INTRODUCTION
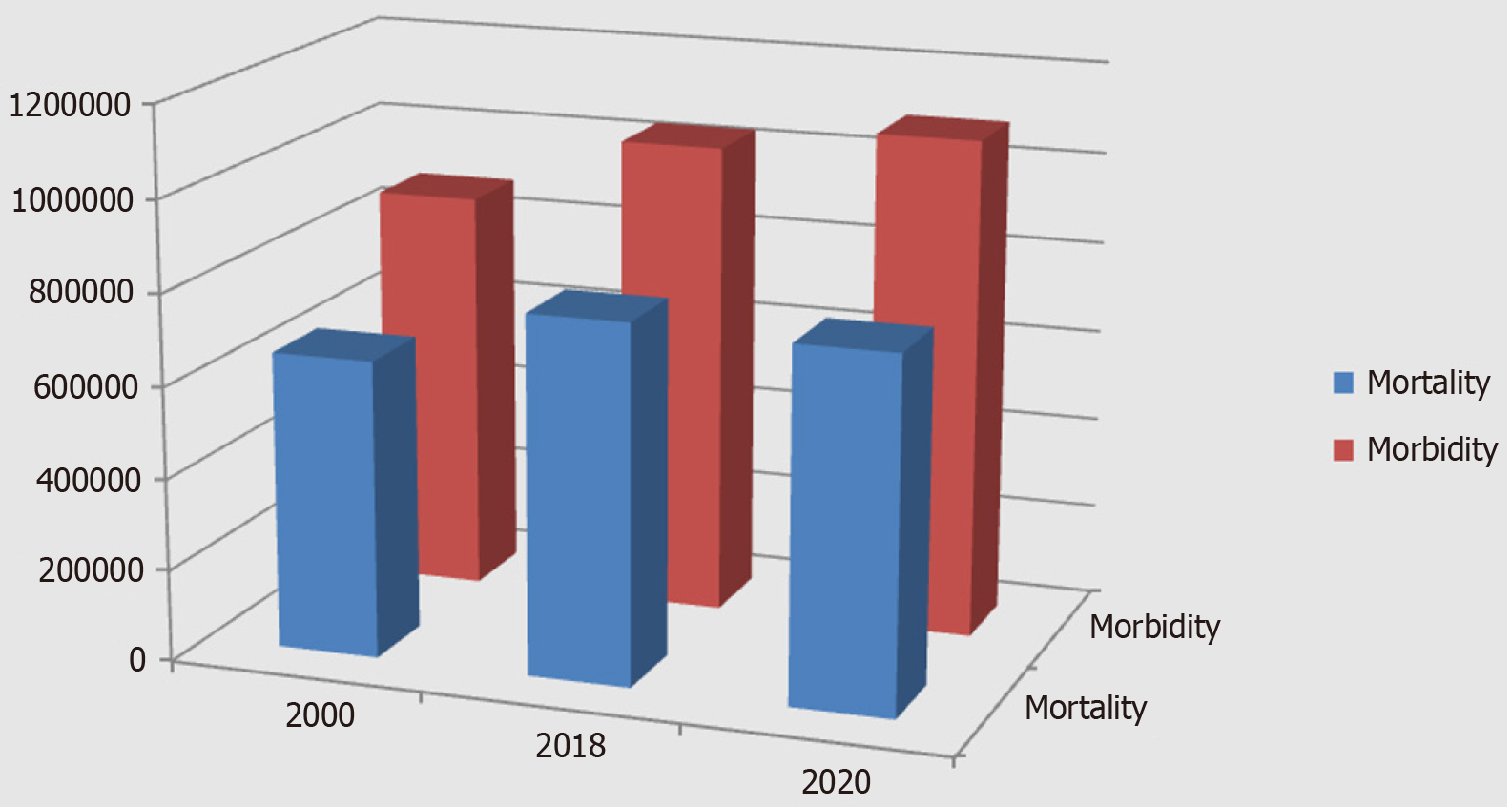
In 2000, 880000 people worldwide were diagnosed with stomach cancer and 650000 died from it[1]. Since then, there has been a steady increase in global statistical indicators of morbidity and mortality for stomach cancer. For example, in 2018, stomach cancer was the second leading cause of cancer-related death and the sixth most common cancer in the world. A total of 1033701 new cases of stomach cancer were reported in 2018, and 782685 patients have died from the disease (Figure 1). This number of deaths from stomach cancer accounts for about 8.2% of global cancer deaths[2]. In 2020, 1089103 people developed stomach cancer in the world, and 768793 of them died[3]. The disease still holds a leading position. Fifty percent of patients with stomach cancer and deaths from stomach cancer are residents of China[4]. The prognosis for the next few years is negative, and a further increase in the incidence of stomach cancer is expected[5]. The difference between the indicators of global population growth, the incidence of stomach cancer, and the death rate from stomach cancer is significant (P = 0.000). Therefore, it is necessary to distinguish between absolute and relative indicators of the 20-year dynamics of the incidence of stomach cancer and mortality from the disease. For 20 years, world statistics on stomach cancer have been characterized by an increase in such indicators as morbidity and mortality (in absolute terms). At the same time, the world's population has been characterized by an even more pronounced growth over the same period. Consequently, relative to the global population, there is a decrease in the incidence of stomach cancer and a more significant decrease in mortality from stomach cancer. Since 2020, new screening technologies for the detection, diagnosis, and treatment of precancerous conditions of the stomach have appeared and achieved perfection. First of all, this is the appearance of highly sensitive and highly specific serological markers for gastric precancerous conditions (atrophic gastritis), which allow effective screening of precancerous gastric pathology in the population of any number belonging to the risk group (40 +). Narrow-spectrum and magnifying endoscopy, using modern endoscopic equipment such as Olympus EXERA III GIF-190 HQ, Olympus EVIS X1, has achieved incredible success in the diagnosis of precancerous diseases and precancerous changes in the gastric mucosa. Narrowband imaging, texture and color enhancement imaging, red dichromatic imaging, and other image-enhanced technologies (image-enhanced endoscopy) have become frequently used in practical healthcare. In addition, the active introduction of computer detection using artificial intelligence in endoscopy has begun. An increase in magnification of 540 times has become available during endoscopic examination of the gastric mucosa (confocal laser endomicroscopy of the upper digestive tract allows a magnification to be increased to a thousand times). As a result, the diagnostic capabilities for detecting precancerous changes in the gastric mucosa have significantly increased[6].
Figure 1 Dynamics of morbidity and mortality from 2000 to 2020 (statistics on stomach cancer).
The dynamics of global statistics on morbidity and mortality due to gastric cancer show unsatisfactory results (Figure 1): (1) There is a decrease in the incidence and mortality of stomach cancer, relative to the growth of the world's population, from 2000 to 2020; (2) The positive dynamics of the incidence and mortality of stomach cancer is very poor, relative to the growth of the world's population (morbidity decreased by 3.5% and mortality by 9%) for 20 years; and (3) This indicates the low effectiveness of preventive and therapeutic measures for stomach cancer, which is associated with the insufficient implementation of modern screening and diagnostic technologies.
There are substantial, high-quality data supporting the effective prevention of gastric cancer that are derived from international studies[7]. Health systems use traditional primary and secondary prevention methods for gastric cancer. Primary prevention of gastric cancer includes mass screening and treatment programs for Helicobacter pylori (H. pylori) infection (“test and treat”), reducing consumption of tobacco and alcohol, and obesity control[8-11]. However, the role of other factors, such as lifestyle, diet, and drug use is still under debate in gastric carcinogenesis[12]. Secondary prevention of gastric cancer includes the diagnosis of early gastric cancer using narrow-band endoscopic imaging methods[13,14]. Some authors suggest introducing screening for atrophic gastritis and precancerous changes in the gastric mucosa to determine the level of risk of developing gastric cancer[15]. What type of prevention is this, primary or secondary? Researchers in some countries propose combining primary and secondary prevention methods to increase the effectiveness of gastric cancer prevention[15,16]. The financial burden makes it very difficult to implement various preventive actions in the population[17,18,19]. A consensus protocol for use in gastric cancer prevention, the global gastric cancer prevention strategy, is currently needed because traditional primary and secondary prevention methods are not effective.
CLASSIFICATION AND FEATURES OF GASTRIC CANCER
Lauren's classification defines two types of gastric cancer: Intestinal-type and diffuse-type[20]. The role of H. pylori in the carcinogenesis of gastric cancer of intestinal and diffuse types has been confirmed by many authors[21,22]. A comparative study of the entire complex of clinical, morphological, prognostic, and immunohistochemical characteristics and parenchymal-stromal relationships in gastric cancer allows us to confirm the feasibility of identifying three main histological classification forms of gastric cancer: Intestinal, diffuse, and mixed. The additional identification of the mixed form of gastric cancer includes significant, early unclassified tumors of complex structure with multidirectional differentiation of the cancerous epithelium according to Correa's cascade type[23,24].
STEPS OF PROTOCOL FOR GLOBAL STRATEGY FOR PREVENTION OF GASTRIC CANCER
The absence of symptoms is an important characteristic of early gastric cancer and precancerous diseases and changes. Such patients are not examined by modern endoscopic methods, such as confocal laser endomicroscopy, narrow-band imaging, and magnifying endoscopy. Modern endoscopic methods are not used for primary screening of early gastric cancer and precancerous lesions due to the high cost of equipment and sensors, as well as the need for trained specialists to interpret images. Invasive endoscopic methods can only be used to verify the diagnosis of precancerous changes and early gastric cancer in patients identified by screening methods. The population has no motivation for examination using invasive endoscopic methods in the absence of symptoms. The identified precancerous pathology (atrophic gastritis) by serological screening is the basis for motivating the patient to continue examination using endoscopic methods with biopsy. Morphological verification of precancerous pathology with a high risk of developing gastric cancer motivates the patient for annual endoscopic monitoring. Morphological examination of biopsy specimens is the “gold” standard for diagnosis, despite the high level of resources of modern endoscopic technologies. Morphological examination of biopsy specimens may not always be the reference standard for diagnosis: Example 1: Morphological diagnosis of the focus of gastric cancer is the reference standard for diagnosis; Example 2: Morphological diagnosis of atrophic gastritis is the reference standard for diagnosis; and Example 3: Morphological examination of biopsy samples of the gastric mucosa is not the standard for determining the level of risk of developing gastric cancer. The risk of developing gastric cancer is proportional to the severity of mucosal atrophy, with both ranging from low to high[25]. Atrophic gastritis is a multifocal atrophic process in the gastric mucosa. The gastric mucosa of different localizations has different degrees of severity of mucosal atrophy. How do you determine the integral severity of mucosal atrophy on the total surface of the stomach? The total surface area of the stomach ranges from 220 ± 27 cm2 to 536 ± 25 cm2. Changes in total gastric volume and total gastric surface area are associated with food intake and gastric emptying. During endoscopy, this is associated with air insufflation[26]. The severity of atrophy of the gastric mucosa is identified in the biopsy specimen in accordance with the morphological visual analogue scale and is determined by the degree of reduction in the number of gastric glands. The area of each biopsy is several mm2. It is impossible to calculate how much surface area of the stomach has mild, moderate, or severe atrophy using a biopsy. Using endoscopic visualization, it is possible to determine the surface area of the stomach with atrophy in accordance with the Kimura-Takemoto classification from C1 to O3. But modern endoscopic methods cannot reveal the severity of atrophy of the gastric mucosa in a specific surface area of the stomach. Endoscopic morphometry is not possible. It is unrealistic to assess the depth (severity) of atrophy of the gastric mucosa using modern endoscopic methods. The risk of developing gastric cancer cannot, therefore, be precisely detailed. The updated Kimura-Takemoto classification of atrophic gastritis is the most optimal way to assess the severity of atrophic gastritis and the risk of developing gastric cancer[27].
CONCLUSION
Global prevention of gastric cancer needs to increase its level of effectiveness. The prevention strategy should include all stages of primary and secondary prevention. The necessary steps to prevent gastric cancer are the following: (1) Maintaining a healthy lifestyle and diet, avoiding smoking and alcohol; (2) Serological screening of H. pylori infections and eradication; (3) Serological screening of atrophic gastritis in the population over 45 years of age and identification of severe atrophic gastritis with a high risk of developing gastric cancer; (4) Verification of atrophic gastritis and precancerous changes in the gastric mucosa using modern endoscopic (confocal laser endomicroscopy, narrow-spectrum imaging, and magnifying endoscopy) and morphological methods among patients with severe atrophic gastritis who were identified using serological screening; (5) Treatment of patients with atrophic gastritis during diagnosis verification; (6) Annual endoscopic and morphological monitoring of patients with atrophic gastritis during permanent treatment; (7) Annual serological monitoring of patients with atrophic gastritis who refused endoscopic and morphological monitoring; and (8) Radical treatment of patients with verified early gastric cancer.
Ways to implement the algorithm for the global strategy for the prevention of gastric cancer (protocol of practical recommendations): (1) State, government, and municipal programs; (2) Departmental programs of health departments; (3) Family doctors for patients who have a contract at the initiative of the doctor; (4) Family doctors for patients with a contract at the patient’s initiative; and (5) Within private healthcare system where both doctors and patients can initiate the implementation of algorithm.