Published online Nov 6, 2024. doi: 10.12998/wjcc.v12.i31.6479
Revised: August 13, 2024
Accepted: August 19, 2024
Published online: November 6, 2024
Processing time: 142 Days and 23.4 Hours
Superficial temporal artery to middle cerebral artery (STA-MCA) bypass is a valuable treatment for preventing ischemia and hemorrhage in occlusive cerebrovascular disease. Anastomosis site dissection is rarely reported among the various bypass-related complications.
In this case report, we describe two patients, who were 63- and 59-years-old with middle cerebral artery occlusion treated by STA-MCA bypass. During bypass surgery, the recipient M4 artery intima was dissected. We sacrificed the dissecting portion, and no complications occurred during the follow-up period. Post
Appropriate recipient artery selection is critical, and if dissection occurs, it is essential to sacrifice the dissecting portion quickly.
Core Tip: The pathological cause of anastomosis site dissection is not precisely understood. We present two rare cases of recipient artery dissection during superficial temporal artery-middle cerebral artery bypass. In order to take appropriate action when these cases occur, more case studies are needed on the causes. We proposed the atherosclerotic change of the recipient artery as the cause.
- Citation: Lee YJ, Park W, Joo SP. Recipient artery dissection during extracranial-intracranial bypass surgery: Two case reports. World J Clin Cases 2024; 12(31): 6479-6485
- URL: https://www.wjgnet.com/2307-8960/full/v12/i31/6479.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i31.6479
Superficial temporal artery to middle cerebral artery (STA-MCA) bypass is one of the direct revascularization procedures used to prevent ischemia and hemorrhage in Moyamoya disease or occlusive cerebrovascular disease[1,2]. Among STA-MCA bypass complications, recipient artery dissection is a very rare phenomenon, and its pathology and appropriate treatments remain unclear[3,4]. Additionally, the literature to date has only mentioned de novo aneurysm formation in the follow-up period, not during surgery[5-7]. Here, we present two cases of cerebral arterial dissection occurring directly after STA-MCA bypass surgery.
Case 1: A 63-year-old male presented intermittent subjective left-arm motor weakness and dizziness suspicious of a transient ischemic attack.
Case 2: A 59-year-old male was admitted to the hospital after experiencing dysarthria, agraphia and finger agnosia.
Case 1: These symptoms occurred 2-3 times a week.
Case 2: The patient presented with slurred speech 2 days ago, and gradually complained of symptoms of Gerstmann syndrome.
Case 1: The patient had a history of hypertension, diabetes and hyperlipidemia.
Case 2: The patient was diagnosed with hypertension and did not take any antihypertensive drugs.
Case 1: The patient did not drink or smoke, and had no relevant family history.
Case 2: The patient had no history of alcohol consumption. He had a smoking history of approximately 10 cigarettes/day for 30 years. He had no family history of similar disease.
Case 1: The patient did not present obvious motor weakness in our institution.
Case 2: The patient stuttered severely and had difficulty expressing himself, but motor weakness was not evident. The National Institute of Health Stroke Scale score was 1 in this patient.
Cases 1 and 2: Routine clinical biochemistry showed normal results.
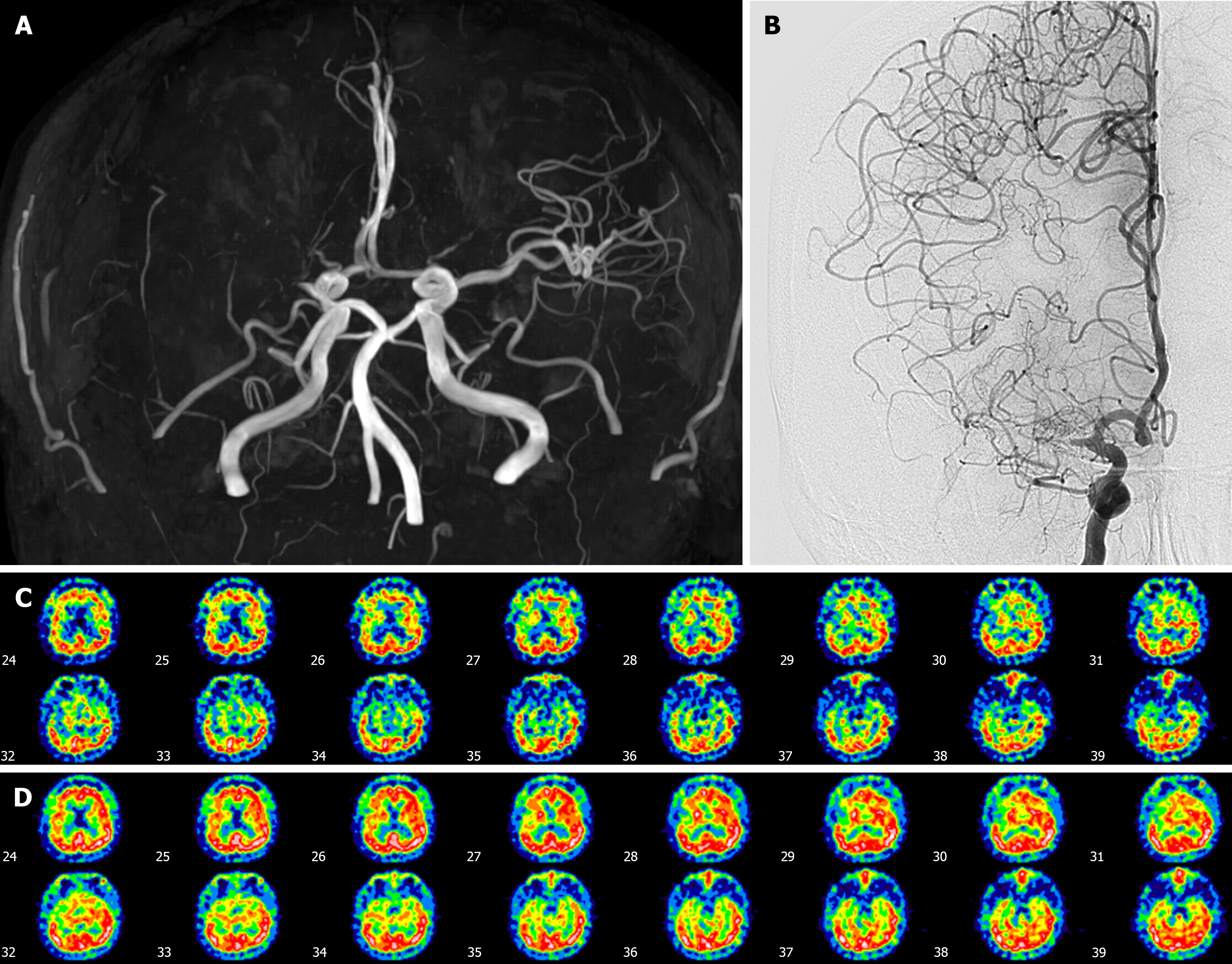
Case 1: Brain magnetic resonance angiography (MRA) and Digital subtraction angiography revealed complete occlusion of the proximal right middle cerebral artery (MCA) with no transdural collateral vessel involvement (Figure 1A and B). Diamox brain single photon emission computed tomography (SPECT) revealed diminished baseline perfusion and poor cerebrovascular reserve in the right frontal lobe (Figure 1C).
Case 2: Brain MRA showed acute infarction involving the left MCA territory and total occlusion of the left internal cerebral artery (ICA) at the origin site. Diamox brain SPECT revealed diminished vascular reserve in the left ICA territory.
Proximal right MCA occlusion was diagnosed according to brain imaging.
Left MCA territory infarction with left ICA occlusion was diagnosed.
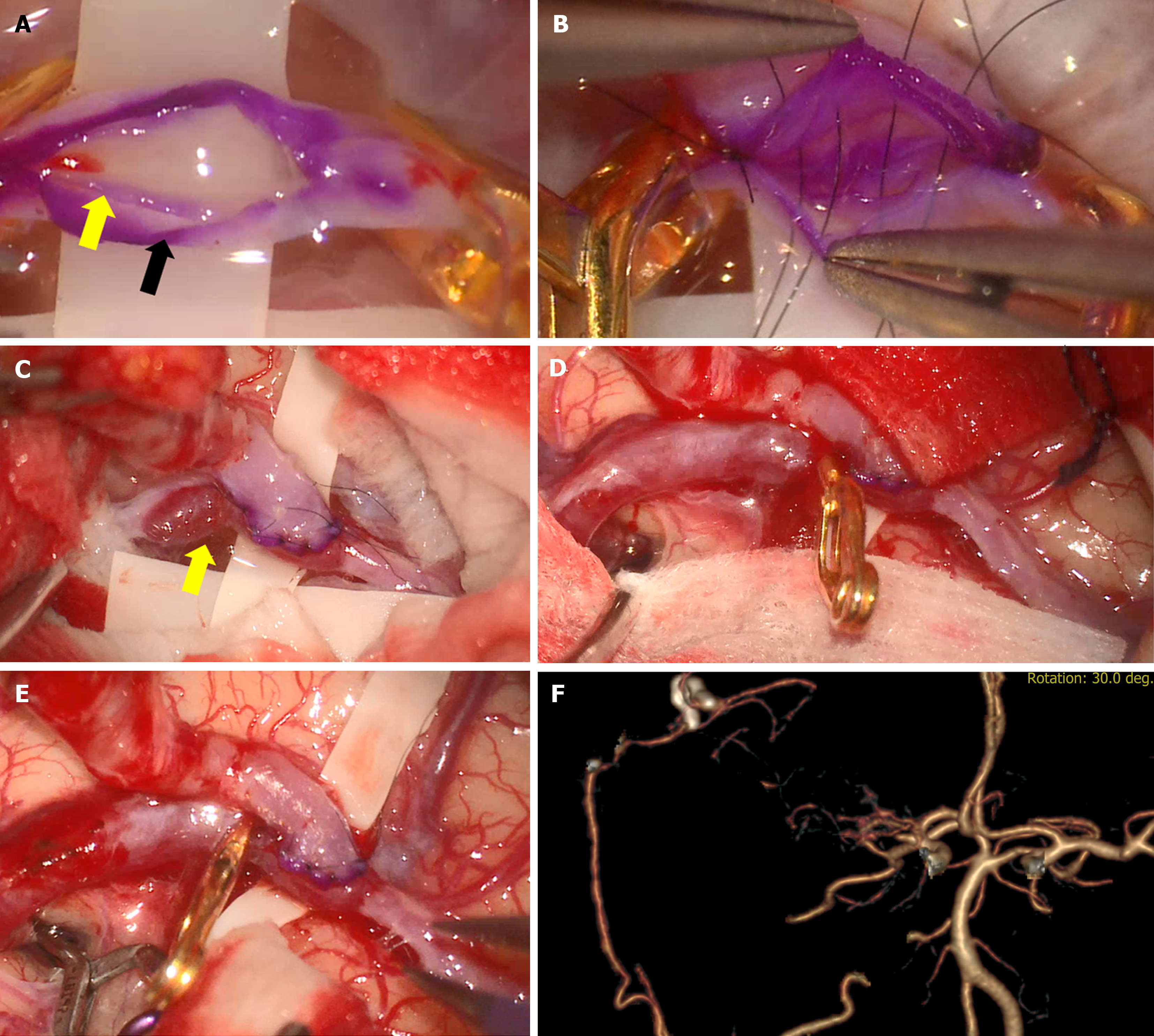
His neurologic symptoms recovered and were not severe, but considering the decreased perfusion, we performed revascularization with a right-sided STA-MCA bypass. The operation was performed through a single incision following the parietal branch of the superficial temporal artery and then curving toward the occipital site, including Chater’s point. A circular right temporal craniotomy was performed, and the appropriate M4 branch was selected as the recipient artery. After puncturing the recipient artery, an arteriotomy was undertaken, and the intima of the artery with sclerotic change was separated (Figure 2A). As there was no other appropriate recipient vessel, end-to-side anastomosis was undertaken using 10-0 nylon to prevent separation of the intima, media, and adventitia (Figure 2B).
Unfortunately, the intima was torn during the bypass procedure despite trying to suture the recipient artery’s full thickness. After removing the temporary clip, dissection occurred in the recipient M4 artery, where the intima was separated (Figure 2C). While we considered management options, the dissection gradually progressed proximally; to prevent rupture, we trapped the dissecting aneurysm using clips (Figure 2D and E). Intraoperative indocyanine green angiography confirmed that patency was well maintained at the anastomosis site, excluding the trapping area (Figure 2F).
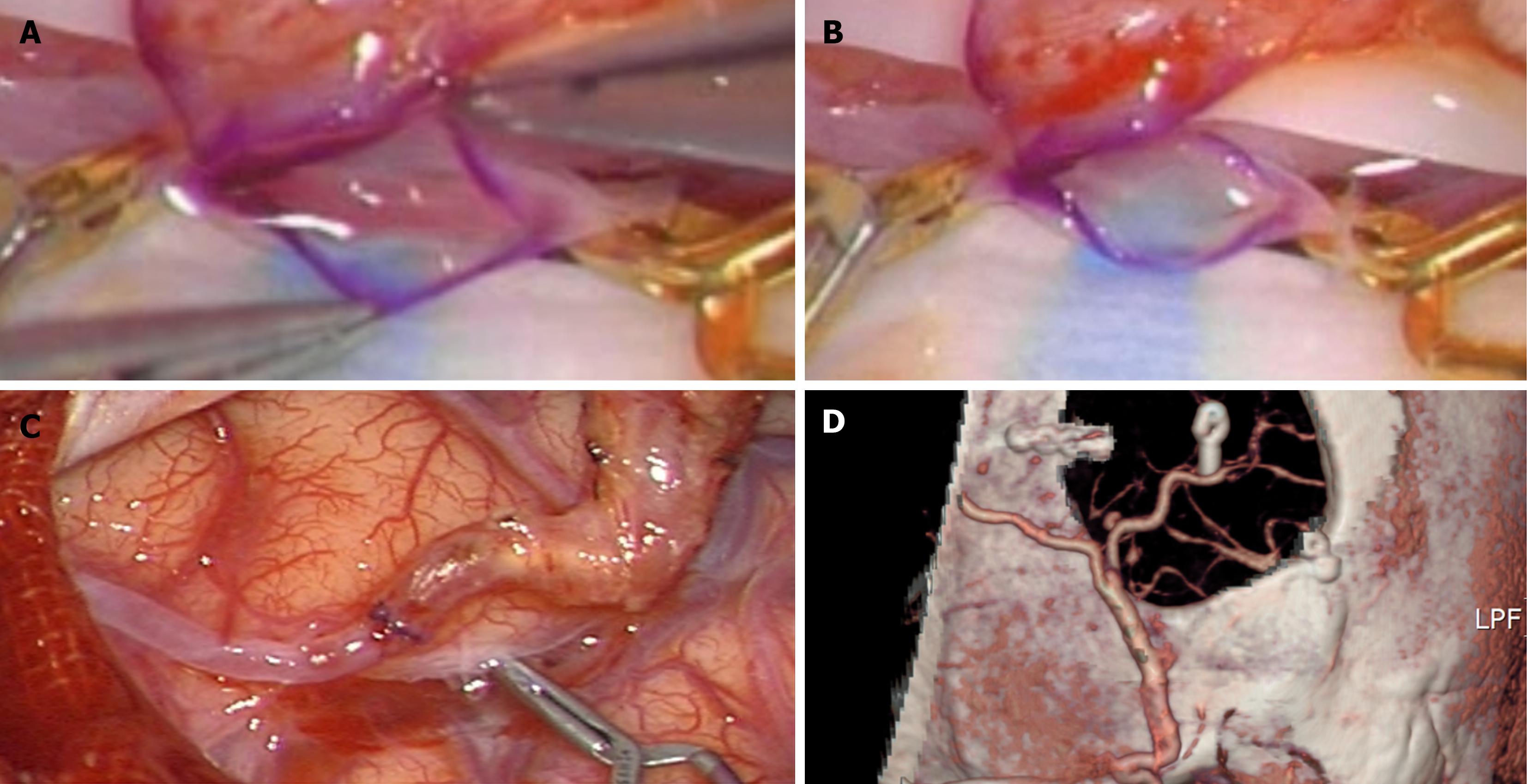
The patient underwent a left STA-MCA bypass procedure. After a circular temporal craniotomy, we performed an arterial puncture of the appropriate M4 branch with a manteaux syringe. When arteriotomy was performed using a micro-scissor, the arterial wall was dissected due to atherosclerosis, and only adventitia was excised (Figure 3A and B). Intimal and medial layers remained intact. The distal margin of the dissected portion was clipped and sacrificed, and end-to-end anastomosis was performed on the contralateral normal recipient artery and STA (Figure 3C and D).
The patient had an uncomplicated postoperative course, and postoperative brain SPECT confirmed an improving state of perfusion in the right frontal lobe.
Postoperative perfusion computed tomography showed a decreased extent of delayed time to peak perfusion in the left MCA territory with relatively well-preserved cerebral volume and flow. The patient was discharged without any complications.
Anastomotic aneurysm is an extremely rare complication that occurs after end-to-side anastomosis of the STA-MCA bypass[4,6,8-11]. Suggested possible causes include hypertension, increased hemodynamic stress due to artificial T-shaped vasculature, and traumatic brain injury during surgical manipulation[6,12]. This bypass-related anastomotic aneurysm is a more commonly reported complication in cardiovascular and peripheral vascular bypass surgery than in STA-MCA bypass surgery[13]. According to Potts et al[4], 24 cases of aneurysm formation at or adjacent to extracranial to intracranial (EC-IC) bypass anastomotic sites have been reported so far, and all were discovered during postoperative follow-up. The period between initial surgery and aneurysm occurrence ranged from less than one month to 21 years. No dissecting aneurysm has been reported immediately after bypass, as was described in our first case. In our second case, we did not attempt bypass because the intima was separated from the adventitia at the incision stage. If anastomosis had been performed, suturing the intima and adventitia together, a pseudoaneurysm would have occurred, as in the first case.
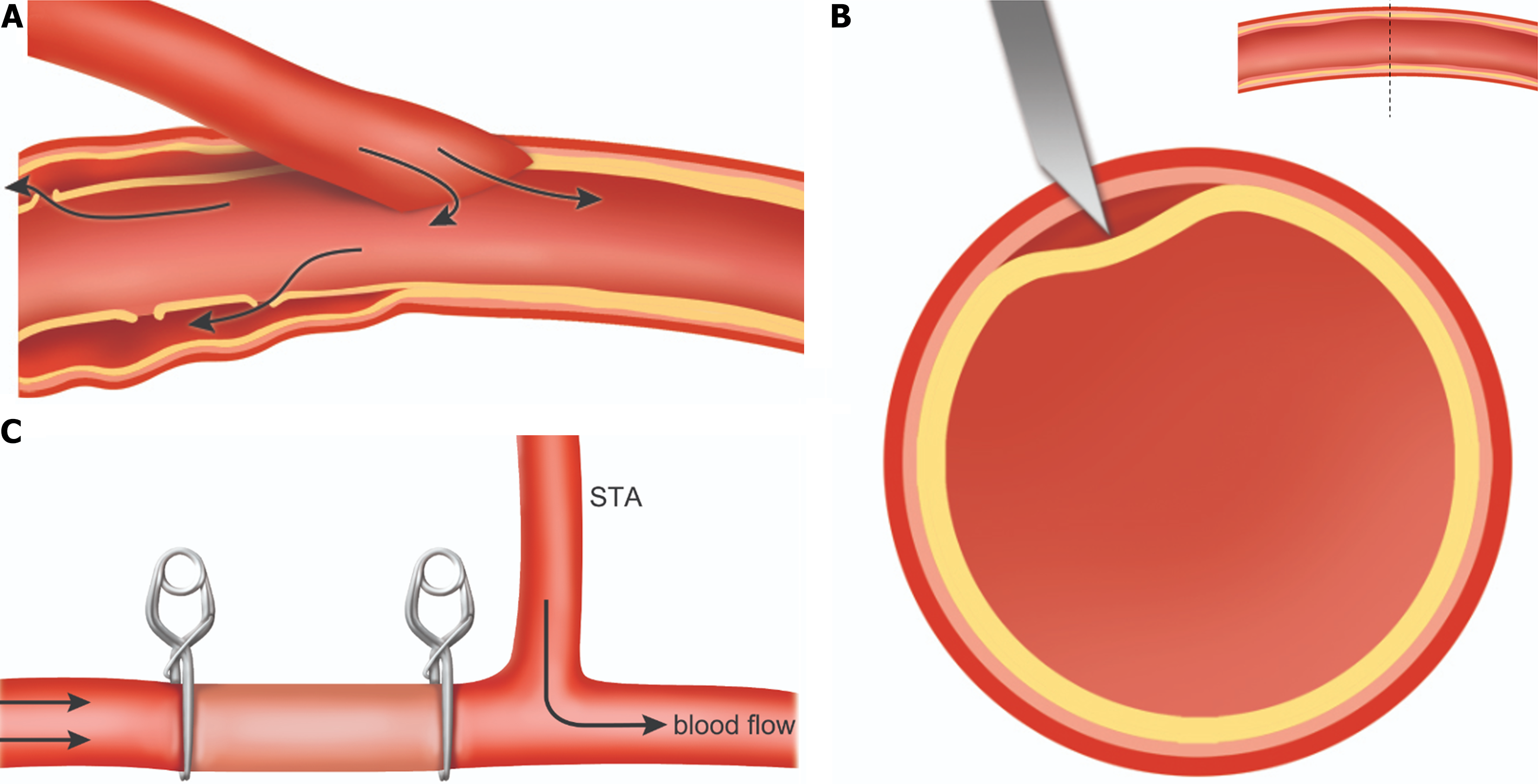
The cause of anastomotic dissection after STA-MCA bypass remains unclear. The causes mentioned above may explain the dissection during the follow-up period after bypass surgery. However, it is insufficient to explain dissection during surgery, as occurred in our cases. We considered intra-operative recipient arterial dissection in two aspects. First, atherosclerotic changes in the recipient artery may cause dissection. Previous studies have demonstrated that atherosclerosis is involved in intracranial dissecting aneurysms[14,15]. Atherosclerosis makes the intima fragile and prone to separation, so dissection may occur due to changes in flow after bypass surgery. This can be expressed in a schema as described in Figure 4A. After the intima is dissected, the dissection may tend to gradually proceed in the direction of the deformed flow along the fragile part due to atherosclerosis under adventitia.
The second cause of recipient artery dissection is when the artery is not punctured to the intima before performing arteriotomy. The method of preparing a recipient artery is to puncture the artery using a small needle and then perform an arteriotomy using micro-scissors. If the entire wall is not incised at once, then the intima is separated, as described in our second case. It may be the operator’s mistake, but if there is an atherosclerotic change in the recipient artery, the intima may not be punctured immediately (Figure 4B). Therefore, when selecting an appropriate recipient artery for bypass, it is critical to check whether there are atherosclerotic changes in the arterial wall.
When a dissecting aneurysm occurs, various management methods, such as clip reconstruction, excision of the aneurysmal anastomotic site, new EC-IC bypass, and STA ligation, have been attempted[4]. The best treatment for dissection remains undetermined, but if dissection occurs during surgery, as in our two cases, another approach should be considered. The area of dissection must be sacrificed quickly before the pseudoaneurysm progresses. One may also attempt bypass surgery by selecting a new recipient artery. However, in our experience, we have confirmed improved perfusion even if end-to-end anastomosis is performed on the non-sacrificed area. We believe this is due to the existing blood flow, though the sacrificed portion is maintained, and blood flow to the bypass site may improve, as demonstrated in Figure 4C. A double barrel bypass may also be considered to improve perfusion further if other appropriate M4 artery segments are available. Due to its rarity and lack of established optimal treatment modalities, this case provides important data for future studies of dissections occurring during EC-IC bypass. To overcome the limitation of small sample size, future studies using additional data are needed.
We report two extremely rare cases of recipient artery dissection during STA-MCA bypass surgery. The most critical decision is appropriate recipient artery selection, and if atherosclerotic changes are seen, one must account for the possibility of dissection. Additionally, if arterial dissection occurs, one must sacrifice the dissected portion quickly and perform an end-to-end bypass on the remaining vessel. The outcomes using this management plan are generally good.
| 1. | Yoshida K. [Evidence of STA-MCA Bypass-History and Overseas Articles]. No Shinkei Geka. 2022;50:735-744. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Schaller B. Extracranial-intracranial bypass surgery to reduce the risk of haemodynamic stroke in cerebroocclusive atherosclerotic disease of the anterior cerebral circulation - a systematic review. Neurol Neurochir Pol. 2007;41:457-471. [PubMed] |
| 3. | Eguchi H, Arai K, Kawamata T. Aneurysm appearing at the anastomosis site 11 years after superficial temporal artery-middle cerebral artery bypass surgery: moyamoya disease with a rapidly growing aneurysm. Illustrative case. J Neurosurg Case Lessons. 2023;6. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Potts MB, Horbinski CM, Jahromi BS. Rapid Development of an Aneurysm at the Anastomotic Site of a Superficial Temporal Artery to Middle Cerebral Artery Bypass: Case Report and Literature Review. World Neurosurg. 2019;128:314-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Ravina K, Patel B, Yim B. Spontaneous development and involution of a de novo pseudoaneurysm at the superficial temporal artery-middle cerebral artery bypass anastomotic site in a patient with moyamoya disease: illustrative case. J Neurosurg Case Lessons. 2024;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 6. | Yokota H, Yokoyama K, Noguchi H. De Novo Aneurysm Associated with Superficial Temporal Artery to Middle Cerebral Artery Bypass: Report of Two Cases and Review of Literature. World Neurosurg. 2016;92:583.e7-583.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Marcus HJ, Patel HC, Kirkpatrick PJ. Graft aneurysm complicating an extracranial-intracranial bypass. Br J Neurosurg. 2009;23:548-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Morgan M, Besser M, Tuck R. Pseudoaneurysm complicating superficial temporal artery-superior cerebellar artery bypass. Surg Neurol. 1986;26:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Sasaki T, Kodama N, Itokawa H. Aneurysm formation and rupture at the site of anastomosis following bypass surgery. Case report. J Neurosurg. 1996;85:500-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Nishizawa S, Yokoyama T, Sugiyama K, Yokota N. Intracerebral hemorrhage from a ruptured pseudoaneurysm after STA-MCA anastomosis--case report. Neurol Med Chir (Tokyo). 2000;40:408-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Aoki T, Yoshitomi M, Yamamoto M, Hirohata M, Morioka M. Ruptured de novo aneurysm arising at a site remote from the anastomosis 14 years after superficial temporal artery-middle cerebral artery bypass: a case report. Neurosurgery. 2012;71:E905-E909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Kono K, Masuo O, Nakao N, Meng H. De novo cerebral aneurysm formation associated with proximal stenosis. Neurosurgery. 2013;73:E1080-E1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Pogorzelski R, Fiszer P, Toutounchi S, Szostek MM, Krajewska E, Jakuczun W, Tworus R, Skórski M. Anastomotic aneurysms after arterial reconstructive operations--literature review. Pol Przegl Chir. 2014;86:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Sakata N, Takebayashi S, Kojima M, Masawa N, Suzuki K, Takatama M, Kusumi Y, Mitsumata M. Different roles of arteriosclerosis in the rupture of intracranial dissecting aneurysms. Histopathology. 2001;38:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Nakayama Y, Tanaka A, Kumate S, Tomonaga M, Takebayashi S. Giant fusiform aneurysm of the basilar artery: consideration of its pathogenesis. Surg Neurol. 1999;51:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |