Published online Nov 6, 2024. doi: 10.12998/wjcc.v12.i31.6472
Revised: August 14, 2024
Accepted: August 21, 2024
Published online: November 6, 2024
Processing time: 172 Days and 16 Hours
Streptococcus gallolyticus subspecies pasteurianus (SGSP) is a rare pathogen res
A 45-day-old female infant presented with two episodes of high fever (maximum temperature: 39.5 °C) and two generalized grand mal seizure episodes that lasted over ten seconds and self-resolved without concomitant symptoms. Postadmission, the patient’s C-reactive protein level was 40.73 mg/L, white blood cell count was 13.42 × 109/L, neutrophil ratio was 78.4%, procalcitonin level was 7.89 μg/L, cerebrospinal fluid (CSF) white cell count was 36 × 106/L, multinucleated cell ratio was 95.2%, and protein concentration was 0.41 g/L. Blood and CSF culture revealed that the pathogen was SGSP. The bacterium was sensitive to ampicillin, furazolidone, penicillin, lincomycin, moxifloxacin, rifampicin, vancomycin, and levofloxacin but resistant to clindamycin and tetracycline. Sputum culture revealed the presence of MRSA, which was sensitive to vanco
SGPSP-induced infant bacterial meningitis and sepsis should be treated with prompt blood and CSF cultures, and a sensitive antibiotic therapy to ensure a favorable prognosis.
Core Tip: We present a case of infant meningitis and sepsis caused by Streptococcus gallolyticus subspecies pasteurianus (SGSP), accompanied by bronchopneumonia induced by multidrug-resistant Staphylococcus aureus. The etiological classification and drug resistance profiles of the pathogens were confirmed through timely cultures of blood, cerebrospinal fluid, and sputum. The patient was effectively treated with a combined antibiotic anti-infection regimen, had no complications or sequelae and showed normal growth and development after discharge. Since SGSP is a rare pathogen that causes bacterial meningitis in infants, with unclear mechanisms and routes of infection, this report can provide a reference for the treatment and study of similar cases.
- Citation: Zou D, Li F, Jiao SL, Dong JR, Xiao YY, Yan XL, Li Y, Ren D. Infantile bacterial meningitis combined with sepsis caused by Streptococcus gallolyticus subspecies pasteurianus: A case report. World J Clin Cases 2024; 12(31): 6472-6478
- URL: https://www.wjgnet.com/2307-8960/full/v12/i31/6472.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i31.6472
Bacterial meningitis is a significant risk factor for mortality and disability in infants, and infant bacterial meningitis and sepsis are commonly caused by Group B Streptococcus and Escherichia coli[1]. Streptococcus gallolyticus subspecies pasteurianus (SGSP) is a common commensal bacterium in the gastrointestinal tract of humans and cattle. It is a rare pathogen that causes neonatal sepsis and meningitis, and it may be significantly overlooked because it is not included in routine Group B Streptococcus screening[2]. Timely and accurate identification of pathogens can enable more precise anti-infective treatment, improve cure rates, and improve patient prognosis. Therefore, we report a case of infant meningitis and sepsis caused by SGSP, accompanied by bronchopneumonia induced by multidrug-resistant Staphylococcus aureus (MRSA), aiming to increase clinicians' awareness of this pathogen and to emphasize the clinical manifestations of fever and convulsions in infants.
A 45-day-old female infant was admitted due to fever and two convulsive episodes prior.
Approximately 5 hours prior to admission, the infant developed a fever (temperature 38.5 °C) without any identifiable reason, and her parents treated her with cold packs. Four hours prior to admission, the child experienced her first convulsion, which was characterized by staring, cyanosis of the lips and mouth, clenching of the teeth, nuchal rigidity, tonic clonus of the limbs, and the inability to respond to calls. The seizure episode lasted for approximately 10+ seconds and then resolved on its own, with a favorable mental response and fever. Two hours prior to admission, the child had another convulsion, which also presented as a grand mal seizure and spontaneously resolved after a few seconds. The patient’s temperature measured immediately after the seizure was 39.5 °C. In response, the patient’s parents gave her oral acetaminophen to alleviate the fever. Prior to admission to our hospital, the patient had no other accompanying symptoms.
One week prior to admission, the infant experienced an infection in the umbilical area. The parents administered penicillin topically, which resulted in resolution of the infection.
The infant was born at full term via cesarean section, with a birth weight of 4.15 kg. This was the mother’s second pregnancy and second delivery (G2P2). There was no asphyxiation or birth injury. She had been formula-fed since birth. Her development was normal, and she had the ability to momentarily raise her head at the time of presentation. The infant was given all vaccinations, including the Bacillus Calmette–Guérin vaccine and two doses of hepatitis B vaccines, in accordance with the immunization schedule. The mother had a healthy pregnancy, and both parents, along with the infant’s older brother, were in good health.
On admission, physical examination revealed the following: Body temperature, 36.5 °C; pulse rate, 131/min; respiratory rate, 36/min; body length, 59 cm; and weight, 5.25 kg. The patient was alert and responsive, with a ruddy complexion, stable breathing, and coarse breath sounds in both lungs; a few coarse moist rales could be auscultated in both lungs. The anterior fontanelle measured approximately 2.5 cm × 2.5 cm and was slightly bulged. The infant’s pupil, heart, lung, and abdominal examination results were normal. Her navel was not red and had no discharge. Limb muscular strength and tension were normal. There were no abnormal neurological reflex examination findings, and the infant was bilaterally negative for Babinski, Kernig's, and Brudzinski's signs.
After admission, comprehensive laboratory tests, including TORCH, Epstein-Barr virus, Mycoplasma pneumoniae, Chlamydia pneumoniae immunoglobulin M, and influenza A/B antigen screenings, were performed immediately, and the results were all negative. Liver and kidney function, electrolyte levels, cardiac markers, and routine urinalysis and stool tests did not reveal any abnormalities. Blood cultures and sputum cultures were also promptly initiated. A cerebrospinal fluid (CSF) examination was conducted 9 hours after admission, and the CSF obtained via lumbar puncture was clear.
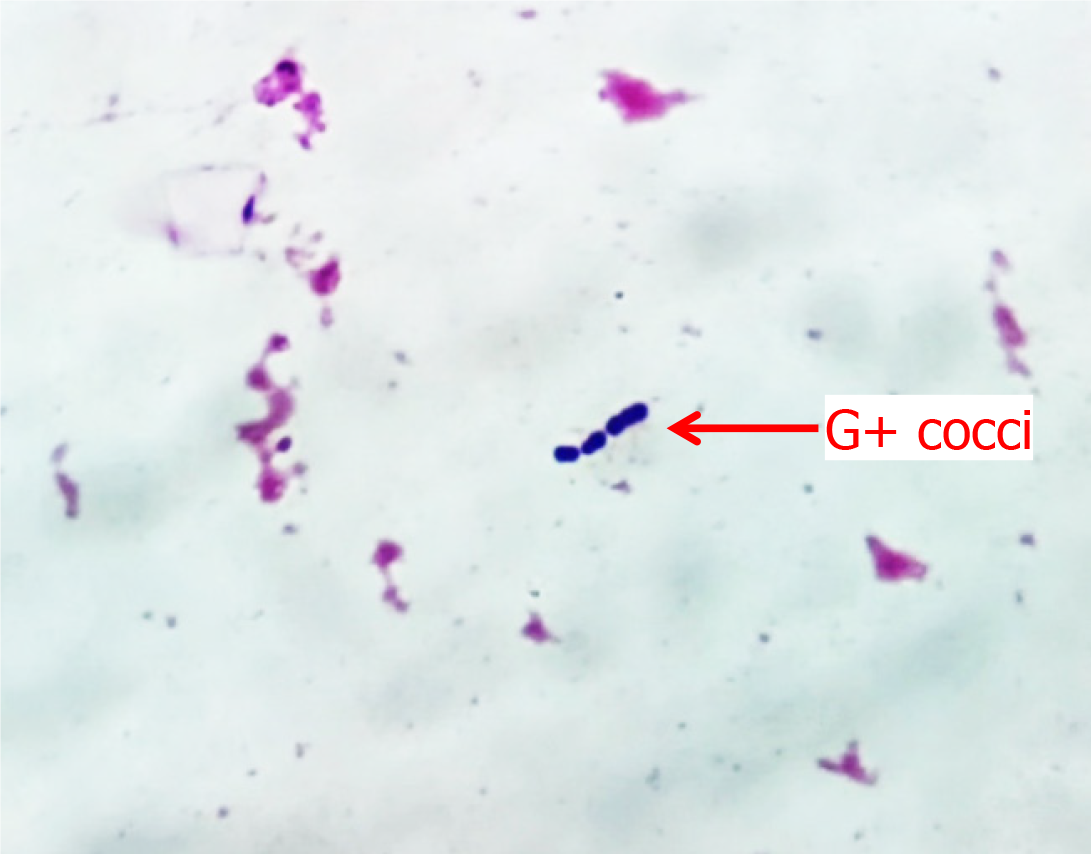
On Day 1, the infant's blood culture revealed gram-positive cocci arranged in short chains, as shown in Figure 1. On Day 3, the causative agent was detected as SGSP from the blood and CSF cultures via the VITEK 2 Compact System of bioMerieux, Inc. The bacterium was sensitive to ampicillin, furantoin, penicillin, lincomycin, moxifloxacin, tylosin, vancomycin, and levofloxacin, but it was resistant to clindamycin and tetracycline. From the sputum culture, MRSA was sensitive to vancomycin, suggesting a community-acquired infection. The relevant test results obtained during the hospitalization of the infant are shown in Table 1.
| Parameters | Day 1 | Day 2 | Day 4 | Day 9 | Day 12 |
| C-reactive protein level (mg/L) | 40.73 | 83.08 | 13.36 | 64.78 | 8.07 |
| White blood cell count (× 109/L) | 13.42 | 10.75 | 10.69 | 17.78 | 12.36 |
| Neutrophil ratio (%) | 78.4 | 69.2 | 51.0 | 69.0 | 28.2 |
| Procalcitonin (μg/L) | 7.89 | 11.20 | 3.16 | 0.18 | / |
| CSF white blood cell count (× 106/L) | 36 | / | 100 | / | 0 |
| CSF multinucleated cell ratio (%) | 95.2 | / | 89.0 | / | 0 |
| CSF glucose (mmol/L) | 3.53 | / | 2.92 | / | 2.66 |
| CSF protein concentration (g/L) | 0.41 | / | 0.54 | / | 0.36 |
| Blood culture result | G+ cocci | / | Neg | / | / |
| CSF culture result | G+ cocci | / | Neg | / | Neg |
| Urine and stool tests | NA | NA | / | NA | / |
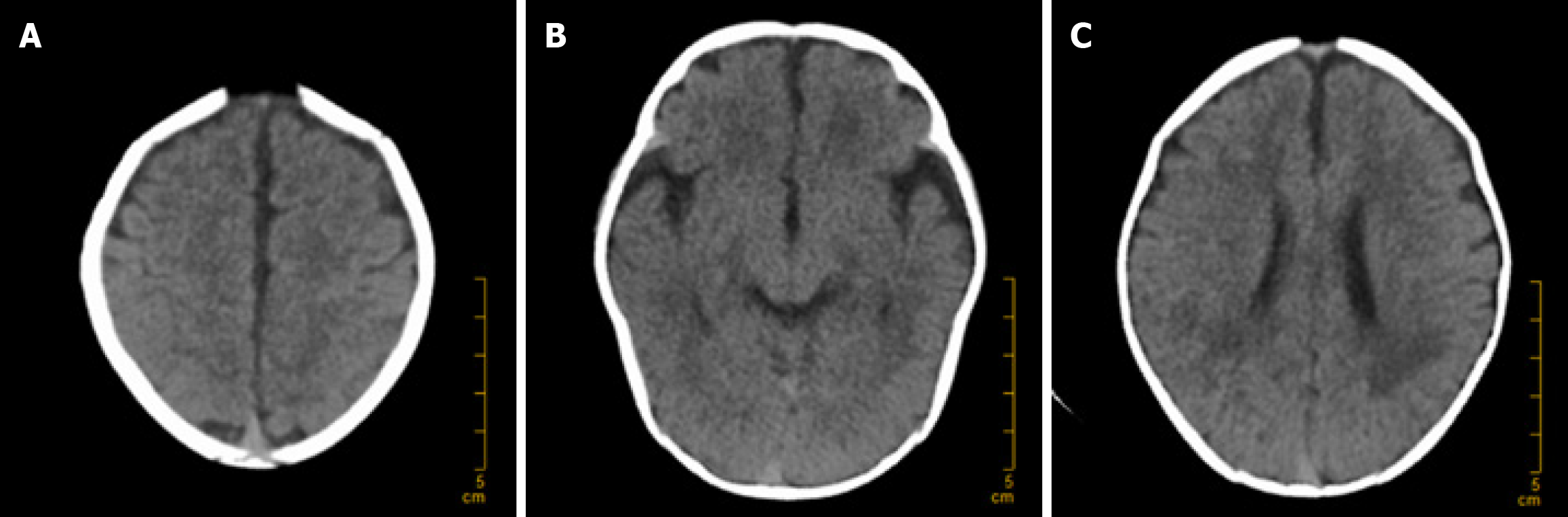
On Day 1, bedside chest X-ray, cranial ultrasound, and echocardiography were performed, with no evidence of endocarditis or related diseases. The dynamic electroencephalogram was normal. On Day 4, ultrasound of the brain, abdomen, urinary system, and gastrointestinal tract was conducted, revealing no purulent foci. On Day 8, a brain computed tomography (CT) scan was performed, which revealed no complications such as hydrocephalus or subdural effusion, as depicted in Figure 2.
The patient was diagnosed with meningitis and sepsis caused by SGSP, accompanied by bronchopneumonia induced by MRSA.
Upon admission, based on the infant's elevated C-reactive protein level (CRP), white blood cell count, procalcitonin level, and history of convulsions, we preliminarily considered the risk of intracranial bacterial infection. Therefore, we empirically administered ceftriaxone (100 mg/kg/d) for anti-infection and mannitol to reduce intracranial pressure. Ten hours after admission, the infant experienced two more seizures, both of which were generalized tonic-clonic seizures. After mitigation, the infant exhibited poor responsiveness, increased muscle tone in all four limbs upon stimulation, a rapid respiratory rate (approximately 80 beats/min), an accelerated heart rate (approximately 190 beats/minute), and a body temperature of 38.3 °C. The infant presented symptoms of septic shock. Immediate endotracheal intubation was performed, and ventilator-assisted breathing was implemented. After discussion among physicians, vancomycin (10 mg/kg/dose, q6h) was empirically added in combination with ceftriaxone for anti-infective therapy.
On Day 2, the infant did not experience any further convulsions but had recurrent fever, cough, and difficulty breathing. The infant also displayed symptoms of anemia, which resolved after a blood transfusion, resulting in normal facial coloration. On Day 3, the infant's body temperature returned to normal. On Day 4, follow-up tests revealed a significant decrease in the white blood cell count, CRP level, and procalcitonin level in blood samples compared with previous results, suggesting that the combined therapy was effective. The vancomycin concentration in the blood was 16.3 μg/mL, which was within the therapeutic reference range. Therefore, we continued with this treatment regimen. Subsequent blood cultures, CSF cultures, and sputum cultures revealed no bacterial growth. As the infant's blood oxygen saturation was normal and spontaneous respiration was adequate, she was successfully weaned off the ventilator on this day.
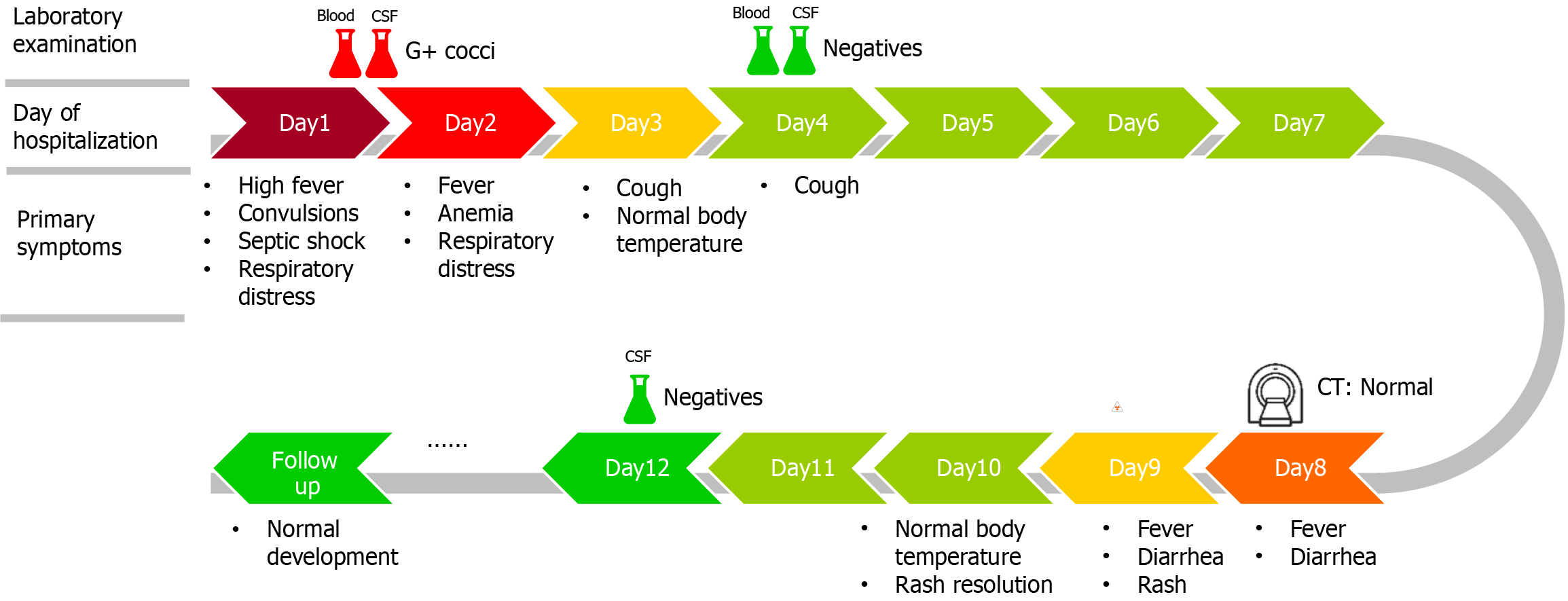
On Day 8, the infant developed a high fever (38.3 °C) accompanied by symptoms of diarrhea. We were vigilant for complications such as subdural effusion and performed a brain CT scan, which revealed no signs of hydrocephalus or subdural effusion, as shown in Figure 2. Routine stool examination revealed no red or white blood cells, and tests for rotavirus and adenovirus were negative. Symptomatic treatment with oral montmorillonite powder was administered, and after two days, the stool returned to normal. On Day 9, the infant developed a generalized erythematous rash, which was considered to be a delayed drug-induced allergic reaction. After the administration of ceftriaxone ceased, the rash significantly subsided. On Day 10, the infant's body temperature returned to normal. On Day 12, follow-up blood routine and CSF cultures revealed no significant abnormalities. The infant was discharged upon the family's request. The main symptoms of the patient over the course of hospitalization are depicted in Figure 3.
At present, the child is 1 year and 5 months old, and no growth retardation or neurological sequelae have been found during follow-up.
SGSP is a Lancefield group D Streptococcus, formerly known as Streptococcus bovis biotype II/2 or Streptococcus pasteurianus until its reclassification in 2003. SGSP infections primarily affect immunocompromised populations, including elderly individuals, pregnant women, and neonates, and can lead to gastrointestinal malignancies, diabetes, infectious endocarditis, intrauterine infections, urinary tract infections, pediatric meningitis, and bacteremia[3]. A 12-year data analysis conducted in France revealed that only 0.5% of pediatric bacterial meningitis cases caused by Streptococcus bovis, with further analysis revealing that 80% of the causative strains were of the SGSP subtype[4]. In a study on the microbiological and clinical characteristics of SGSP conducted in China, 45 cases of SGSP infection from eight hospitals across six provinces in China from 2011 to 2017 were analyzed. Only one case of late-onset pediatric meningitis was identified (approximately 2%), indicating that although SGSP is geographically widespread, it is a rare pathogen that causes pediatric disease[3]. Owing to the limited number of reports of SGSP infections, this restricts the possibility of conducting large-scale epidemiological studies, which may lead to an underestimation of the true incidence of infections caused by this bacterium. However, case reports and literature reviews are increasing our understanding and awareness of this bacterium[5-8].
The pathogenesis of bacterial meningitis involves pathogenic bacteria invading the bloodstream through the respiratory tract, digestive tract, or lower reproductive system. These bacteria then persist in the bloodstream, traverse the blood-cerebrospinal fluid barrier, and invade the subarachnoid space, ultimately leading to meningeal inflammation. In infants older than 3 months, bacterial meningitis is mainly caused by Neisseria meningitidis and Streptococcus pneumoniae, while in infants younger than 3 months, the main pathogens include group B streptococci, Neisseria meningitidis, Escherichia coli, and Streptococcus pneumoniae[9]. Because SGSP is a rare etiological agent causing bacterial meningitis in children, the risk factors for its pathogenesis are not fully understood at present. In terms of transmission routes, vertical transmission through maternal vaginal colonization and intrauterine infection has been implicated[10,11]. Prematurity, which is related to the immature blood-CSF barrier, appears to be a risk factor for SGSP infection[12]. However, retrospective studies have also indicated that special attention should be given to the risk of meningitis in children between 28 days and 2 years of age, especially those born prematurely (with an average gestational age of 32.5 weeks)[4]. Li et al[7] proposed the hypothesis that SGSP present in the gut of children could invade the bloodstream during periods of weakened immune function. Chang et al[13] reported the horizontal transmission route caused by gut colonization in infant caregivers. In this case, the full-term infant was born via cesarean section to a woman with a healthy pregnancy, and was 7 weeks old at the time of admission, thus making vertical transmission an unlikely source of infection. Prior to admission, the infant experienced an umbilical infection that resolved spontaneously after the topical application of penicillin, with the umbilical area being normal during hospitalization. Therefore, we consider that the infant may have contracted SGSP bacterial infection from the environment after the immune barrier was compromised. Concurrently, MRSA from the environment also invaded, leading to bronchopneumonia. On the eighth day of hospitalization, the infant experienced fever and diarrhea recurrence. Apart from elevated blood inflammatory marker levels, no other bacterial infections were detected. The possible causes are considered to be dysbiosis of the gut microbiota due to antibiotic use, or other hospital-acquired bacterial infections.
The common clinical manifestations of neonatal bacterial meningitis are fever and convulsions. Compared with older children, febrile infants under three months of age have higher rates of invasive bacterial infections[14]. The patient in this case was admitted primarily for fever and seizures, which are not specific compared with the clinical manifestations of meningitis caused by other bacterial infections. However, the patient had seizures, and her blood inflammation markers such as CRP and procalcitonin were significantly elevated, indicating the need for a CSF examination[14]. As a result, we conducted a CSF examination upon the patient admission and cultured bacteria, providing a basis for subsequent precision anti-infective therapy, which also helps to shorten the treatment duration and enhance therapeutic outcomes.
SGSP has good sensitivity to penicillin, ampicillin, cefotaxime, meropenem, and vancomycin, and good outcomes can be obtained with single or combined drug treatment[3,6]. Based on this infant’s history of fever and convulsions, we were alerted to potential penicillin-resistant bacterial infection. Ceftriaxone was our initial treatment for single-agent anti-infective therapy upon admission. As the infant experienced septic shock 10 hours after admission, the anti-infective treatment plan of vancomycin combined with ceftriaxone was actively used after discussion among the doctors. After subsequent laboratory testing revealed the pathogen's resistance profile, we found that our treatment approach was appropriate. In the later stages of treatment for this patient, a drug-induced rash appeared, which resolved after discontinuation of the ceftriaxone. We consider this to be a normal side effect of the medication. The treatment duration lasted for 12 days, and favorable therapeutic outcomes were achieved.
Bacterial meningitis is a significant cause of morbidity and mortality in neonates and children[15]. Common complications of bacterial meningitis in children include subdural effusion, epilepsy, hydrocephalus, hearing loss, and intracranial hemorrhage[16]. Although a cases of SGSP infection had presented with delayed central nervous system complications, no neurological sequelae were found during follow-up[17]. This suggests that the majority of pediatric patients with SGSP infection have a favorable prognosis[7]. At present, the child is 1 year and 5 months old, and no growth retardation or long-term neurological sequelae have been found during follow-up, and her hearing was normal, indicating the effectiveness of our treatment plan.
Our retrospective case report also has limitations. Since SGSP may originate from the infant’s caregivers, we did not perform cultivation and identification of intestinal bacteria for the caregivers, leading to uncertainty in the etiology of this case. Moreover, owing to the rarity of this bacterial infection, we are unable to discuss the recent epidemiological trends of SGSP.
We report a case of infant meningitis and septicemia caused by the rare pathogen SGSP, complicated by bron
| 1. | Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27:21-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 592] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 2. | Sim JY, Wang LW, Chow JC, Hsu WY, Chen YC, Chang YH, Chou Y, Chen WY, Tang HJ, Chang TH. Streptococcus gallolyticus - A potentially neglected pathogen causing neonatal sepsis not covered by routine group B streptococcus screening. J Microbiol Immunol Infect. 2021;54:1190-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Li Y, Chen X, Zhang Z, Wang L, Wang J, Zeng J, Yang J, Lu B. Microbiological and clinical characteristics of Streptococcus gallolyticus subsp. pasteurianus infection in China. BMC Infect Dis. 2019;19:791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Beneteau A, Levy C, Foucaud P, Béchet S, Cohen R, Raymond J, Dommergues MA. Childhood meningitis caused by Streptococcus bovis group: clinical and biologic data during a 12-year period in France. Pediatr Infect Dis J. 2015;34:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Iliodromiti Z, Tsaousi M, Kitsou K, Bouza H, Boutsikou T, Pouliakis A, Tsampou E, Oikonomidi S, Dagre M, Sokou R, Iacovidou N, Petropoulou C. Neonatal Sepsis Caused by Streptococcus gallolyticus Complicated with Pulmonary Hypertension: A Case-Report and a Systematic Literature Review. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Wang R, Huang S, Zhang S, Li Y. [Streptococcus gallolyticus meningitis: A systematic review]. Zhongguo Xunzheng Erke Zazhi. 2022;17:350-354. [DOI] [Full Text] |
| 7. | Li J, Yan C, Wei D, Gong X. Streptococcus gallolyticus Subspecies pasteurianus Meningitis in an Infant with Hypothyroidism and Diarrhea. Infect Drug Resist. 2023;16:6217-6223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Jaya-Bodestyne SL, Tan YY, Sultan R, Yeo KT, Kong JY. Clinical Course and Outcomes of Infants with Streptococcus bovis/Streptococcus Gallolyticus subspecies pasteurianus Infection: A Systematic Review and Meta-analysis. Pediatr Infect Dis J. 2024;43:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Okike IO, Ribeiro S, Ramsay ME, Heath PT, Sharland M, Ladhani SN. Trends in bacterial, mycobacterial, and fungal meningitis in England and Wales 2004-11: an observational study. Lancet Infect Dis. 2014;14:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Sasi S, Abid FB, Wilson GJ, Zaqout A, Nair AP, Chitrambika P. Intrauterine infection and postpartum bacteremia due to Streptococcus gallolyticus subsp gallolyticus: An emerging concern. IDCases. 2022;29:e01562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Binghuai L, Wenjun S, Xinxin L. Intrauterine infection and post-partum bacteraemia due to Streptococcus gallolyticus subsp. pasteurianus. J Med Microbiol. 2013;62:1617-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Orbea M, Desai N, Foster C. Invasive Streptococcus Gallolyticus Infections in Infants At Texas Children's Hospital: A 9-Year Retrospective Review. Pediatr Infect Dis J. 2022;41:e494-e497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Chang TH, Hsueh PR, Huang YT, Chen PY, Tang HJ, Chen JM. Prolonged Streptococcus gallolyticus subsp. pasteurianus gut colonization in healthcare workers and potential transmission role in neonatal sepsis. J Microbiol Immunol Infect. 2023;56:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Pantell RH, Roberts KB, Adams WG, Dreyer BP, Kuppermann N, O'Leary ST, Okechukwu K, Woods CR Jr; SUBCOMMITTEE ON FEBRILE INFANTS. Evaluation and Management of Well-Appearing Febrile Infants 8 to 60 Days Old. Pediatrics. 2021;148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 277] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 15. | Kim KS. Acute bacterial meningitis in infants and children. Lancet Infect Dis. 2010;10:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 16. | Subspecialty Group of Infectious Diseases, the Society of Pediatrics, Chinese Medical Association; Editorial Board, Chinese Journal of Pediatrics. [Expert consensus on the diagnosis and management of bacterial meningitis complications in children]. Zhonghua Er Ke Za Zhi. 2023;61:108-116. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Park JW, Eun SH, Kim EC, Seong MW, Kim YK. Neonatal invasive Streptococcus gallolyticus subsp. pasteurianus infection with delayed central nervous system complications. Korean J Pediatr. 2015;58:33-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |