Published online Nov 6, 2024. doi: 10.12998/wjcc.v12.i31.6500
Revised: August 9, 2024
Accepted: August 16, 2024
Published online: November 6, 2024
Processing time: 124 Days and 17.9 Hours
Miller fisher syndrome (MFS) is a variant of Guillain-Barré syndrome, an acute immune-mediated peripheral neuropathy that is often secondary to viral infections. Anti-ganglioside antibodies play crucial roles in the development of MFS. The positive rate of ganglioside antibodies is exceptionally high in MFS patients, particularly for anti-GQ1b antibodies. However, the presence of other ganglioside antibodies does not exclude MFS.
We present a 56-year-old female patient who suddenly developed right blepharoptosis and progressively worsening vision in both eyes. There were flu symptoms prior to onset, and a coronavirus disease 2019 test was positive. On physical examination, the patient exhibited bilateral extraocular muscle paralysis, weakened reflexes in both limbs, and impaired coordination. The cerebrospinal fluid examination results showed no obvious abnormalities. Bilateral peroneal nerve F-waves were not extracted. Serum anti-GD1b IgG and anti-GT1a IgG antibodies were positive. The patient received intravenous methylprednisolone (1000 mg/day), with the dosage gradually decreased. Additionally, intravenous high-dose immunoglobulin treatment was administered for 5 days (0.4 g/kg/day) from day 2 to day 6 of hospitalization. The patient’s symptoms improved after treatment with immunoglobulins and hormones.
Positive ganglioside antibodies may be used as supporting evidence for the diagnosis; however, the diagnosis of MFS is more reliant on clinical symptoms.
Core Tip: This case study reports a unique presentation of Miller fisher syndrome with positive anti-GQ1b and anti-GT1a antibodies alongside multiple autoimmune markers. While ganglioside antibodies offer supportive evidence, Miller fisher syndrome diagnosis primarily relies on clinical manifestations, emphasizing the importance of comprehensive clinical assessment in diagnosing this immune-mediated neuropathy.
- Citation: Wei CQ, Yu X, Wu YY, Zhao QJ. Miller fisher syndrome with positive anti-GQ1b/GT1a antibodies associated with COVID-19 infection: A case report. World J Clin Cases 2024; 12(31): 6500-6505
- URL: https://www.wjgnet.com/2307-8960/full/v12/i31/6500.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i31.6500
Miller fisher syndrome (MFS), an autoimmune nerve disorder, is a variant of Guillain-Barré syndrome (GBS)[1]. Its classic symptoms are ocular paralysis, ataxia, and reduced tendon reflexes[2]. MFS has been linked to coronavirus disease 2019 (COVID-19) infection with symptom severity ranging from mild to severe[3]. COVID-19 can harm nerve tissue directly or trigger an immune response leading to GBS[4]. Antibodies play a key role in MFS development, with anti-GQ1b antibodies being most common[5]. A patient with MFS presented with ophthalmoplegia, ataxia, weakened reflexes, blurred vision, and eyelid drooping. Anti-GQ1b/GT1a antibodies were positive. From this case and literature review, we discuss the pathophysiology and possible treatment implications for this presentation.
MFS patients presented with ophthalmoplegia, ataxia, weak reflexes, blurred vision, drooping eyelids, and positive anti-GQ1b/GT1a antibodies. We discuss pathophysiology and therapeutic implications through this case and a literature review.
Right eyelid ptosis and blurred vision for one week.
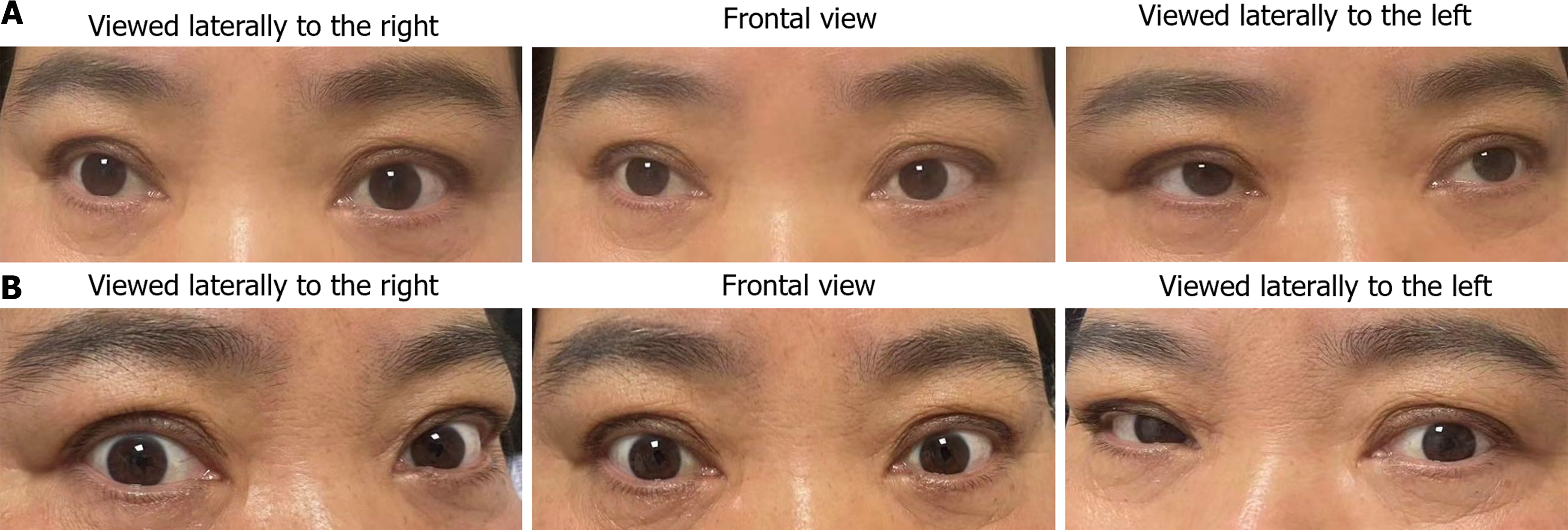
A 56-year-old woman was hospitalized on March 19, 2024, with the chief complaint of “right eyelid ptosis and blurred vision for one week”. She had a history of headache, nasal congestion, body aches, and fever one week before admission. The COVID-19 self-test was positive. After antiviral and antipyretic treatment, the above symptoms improved. She subsequently developed weakness in lifting her right eyelid, and the movement of both eyeballs was restricted in all directions. The patient’s visual acuity gradually deteriorated in both eyes, prompting a visit to an ophthalmology department. No abnormalities were detected during the fundus examination. Head magnetic resonance imaging (MRI) revealed bilateral swelling of the optic nerve and extraocular muscles, and the patient was referred to a neurology department. During the course of the disease, the patient experienced walking instability. There were no signs of unconsciousness, and no presentations of numbness, or weakness.
The patient had a history of hypertension but denied drug abuse or toxic exposure. Finally, there was no indication of hereditary disorders in their family background.
The patient reports having no history of smoking or alcohol consumption. She is currently employed as a worker in a freezing factory, where he has been exposed to cold temperatures and potentially cold-related occupational hazards. Regarding his family history, the patient denies any significant genetic diseases among his immediate family members, suggesting no known predisposition to hereditary conditions.
The patient’s body temperature and mental state were normal upon admission. On physical examination, the bilateral eyeballs were essentially fixed, moving horizontally by approximately 2 mm; the bilateral pupil light reflex was symmetrically weakened and the pharyngeal reflex was decreased. Despite her reflexes of knee and ankle were bilaterally reduced, she was able to move her extremities spontaneously. The perceptions of her face, trunk, and extremities were intact, however the coordinating functions of her limbs were impaired bilaterally. Though with the presence of normal bilateral flexor plantar responses, the patient was not capable of walking properly. As well, the rectovesical sphincter functioned normally, and there were no visible involuntary movements.
Results of laboratory examinations, including routine blood, urine, coagulation, glucose, lipid, electrolyte, kidney, liver, and tumor marker, were all within normal limits. Serological inspections of human immunodeficiency virus, Treponema pallidum, eosinophils, hepatitis B, and hepatitis C virus were negative. The level of thyroid hormones were also sustained in normal range. Moreover, cerebrospinal fluid (CSF) viral serology, Gram staining, and culture were negative.
The lumbar puncture revealed a CSF pressure of 130 mmH2O. CSF analysis revealed a white blood cell count of 13 × 106/L, a protein level of 367.9 mg/L, a chloride activity of 117 mmol/L, and a sugar concentration of 3.79 mmol/L.
On admission day, the patient was treated with intravenous methylprednisolone (1000 mg/day) for five days, with the dosage gradually decreasing in subsequent days. Simultneously, during hospitalization days 2 to 6, she was also received intravenous high-dose immunoglobulin (IVIg) treatment (0.4 g/kg/day). Serum antibodies were measured via Western blot analysis for gangliosides (anti-GM1, anti-GM2, anti-GM3, anti-GM4, anti-GD1a, anti-GD1b, anti-GD2, anti-GD3, anti-GT1a, anti-GT1b, anti-GQ1b, and anti-sulfatide IgG and IgM), and the results revealed anti-GT1a IgG (+) and anti-GQ1b IgG (+) (Supplementary Figure 1 and Supplementary Table 1). To rule out myasthenia gravis, enzyme linked immu
Head MRI showed bilateral swelling of the optic nerve and extraocular muscles. No clinical aberrations are found in chest computed tomography and electrocardiogram. Cardiac ultrasonography showed no abnormal morphological structures. Chest computed tomography revealed no apparent abnormalities. A sensory nerve conduction study, including action potentials and conduction velocity, was normal. Bilateral peroneal nerve F-waves were not extracted, indicating proximal injury (Table 1). Furthermore, no obvious abnormalities were observed via electro-encephalography.
| Nerve | Side | F-Lat | M-Lat | F-M Lat | F-Occurr | Distance | FWCV | |
| Peroneal nerve | Left | 0.0 ms | 0.0 ms | 0.0 ms | 0/20 | 0 | 0 | 0 |
| Tibial nerve | Left | 46.0 ms | 4.2 ms | 41.8 ms | 20/20 | 100 | 1200 mm | 58.8 m/s |
| Peroneal nerve | Right | 0.0 ms | 0.0 ms | 0.0 ms | 0/20 | 0 | 0 | 0 |
| Tibial nerve | Right | 47.7 ms | 3.8 ms | 43.9 ms | 17/20 | 85 | 1200 mm | 55.9 m/s |
MFS.
On admission day, the patient was treated with intravenous methylprednisolone (1000 mg/day) for five days and with dosage gradually decreasing in subsequent days. Simultneously, during hospitalization days 2 to 6, she was also received IVIg treatment for 5 days (0.4 g/kg/day).
The patient was discharged with improved symptoms on the 17th day of admission (Figure 1). She was administered prednisone orally, with the prednisone dose gradually reduced.
According to the most recent comprehensive study, up to 36.4% of people who had contracted COVID-19, a novel coronavirus that can give rise to highly contagious respiratory illness, experienced neurological symptoms[6]. GBS is the most common neuromuscular complication of COVID-19, occurring in both para-infectious and postinfectious patterns[7]. Clinically, a large proportion of patients with COVID-19 related GBS present as acute inflammatory demyelinating polyneuropathy, while 11% of them manifest as acute motor axonal neuropathy, and 7% as acute motor-sensory axonal polyneuropathy[8]. MFS is considered a GBS subtype, accounting for only 5% of GBS cases[9]. Both GBS and MFS have been reported as initial presentations of COVID-19 infection[10].
The diagnosis of MFS is usually clinical[11], and there is no difference between COVID-19-related and classical cases. Anti-ganglioside antibodies play a key role in the pathophysiological process of GBS and MFS. A positive serology of anti-GQ1b antibodies that has a sensitivity of 85%[12] would be able to support the diagnosis under most circumstances. It is an autoantibody against the ganglioside GQ1b, which is found abundant in the para-nodal region of the nodes of Ranvier along the central and peripheral myelinated axons[9]. Furthermore, serum anti-GT1a antibodies can also be detected in patients with GBS who develop bulbar palsy[13], and the existence of this monospecific antibody is considered indispensable for the pathogenosis of bulbar paralysis as it shows no cross-reaction with GQ1b[14]. Moreover, the abundance of GT1a gangliosides in the glossopharyngeal and vagus nerves further account for this assumption[14]. Bulbar palsy may therefore be associated with serum anti-GT1a IgG antibodies. In this study, we report a case of MFS overlapped with GBS, the serum of whom demonstrated positive anti-GQ1b and anti-GT1a IgG. Our patient initially presented with bulbar palsy, ophthalmoplegia, diplopia, and areflexia, and these clinical features may be explained, at least in part, by the presence of both anti-GQ1b and anti-GT1a IgG antibodies.
MFS/GBS cases with acute ophthalmoplegia as the initial symptom have rarely been reported in the literature. Some researchers have put forward the notion of “anti-GQ1b antibody syndrome”. Extraocular paralysis and ataxia can be caused by antibodies against GQ1b, which is highly expressed in the muscular spindles of both extraocular and skeletal muscles. The anti-GQ1b syndrome is composed of six types: Typical MFS, incomplete MFS, GBS, Bickerstaff’s brainstem encephalitis, pharyngocervical brachial variant, and overlap syndrome. Anti-GQ1b antibody syndrome can be diagnosed by the presence of anti-GQ1b IgG antibodies[15]. Consistent with our findings, the anti-GQ1b antibody was positive in our patient, hence cross-reactivity between these antibodies and gangliosides (sialic acid-containing glycosphingolipids located on neuronal cell surfaces) occurs, eventually resulting in autoimmune destruction of myelin sheaths and axons. COVID-19 can either damage nervous tissue directly or trigger the host immune system, which consequently leads to the occurrence of certain autoimmune diseases like GBS[5]. Additionally, in clinical setting, Gadolinium-enhanced MRI of the brain has been considered as an excellent confirmatory test for MFS. Patients with MFS present with pathologic gadolinium-enhancing lesions in cranial nerves, including specifically the bilateral oculomotor, facial, and abducens nerves as well as in the cauda equina[16]. A bilateral enhancement of the cranial nerves of the oculomotor domain and the abducens domain supported the diagnosis of MFS in our patient. IVIg and plasma exchange therapy are used to treat GBS, with a majority of patients achieving gradual recovery after treatment[17]. Regardless of the underlying infectious cause, the therapeutic strategies for MFS remains the same.
In summary, the diagnosis of MFS depends largely on clinical presentations as well as certain elevated serum antibodies. Although the positivity rate of ganglioside antibodies, particularly anti-GQ1b antibodies, is exceptionally high in MFS patients, the presence of other types of ganglioside antibodies does not exclude the possibility of MFS. Finally, the diagnostic criteria for MFS should not solely be based on their positive status, but should also take into account other related factors, specifically, the presence of ganglioside antibodies. Our findings may provide insight and possibly explain the connection between these entities.
The patient’s legal guardian provided written informed consent for the treatment.
| 1. | Shahrizaila N, Lehmann HC, Kuwabara S. Guillain-Barré syndrome. Lancet. 2021;397:1214-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 344] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 2. | Al Othman B, Raabe J, Kini A, Lee AG. Update: the Miller Fisher variants of Guillain-Barré syndrome. Curr Opin Ophthalmol. 2019;30:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Martins-Filho PR, Pereira de Andrade AL, Pereira de Andrade AJ, Moura da Silva MD, de Souza Araújo AA, Nunes PS, Santos VS, Ferreira LC, de Aquino Neves EL, Quintans-Júnior LJ. Miller Fisher Syndrome in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Systematic Review. J Clin Neurol. 2021;17:541-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1219] [Cited by in RCA: 1255] [Article Influence: 251.0] [Reference Citation Analysis (0)] |
| 5. | Cutillo G, Saariaho AH, Meri S. Physiology of gangliosides and the role of antiganglioside antibodies in human diseases. Cell Mol Immunol. 2020;17:313-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 6. | Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4761] [Cited by in RCA: 4704] [Article Influence: 940.8] [Reference Citation Analysis (0)] |
| 7. | Khan F, Sharma P, Pandey S, Sharma D, V V, Kumar N, Shukla S, Dandu H, Jain A, Garg RK, Malhotra HS. COVID-19-associated Guillain-Barre syndrome: Postinfectious alone or neuroinvasive too? J Med Virol. 2021;93:6045-6049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Finsterer J, Scorza FA. Guillain-Barre syndrome in 220 patients with COVID-19. Egypt J Neurol Psychiatr Neurosurg. 2021;57:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 9. | Teener JW. Miller Fisher's syndrome. Semin Neurol. 2012;32:512-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Aldabain L, Haddaden M, Farooqi R, Alissa M. COVID-19 Presenting As Miller Fisher Syndrome in a Patient With a History of Guillain-Barré Syndrome: A Case Report. Cureus. 2022;14:e26588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Chafiq K, Hadzine Y, Elmekkaoui A, Benlenda O, Rajad H, Wakrim S, Nassik H. Navigating the complexity of Guillain-Barré syndrome and Miller-Fisher syndrome overlap syndrome: a pediatric case report. Pan Afr Med J. 2024;47:127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 12. | Nishimoto Y, Odaka M, Hirata K, Yuki N. Usefulness of anti-GQ1b IgG antibody testing in Fisher syndrome compared with cerebrospinal fluid examination. J Neuroimmunol. 2004;148:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Yamashita H, Koga M, Morimatsu M, Yuki N. Polyneuritis cranialis related to anti-GT1 a IgG antibody. J Neurol. 2001;248:65-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Nagashima T, Koga M, Odaka M, Hirata K, Yuki N. Clinical correlates of serum anti-GT1a IgG antibodies. J Neurol Sci. 2004;219:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Yoon L, Kim BR, Kim HY, Kwak MJ, Park KH, Bae MH, Lee Y, Nam SO, Choi HY, Kim YM. Clinical characterization of anti-GQ1b antibody syndrome in Korean children. J Neuroimmunol. 2019;330:170-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Kiphuth IC, Saake M, Lunkenheimer J, Dörfler A, Schwab S, Kollmar R. Bilateral enhancement of the cranial nerves III-XII in severe Miller-Fisher syndrome. Eur Neurol. 2009;62:252-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | van Doorn PA. Diagnosis, treatment and prognosis of Guillain-Barré syndrome (GBS). Presse Med. 2013;42:e193-e201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |