Published online Nov 6, 2024. doi: 10.12998/wjcc.v12.i31.6486
Revised: August 19, 2024
Accepted: August 28, 2024
Published online: November 6, 2024
Processing time: 125 Days and 19.6 Hours
Primary cutaneous anaplastic large cell lymphoma (PC-ALCL) poses significant diagnostic difficulties due to its similarity in the appearance of skin lesions with chronic inflammatory disorders and other dermatological conditions. This study aims to investigate these challenges by conducting a comprehensive analysis of a case presenting with PC-ALCL, emphasizing the necessity of accurate differentiation for appropriate management.
An 89-year-old female patient with diabetes and hypertension presented with arm and abdominal ulcerated mass lesions. Diagnostic procedures included skin biopsies, histopathological assessments, and immunohistochemistry, complemented by advanced imaging techniques to confirm the diagnosis. The patient’s lesions were determined as PC-ALCL, characterized by necrosis, chronic inflammation, and a distinct immunophenotypic profile, including CD30, CD3, CD4, and EBER, CD56, MUM-1, Ki 67-positive in > 80% of tumor cells, CD10, but negative for anaplastic lymphoma kinase, CD5, CD20, PAX-5, Bcl-2, Bcl-6, CD8, and CD15. Recurrence was not reported at the 6-month follow-up.
Accurate PC-ALCL differentiation from similar conditions is crucial for effective management and requires a multidisciplinary approach.
Core Tip: This case report describes the diagnostic complexities and management strategies for primary cutaneous anaplastic large cell lymphoma (PC-ALCL), emphasizing the difficulties posed by its necrosis and chronic inflammation presentation. This report underscores the necessity of distinguishing PC-ALCL from similar chronic inflammatory conditions by detailing a patient’s journey from initial misdiagnosis to the tailored multidisciplinary approach that follows. This case illustrates the critical role of precise diagnostic techniques in guiding effective treatment plans for dermatological malignancies through histopathological examinations and immunophenotyping.
- Citation: Kim JM, Choi WY, Cheon JS. Diagnostic and management challenges in primary cutaneous anaplastic large cell lymphoma with necrosis, inflammation, and surgical intervention: A case report. World J Clin Cases 2024; 12(31): 6486-6492
- URL: https://www.wjgnet.com/2307-8960/full/v12/i31/6486.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i31.6486
The fifth edition of the World Health Organization Classification of Tumors published in 2022 categorized primary cutaneous anaplastic large cell lymphoma (PC-ALCL) under primary cutaneous T-cell lymphomas[1]. Primary cutaneous T-cell lymphomas are categorized as non-Hodgkin lymphoma. They are initially characterized by the presence of cutaneous T-cells, with no evidence of systemic disease upon diagnosis[2]. Most patients with PC-ALCL demonstrate solitary or grouped nodules in the realm of cutaneous T-cell lymphomas, where T-cells are capable of infiltrating the skin. These nodules, which typically develop over weeks to months, measure up to several centimeters. This particular infiltration pattern, while indicating cutaneous T-cell lymphomas, mirrors the infiltration observed in inflammatory disorders, which complicate the early-stage diagnosis due to the similarities in symptom presentation[3]. Chronic inflammatory skin disorders are defined by continuous inflammatory responses, where immune cells, including T-cells, infiltrate the skin and other tissues[4]. This causes persistent inflammation and subsequent tissue damage, a phenomenon that closely resembles the clinical features of PC-ALCL. The overlapping clinical presentations pose significant diagnostic difficulties. We aim to present a case of simultaneous localized mass lesions with arm and abdominal PC-ALCL ulcers. Skin biopsies from these areas were performed, with the biopsies from the arm and the abdomen revealing necrosis and chronic inflammation, respectively. This differentiation in biopsy results emphasizes the diagnostic challenges and highlights the complexity of managing distinct cutaneous lymphoma presentations.
An 89-year-old female patient presented with persistent arm and abdominal skin lesions, not responding to conventional treatment.
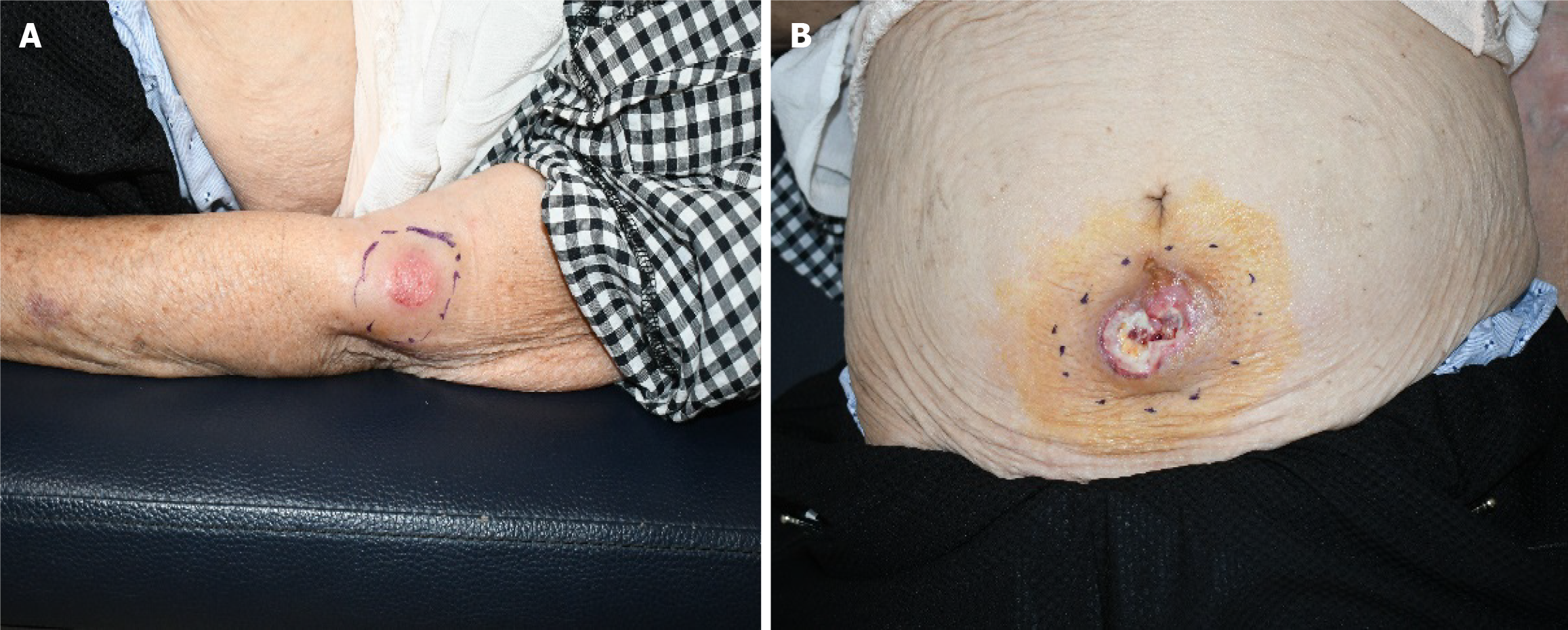
She reported no improvement in her wound condition despite a month of antiseptic treatment at a local hospital (Figure 1). She was referred to our department for specialized wound management after an initial biopsy at a local hospital that indicated necrotic tissue. A subsequent skin biopsy was performed upon a re-evaluation in our facility, causing a refined diagnosis by our pathology team. The histopathological analysis revealed chronic inflammation in addition to necrosis. She reported no systemic symptoms, such as fever, weight loss, or malaise, and no evidence indicated palpable lymphadenopathy.
She reported a medical history of diabetes and hypertension.
No relevant family history contributed to the current condition.
Physical examination revealed a 4 cm × 4 cm mass with a 3 cm × 3 cm skin defect on her arm and a 9 cm × 5 cm mass with a 1.5 cm × 1.5 cm skin defect on her abdomen.
Her laboratory evaluations, including a complete blood count, C-reactive protein, and ESR, all fell within the normal range.
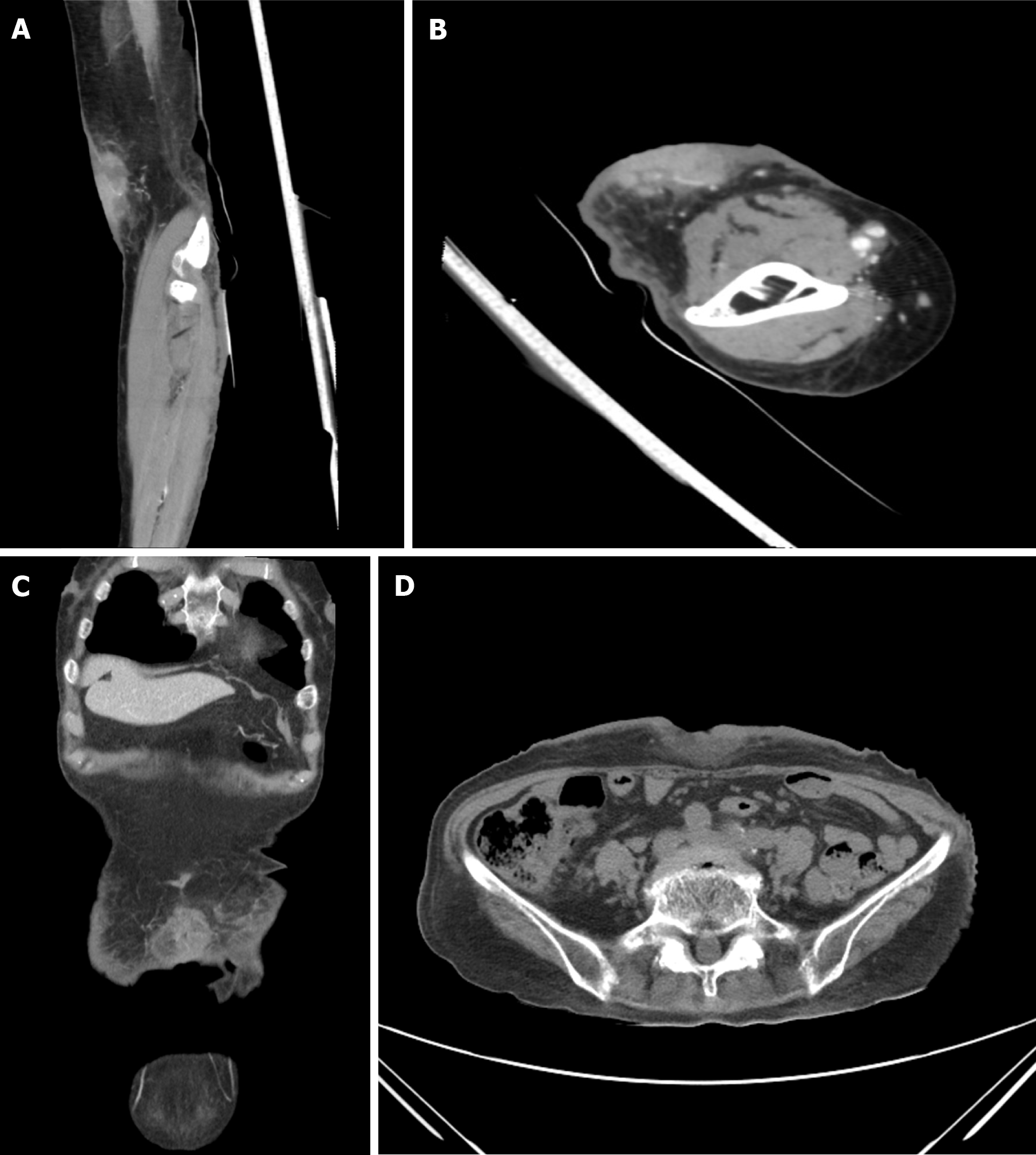
Advanced imaging via contrast-enhanced computed tomography (CT) revealed a 2.9 cm × 1.8 cm × 4.9 cm mass on the left arm, characterized by heterogeneous improvement and a deep subcutaneous layer, as well as an approximately 4.7 cm × 4 cm × 0.7 cm soft tissue defect in the mid-abdomen below the umbilicus.
Additional imaging studies, including an improved thorax CT, were performed following the recommendations of a hematologist/oncologist to assess for metastases. These subsequent imaging examinations revealed no significant results, confirming the absence of additional evidence of cancer metastasis (Figure 2).
PC-ALCL, confirmed by skin biopsy and subsequent histopathological analysis, exhibited necrosis and chronic inflammation alongside specific immunophenotyping results.
The surgical intervention involved abdominal and left arm mass removal, with resection margins of approximately 2 cm and 1 cm, respectively. The resultant defects were closed using an advancement flap covering to guarantee that the surgical resection margins were free from cancer cells.
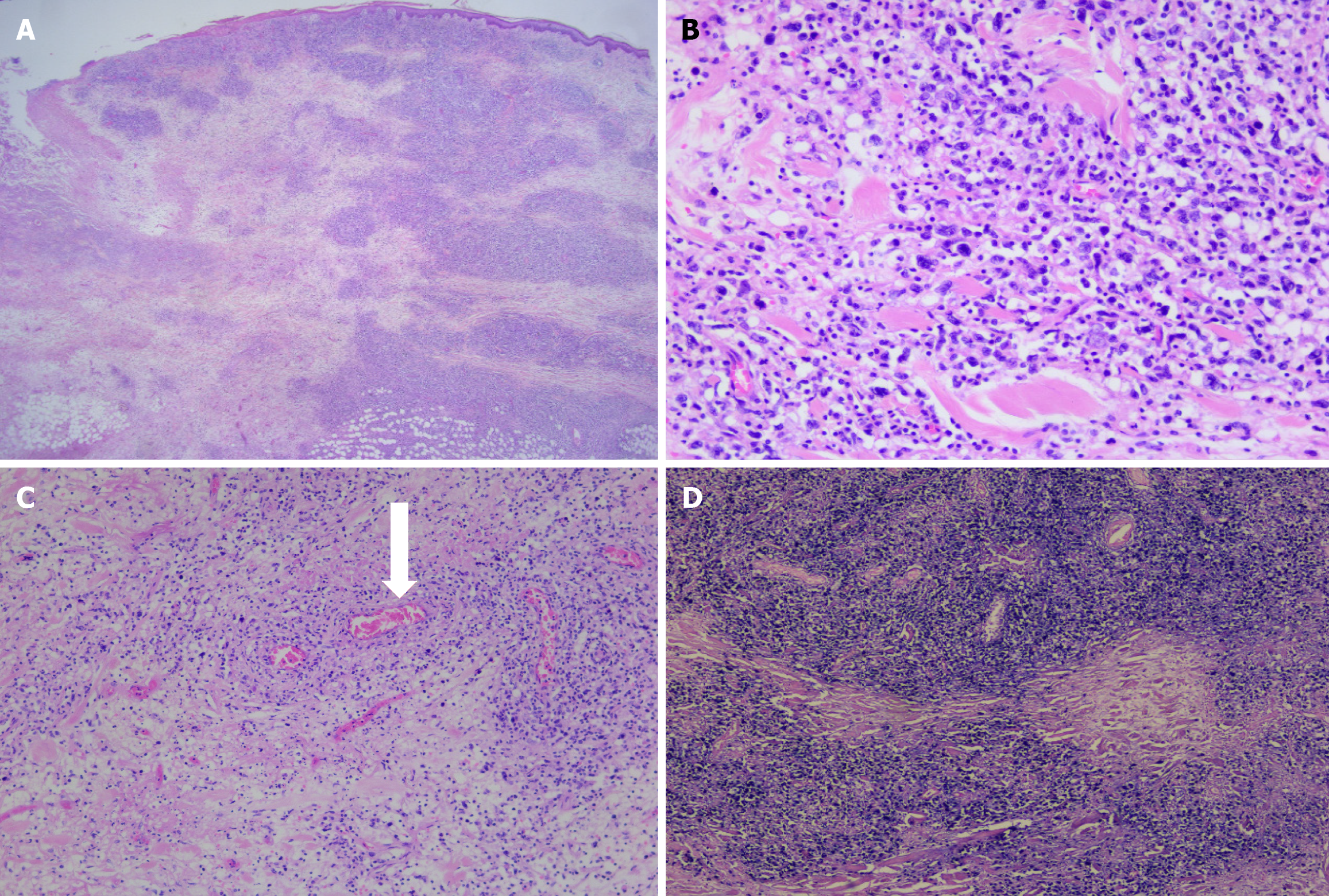
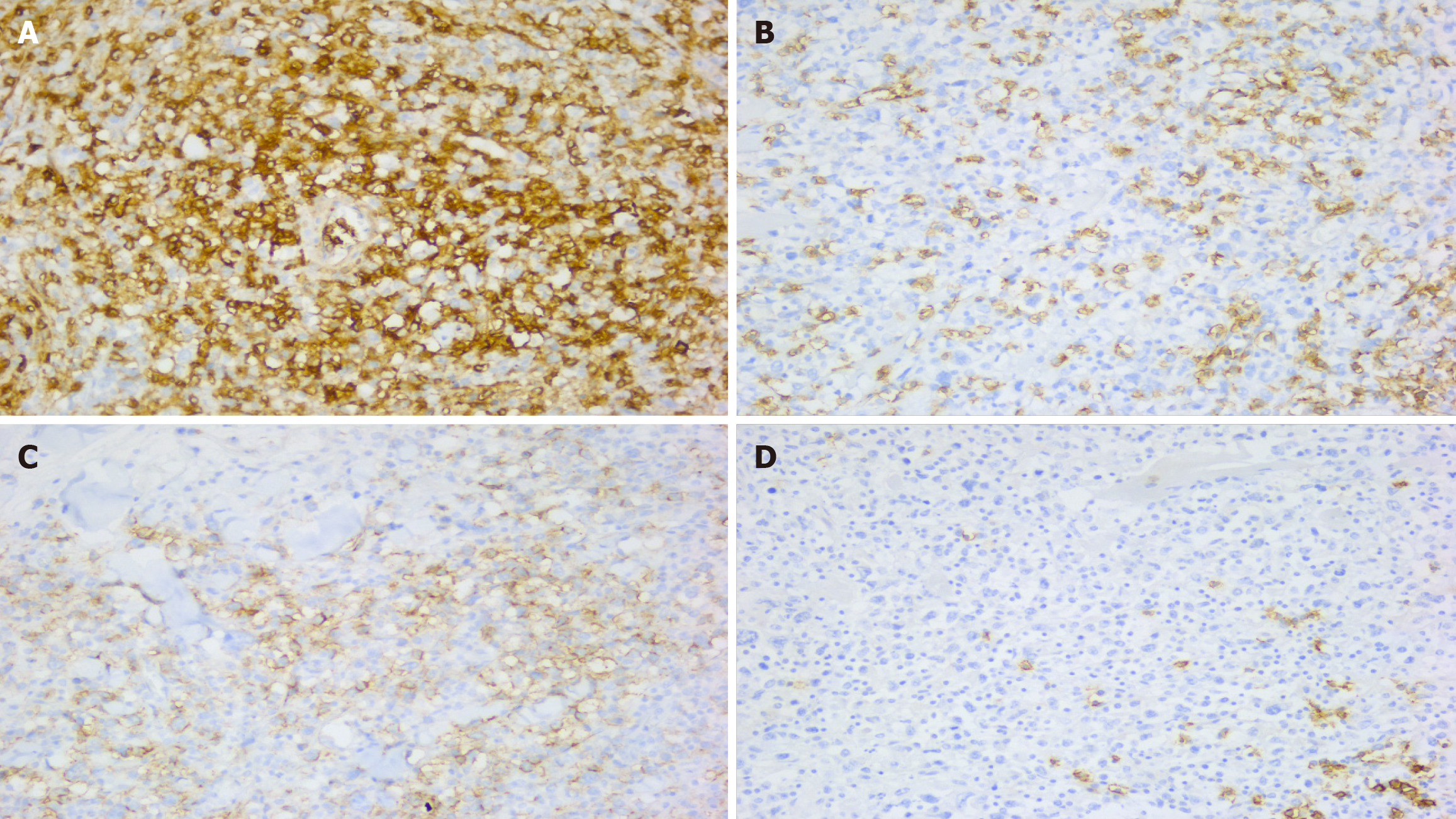
Histologically, the specimen exhibited extensive multifocal geographic tumor necrosis amidst highly pleomorphic lymphoid cell infiltration. These cells were predominantly round-to-oval in shape, characterizing irregular, frequently horseshoe-shaped nuclei. Further, vascular invasion accompanied by surrounding necrosis was evident (Figure 3). Immunohistochemistry and morphometric pathology described lymphoma cells as positive for CD30, CD3, CD4, EBER (in situ hybridization), CD56, MUM-1, and Ki 67, with positivity in > 80% of tumor cells. They were negative for anaplastic lymphoma kinase (ALK), CD5, CD20, PAX-5, Bcl-2, Bcl-6, CD8, and CD15 (Figure 4). The combination of these immunohistochemical markers is crucial in distinguishing this specific type of lymphoma from other neoplastic diseases. The patient demonstrated no signs of recurrence or any notable complications at the 6-month follow-up.
PC-ALCL diagnostic and therapeutic landscape poses compelling difficulties, further complicated by conditions with similar clinical presentations, including chronic inflammatory skin diseases and chronic wounds[3]. This complexity is described by a case where necrotic tissue and chronic inflammation were persistently found in biopsies from a local clinic and our institution, despite > 4 weeks of wound management without improvement. Chronic inflammatory skin disorders, characterized by sustained inflammatory responses and T-cell infiltration into skin tissues, alongside chronic wounds, marked by skin induration caused by factors, such as edema, inflammation, or malignant infiltration, underscore the diagnostic intricacies[4,5].
The management strategy substantially diverges for patients presenting with symptoms mimicking PC-ALCL, such as chronic wounds originating from infections or inflammatory disorders. These conditions frequently require a dual approach of systemic and topical treatments tailored to the underlying etiology, ranging from antibiotics and surgical interventions for abscesses to immunosuppressants or steroid creams for inflammatory conditions such as scleroderma. This comprehensive treatment paradigm aims not just to alleviate symptoms but also to curb chronic stage progressions.
However, the presence of large CD30-positive tumor cells in the dermis, a hallmark of PC-ALCL not observed in chronic wounds, provides a clear diagnostic criterion that helps in distinguishing between these conditions[6]. The differentiation is further complicated by both conditions presenting with similar clinical signs, making histopathological assessment and immunophenotyping critical for accurate diagnosis. Unlike chronic wounds, which lack neoplastic cells, PC-ALCL demonstrates a distinct histopathological profile with clusters of anaplastic large cells that express CD30, emphasizing the importance of tissue biopsy in the diagnostic workflow[1,3,6].
The primary management strategy focuses on localized treatment options, notably radiation therapy and surgical excision, upon confirming a PC-ALCL diagnosis. Radiation therapy, known for its efficacy, appears as the predominant choice due to its remarkable response rate of 100%. This treatment modality not only provides a high success rate but also presents a favorable safety profile, making it a cornerstone in managing single PC-ALCL lesions.
Moreover, the lack of ALK expression in PC-ALCL, as observed in our patient, indicates a more favorable prognosis and helps in distinguishing it from systemic ALCL variants, which frequently express ALK and require different treatment approaches[7]. This distinction is vital not only for prognostic purposes but also for guiding targeted therapy and avoiding treatments inappropriate for PC-ALCL treatment. The role of a multidisciplinary team becomes paramount in diagnosing and managing PC-ALCL, given these complexities, distinguishing it from chronic wounds through a comprehensive assessment that includes detailed clinical assessment, histopathology, immunophenotyping, and, when necessary, molecular studies[8]. This approach ensures that patients receive accurate diagnoses and appropriate and condition-specific treatment strategies, emphasizing the necessity of considering PC-ALCL in the differential diagnosis of chronic, non-healing lesions within 4 weeks of standard care.
Accurately differentiating PC-ALCL from chronic inflammatory disorders and chronic wounds is crucial for ensuring appropriate management and treatment. The case exemplifies the diagnostic difficulties faced in dermatological oncology, highlighting the need for thorough assessment and the use of specific histological and immunophenotypic markers to achieve a definitive diagnosis. Incorporating molecular diagnostics may further refine our ability to differentiate between neoplastic and non-neoplastic conditions as our understanding of cutaneous lymphomas advances, thereby improving patient outcomes through personalized treatment approaches.
Furthermore, the selection of biopsy sites plays a crucial role in the diagnostic process. Sampling tissues from areas affected by seborrhea, the abdominal skin unless it is the only site of lesions, or dead tissue frequently causes generic histological results, even when the sampling is correctly performed[3]. This information emphasizes the need for strategic biopsy site selection to prevent diagnostic ambiguity, especially in conditions presenting with chronic inflammation or wounds as well as in cases suspected of PC-ALCL. Clinicians navigate the complexities of skin disorders through diligent clinical and pathological assessment, thereby ensuring accurate diagnoses and effective treatments suited to each patient’s specific condition.
We extend our sincere gratitude to all individuals who contributed to the completion of this paper. We especially thank the patient who dedicated their time and effort to this research. We also express our gratitude to all healthcare professionals and researchers involved in the study. Additionally, we would like to thank everyone who assisted in the presentation and publication of this paper.
| 1. | Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D, Calaminici M, Chadburn A, Chan JKC, Cheuk W, Chng WJ, Choi JK, Chuang SS, Coupland SE, Czader M, Dave SS, de Jong D, Du MQ, Elenitoba-Johnson KS, Ferry J, Geyer J, Gratzinger D, Guitart J, Gujral S, Harris M, Harrison CJ, Hartmann S, Hochhaus A, Jansen PM, Karube K, Kempf W, Khoury J, Kimura H, Klapper W, Kovach AE, Kumar S, Lazar AJ, Lazzi S, Leoncini L, Leung N, Leventaki V, Li XQ, Lim MS, Liu WP, Louissaint A Jr, Marcogliese A, Medeiros LJ, Michal M, Miranda RN, Mitteldorf C, Montes-Moreno S, Morice W, Nardi V, Naresh KN, Natkunam Y, Ng SB, Oschlies I, Ott G, Parrens M, Pulitzer M, Rajkumar SV, Rawstron AC, Rech K, Rosenwald A, Said J, Sarkozy C, Sayed S, Saygin C, Schuh A, Sewell W, Siebert R, Sohani AR, Tooze R, Traverse-Glehen A, Vega F, Vergier B, Wechalekar AD, Wood B, Xerri L, Xiao W. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36:1720-1748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 1942] [Article Influence: 647.3] [Reference Citation Analysis (0)] |
| 2. | Benner MF, Willemze R. Bone marrow examination has limited value in the staging of patients with an anaplastic large cell lymphoma first presenting in the skin. Retrospective analysis of 107 patients. Br J Dermatol. 2008;159:1148-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Sokołowska-Wojdyło M, Olek-Hrab K, Ruckemann-Dziurdzińska K. Primary cutaneous lymphomas: diagnosis and treatment. Postepy Dermatol Alergol. 2015;32:368-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Girolomoni G. Classifying immunopathological responses in chronic inflammatory skin diseases. J Eur Acad Dermatol Venereol. 2018;32:654-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Eriksson E, Liu PY, Schultz GS, Martins-Green MM, Tanaka R, Weir D, Gould LJ, Armstrong DG, Gibbons GW, Wolcott R, Olutoye OO, Kirsner RS, Gurtner GC. Chronic wounds: Treatment consensus. Wound Repair Regen. 2022;30:156-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 169] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 6. | Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M; ESMO Guidelines Committee. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv30-iv40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 7. | Kadin ME, Carpenter C. Systemic and primary cutaneous anaplastic large cell lymphomas. Semin Hematol. 2003;40:244-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Garbe C, Peris K, Soura E, Forsea AM, Hauschild A, Arenbergerova M, Bylaite M, Del Marmol V, Bataille V, Samimi M, Gandini S, Saiag P, Eigentler TK, Lallas A, Zalaudek I, Lebbe C, Grob JJ, Hoeller C, Robert C, Dréno B, Arenberger P, Kandolf-Sekulovic L, Kaufmann R, Malvehy J, Puig S, Leiter U, Ribero S, Papadavid E, Quaglino P, Bagot M, John SM, Richard MA, Trakatelli M, Salavastru C, Borradori L, Marinovic B, Enk A, Pincelli C, Ioannides D, Paul C, Stratigos AJ. The evolving field of Dermato-oncology and the role of dermatologists: Position Paper of the EADO, EADV and Task Forces, EDF, IDS, EBDV-UEMS and EORTC Cutaneous Lymphoma Task Force. J Eur Acad Dermatol Venereol. 2020;34:2183-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |