mori M, Sakai K, Saito K, Nojima T, Mohri M, Matsubara K, Hayashi S, Yamada M. Hypertrophic Pachymeningitis with Characteristics of Both IgG4-related Disorders and Granulomatosis with Polyangiitis. Intern Med. 2022;61:1903-1906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Yim SH, Yoon JS, Lee CH, Kim J. Hypertrophic Pachymeningitis and Interstitial Lung Disease in IgG4-Related Disease. J Clin Neurol. 2022;18:481-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 27. | Cui Y, Masaki K, Zhang X, Yamasaki R, Fujii T, Ogata H, Hayashida S, Yamaguchi H, Hyodo F, Eto H, Koyama S, Iinuma K, Yonekawa T, Matsushita T, Yoshida M, Yamada K, Kawano M, Malissen M, Malissen B, Kira J. A novel model for treatment of hypertrophic pachymeningitis. Ann Clin Transl Neurol. 2019;6:431-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |