Published online Jan 26, 2024. doi: 10.12998/wjcc.v12.i3.637
Peer-review started: November 8, 2023
First decision: December 5, 2023
Revised: December 8, 2023
Accepted: January 5, 2024
Article in press: January 5, 2024
Published online: January 26, 2024
Processing time: 69 Days and 3.7 Hours
Early initiation of enteral feeding is recognized to play a crucial role in improving the outcomes of treatment of acute pancreatitis. However, the method of administration of enteral nutrition remains debatable. We present the experience of treating a patient with moderate-severe acute pancreatitis, at high risk of progressing to a severe or fatal condition, using a novel method of selective feeding with duodenal isolation.
A 27-year-old female patient presented to the emergency unit of the hospital with a typical manifestation of acute pancreatitis. Despite a conventional treatment, the patient’s condition deteriorated by day 2 of hospitalization. Using an endoscopic approach, a novel catheter PandiCath® was placed to the duodenum of the patient, isolating its segment between the duodenal bulb and the ligament of Treitz. In the isolated area created, a negative pressure was applied, followed by introduction of early selective enteral feeding. The patient’s condition subsequently improved in a rapid manner, and no complications often associated with moderate-to-severe acute pancreatitis developed.
Within 48 h of starting treatment with the novel method, it can prevent the development of multiple organ failure and, when combined with minimally invasive drainage methods, help prevent infection.
Core Tip: Acute pancreatitis represents a common surgical disease; its moderate and severe forms are often associated with development of life-threatening complications. We report a case of acute pancreatitis where a standard treatment was augmented with duodenum decompression using a catheter of special design, further allowing introduction of early selective enteral feeding, leading to rapid improvement of the patient’s condition, with no complications. Our observations suggest that this approach may be beneficial for moderate and severe cases of acute pancreatitis.
- Citation: Kashintsev AA, Anisimov SV, Nadeeva A, Proutski V. Early selective enteral feeding in treatment of acute pancreatitis: A case report. World J Clin Cases 2024; 12(3): 637-642
- URL: https://www.wjgnet.com/2307-8960/full/v12/i3/637.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i3.637
Acute pancreatitis ranks among the top five abdominal surgical diseases worldwide[1,2]. According to the international treatment recommendations for acute pancreatitis, intensive therapy should be complemented with gastric and intestinal drainage and early enteral feeding[3]. However, the method of administering nutrition, whether via oral, gastric, or intestinal route, remains unresolved[4,5]. A new treatment method utilizing the novel catheter PandiCath® has been developed[6,7], offering several potential treatment mechanisms, including selective enteral feeding. The device temporarily isolates and disconnects the duodenum from the stomach and proximal jejunum, preventing acidification of duodenal contents as well as reflux of bacterial flora from the small intestine to the duodenum. Additionally, it creates a negative pressure area in the duodenum to enhance drainage of biological fluids from the hepatic and pancreatic duct systems, preventing bile reflux and reducing intraductal pressure. Finally, by delivering nutrients to the jejunum while draining the stomach, PandiCath® prevents the gastric outlet syndrome. We report a case where this approach augmented a standard treatment for acute pancreatitis. Our findings provide insight into the new promising method of treatment of moderate-to-severe cases of acute pancreatitis.
A 27-year-old female patient was admitted to the hospital in an emergency on March 20, 2023. The clinical presentation of acute pancreatitis was typical. At the time of examination, the patient complained of abdominal pain, nausea, and vomiting. The intensity of the pain assessed using the visual analog scale (VAS) was 10[8].
The patient reported experiencing the above symptoms for the first time on March 19, 2023, after consuming alcohol and fatty food. Negative prognostic factors included type 2 diabetes diagnosed in October 2022 and being overweight (height - 172 cm, weight - 86 kg, body mass index = 29.1).
No previous history.
No personal and family history.
On physical examination, the heart rate was 88 beats per minute, blood pressure was 130/70 mmHg, respiratory rate was 16 per minute, and body temperature was 36.8 °C.
Laboratory analyses did not show significant deviations: White blood cell count - 8.68, hematocrit - 39.9%, blood amylase - 151 IU/L, glucose - 18.39 mmol/L, C-reactive protein (CRP) - 78.3 mg/L, and serum was lipemic.
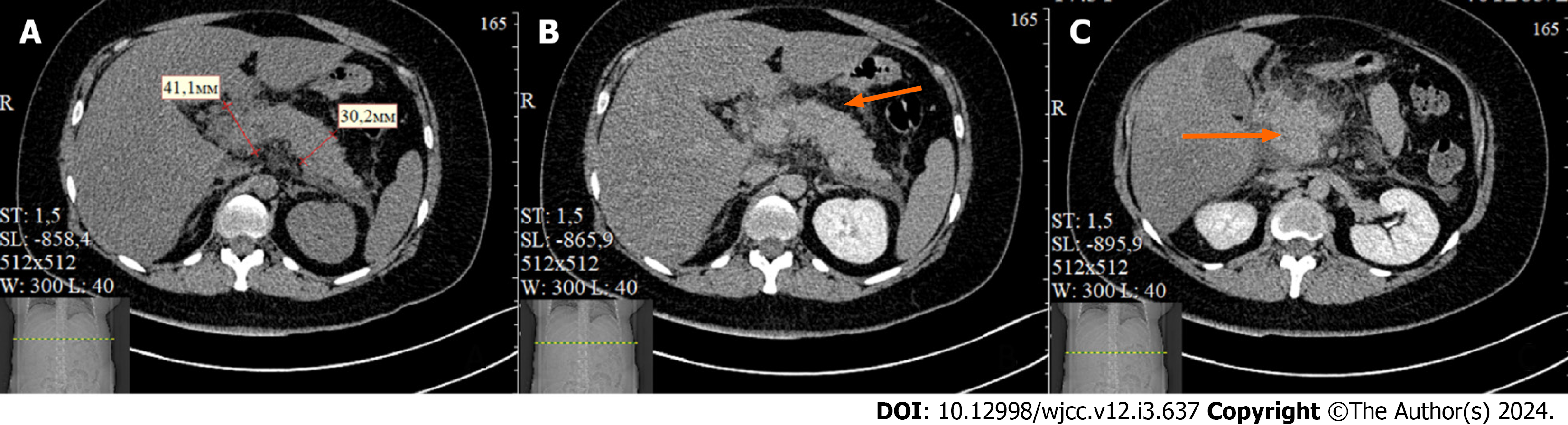
Abdominal ultrasound showed an enlargement of the pancreas with blurred contours. Chest X-ray revealed pleural effusion. Enhanced abdominal computed tomography (CT) showed an enlargement of the pancreas up to 45 mm with formation of the para-pancreatic infiltrate. No necrotic changes were observed in the pancreatic tissue. Accumulation of fluid was detected in the para-pancreatic and para-renal spaces with a thickness of 11-35 mm, as well as pleural effusion (Figure 1). According to the Computed Tomography Severity Index criteria, the patient's score was 6, and according to the Modified Computed Tomography Severity Index, the score was 8, which suggested a moderate-severe or severe form of acute pancreatitis[9].
Acute pancreatitis, severe form; systemic inflammatory response syndrome (SIRS).
Within 24 h from disease onset (on March 20, 2023), the patient was admitted to the intensive care unit (ICU) and active fluid resuscitation therapy was initiated. On March 21, 2023, the patient's condition deteriorated, manifesting with somnolence and toxic encephalopathy - the patient appeared indifferent to the ongoing treatment, requested to be left alone, and expressed suicidal thoughts. SIRS developed, with the following vital signs: Heart rate - 116 beats per minute, respiratory rate - 21 per minute, receiving oxygen inhalation at a rate of 5 Liters per hour, oxygen saturation - 96%, and body temperature - 37.3 °C. Laboratory findings showed leukocytosis - 5.6 and hematocrit - 35.2%, with a shift in the leukocyte formula towards band cells - 34%. The blood amylase level was 276 IU/L, glucose was 16.3 mmol/L, calcium was 1.95 mmol/L, and CRP increased to 432.4 mg/L. The procalcitonin level was 0.41 ng/mL.
Based on the Bedside Index for Severity in Acute Pancreatitis scale, an increase in score from 2 to 3 was observed, which corresponds to moderate-severe pancreatitis with an increased risk of mortality. Patient’s RANSON score was 3, with an estimated risk of mortality of 15%[10]. The patient experienced severe pain with a VAS score of 10. On examination of the upper abdomen, an inflammatory infiltrate and positive peritoneal signs were identified. On a Focused Assessment with Sonography for Trauma ultrasound examination on March 21, abdominal effusion was detected in the subhepatic and intersigmoid spaces, predominantly on the right side, with a maximum thickness of up to 50 mm. Based on the clinical findings, it was decided to perform abdominal drainage under ultrasound guidance. A total of 600 mL of hemorrhagic effusion was obtained, with amylase activity measuring 2079 IU/L. A prolonged epidural anesthesia was applied at 11 am on March 21.
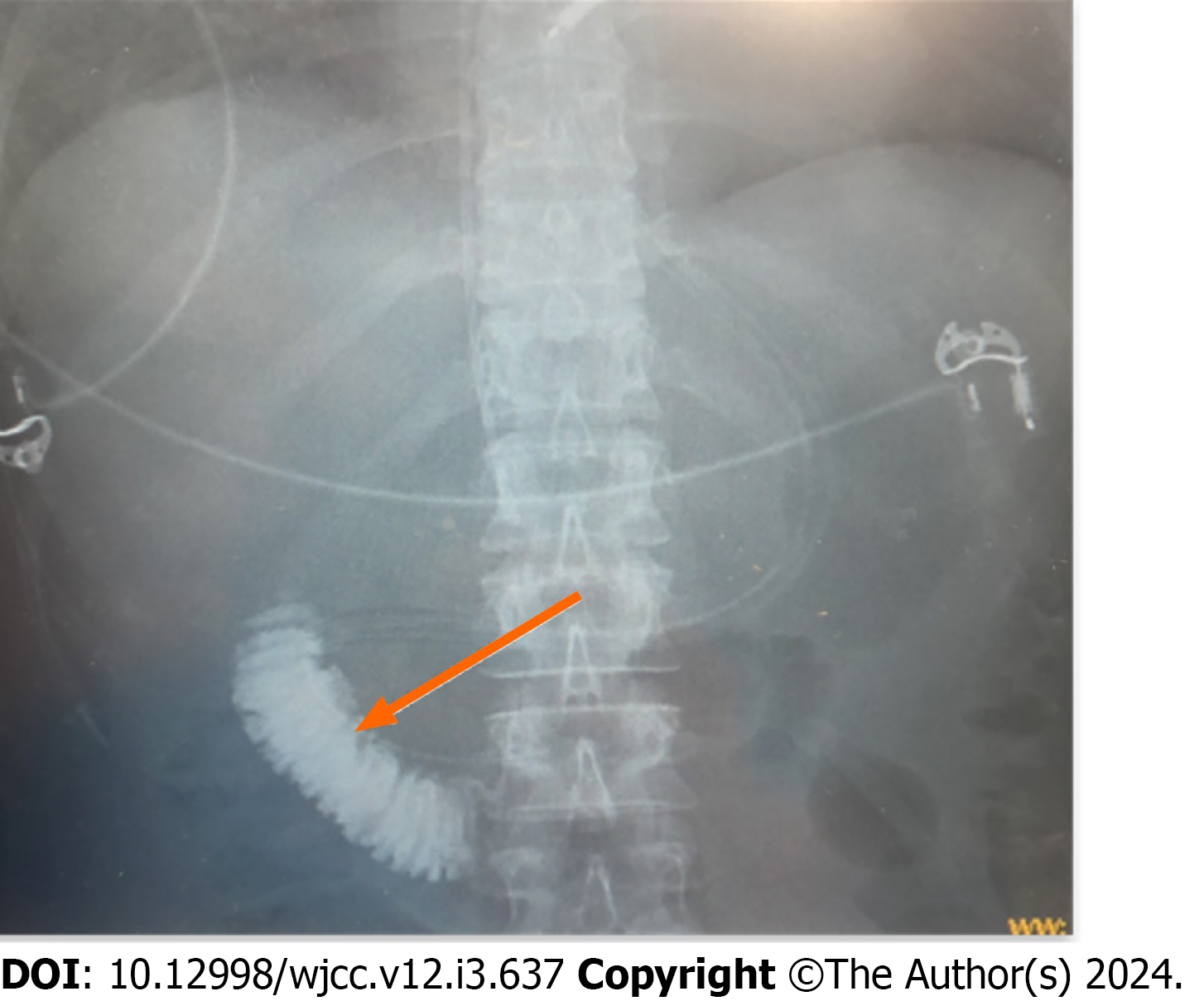
It was then decided that the patient fits to a prospective, parallel-group, open-label, multicenter, centrally randomized study that was conducted from October 2020 to May 2023. The two groups of the study included standard therapy vs standard therapy with the early selective feeding method. The randomization process followed pre-established patient distribution tables. The study protocol was approved by the local and independent ethics committees. Additional insurance for the patients participating in the study was arranged. The study included patients with their first episode of acute pancreatitis, presenting with non-mild forms of the disease of alimentary and biliary etiology without indications for endoscopic retrograde cholangiopancreatography. The randomization occurred within 96 h from the onset of the pancreatitis episode. The objectives and purposes of the study were explained to each patient, who provided voluntary consent before being enrolled. After verifying all inclusion and exclusion criteria for the study and obtaining consent, the patient was assigned, according to the randomization table, to the study group combining standard therapy and early selective enteral feeding. Using an endoscopic approach, at 3 pm on March 21, a PandiCath® catheter was placed isolating a segment of the duodenum between the duodenal bulb and the ligament of Treitz. In the isolated area created, a negative pressure was applied at a level of 80 mmHg using a VAC pump, resulting in the evacuation of duodenal contents mixed with bile and pancreatic juice. Activity of lipase in evacuated biological fluid was 6502 IU/L. The correct placement of the catheter was verified radiologically (Figure 2).
Three hours after the catheter placement, intensity of the pain syndrome decreased and was assessed with a VAS score as 3. On March 22, 2023, it was noted that the patient's mental status improved, and the patient now understood the severity of her condition. Negative thoughts disappeared, and the patient no longer had suicidal thoughts, understanding that prolonged treatment lied ahead.
The administration of a glucose-saline mixture was initiated through the catheter in a selective manner (i.e., into the proximal part of the jejunum) on March 22, 2023, one hour after catheter placement. It was followed 3 h later by the introduction of enteral nutrition mixture rich with microfiber at 30 kcal/kg. Improvement in intestinal peristalsis was observed from March 22, as evidenced by the absence of evacuation of intestinal contents through the catheter.
On March 23, the patient's peristalsis was fully restored, and physiological parameters were close to normal (heart rate - 80 beats per minute, blood pressure - 120/70 mmHg, respiratory rate - 16 breaths per minute). It was decided to discontinue active decompression of the duodenum and to remove the catheter, transitioning the patient to oral feeding, which was well tolerated. The patient's vital signs improved, and the manifestations of SIRS disappeared. The CRP level decreased to 172.3 mg/L, and the concentration of immature forms of leukocytes was reduced to 8%. The severity assessment using the Sequential Organ Failure Assessment score was 0. On March 24, the patient's conditions continued to improve and she was transferred from the ICU to a surgical ward.
On March 27, a change of a drainage catheter was performed under ultrasound guidance to remove the remaining inflammatory effusion from the peritoneal cavity. Bacteriological analysis of the abdominal fluid was negative, indicating an aseptic course of acute pancreatitis. On April 5, the drainage catheter was removed.
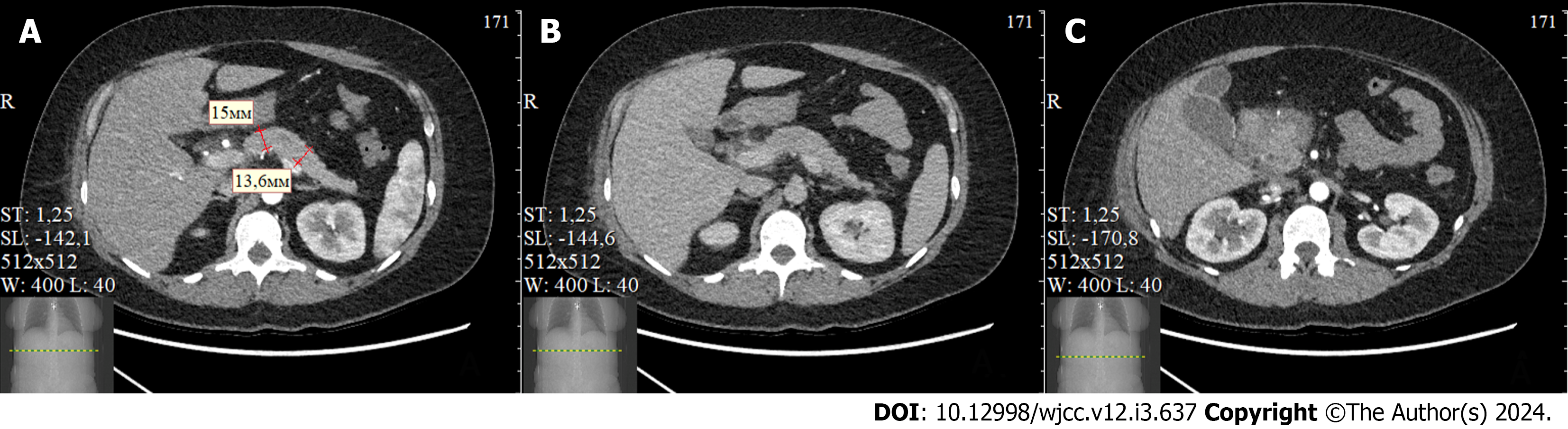
A follow-up CT scan on the 16th day post-admission showed positive progress, with no evidence of necrotic changes in the pancreas, and no signs of infection (Figure 3).
The patient was discharged from the hospital on the 18th day of admission. Three months after discharge, the patient reported no recurrent acute pancreatitis attacks, no further hospitalizations, no abdominal pain, and no fever. The patient experienced no limitations in her somatic status and had returned to normal work routine without any issues.
Within 24 h of hospitalization, the patient receiving standard therapy developed SIRS with a sharp increase in CRP level and other indicators pointing to a poor prognosis and an increased risk of mortality. However, after the addition of the novel therapy, positive dynamics were observed within 48 h, despite the initial presence of factors predisposing to the development of multiple organ dysfunction syndrome.
This case demonstrates a favorable impact of the early selective enteral feeding on the patient's treatment outcome and highlights its potential benefits in preventing severe complications associated with acute pancreatitis. However, certain aspects of the treatment strategy remain subject to discussion, such as the need of peritoneal drainage and the duration of stay in the ICU and overall length of hospitalization. These decisions may vary depending on surgical traditions and treatment strategies adopted by different hospitals.
From a pathophysiological standpoint, the proposed approach confers several therapeutic benefits. Temporarily isolating the duodenum prevents acidification and the entry of mucus and nutrients from the stomach. This effectively inhibits the activation of duodenal enterokinases and trypsinogen. Furthermore, decreased gastric chyme reduces secretion of secretin and cholecystokinin, key players in the development and progression of autolytic aseptic inflammation in the pancreas[11-15]. Preventing the reflux of contents from the small intestine to the duodenum ensures the maintenance of the appropriate composition of microbial flora in respective sections of the gastrointestinal tract. Protection of the duodenum from the passage of gastric and reflux of intestinal contents along with maintaining a negative pressure area in the duodenum prevents the elevation of intraduodenal pressure and stretching of intestinal wall, which otherwise could heighten the risk of enterocyte death compromising the gut’s barrier function and increasing the likelihood of bacterial translocation and para-pancreatic tissue infection. Creation of a negative pressure area in the duodenum also facilitates the outflow of bile and pancreatic juice, thus inhibiting premature activation of enzymes sustaining pancreatic inflammation[11-15]. During digestion, normal outflow of bile and pancreatic juice is facilitated by the coordinated peristalsis and motility of the duodenum and jejunum. However, these mechanisms are disrupted during an acute pancreatitis attack, leading to paresis, which subsequently leads to the development of further severe complications.
Previous research on the impact of enteral feeding on the outcomes of treatment of acute pancreatitis did not reveal significant differences between the various methods of administering nutritional mixtures. Nonetheless, it is important to consider that when the passage of gastric juice, mucus, chyme, and fluid continues through the duodenal lumen, it may trigger humoral stimulation mechanisms that could lead to the exacerbation and prolongation of the inflammatory process and autolysis of the pancreas.
In the case described, we used PandiCath® as a treatment tool but also for collecting a mixture of bile with pancreatic juice, which can be evaluated for various molecular markers. In particular, high activity (6502 IU/L) of lipase, a specific pancreatic enzyme, was detected in this substrate. Thus, the ability of PandiCath® to efficiently collect significant volumes of biofluids could make it a useful diagnostic tool.
The proposed treatment method provides a “humoral rest” of the pancreas, while at the same time local application of negative pressure re-creates normal physiological conditions, facilitating the drainage of bile and pancreatic juice. The essential role of early enteral feeding has been previously proven[3-5]. Application of the early selective enteral feeding aligns well with existing international standards for the treatment of acute pancreatitis and has the potential to improve it. Furthermore, the proposed method can be complemented with targeted delivery of medicines into the isolated area of the duodenum, potentially enhancing its effectiveness. Isolation of the duodenum is achieved by inflating catheter balloons with a liquid. Some authors suggested that applying local pancreatic hypothermia reduces severity of acute pancreatitis[16], and we further suggest that using chilled liquid to inflate balloons of PandiCath® could also help achieve this goal.
Overall, the novel treatment approach described in this report shows promise in improving acute pancreatitis patients’ outcome and can potentially contribute to improving standard of care for this disease. Further studies are necessary to validate and refine the proposed method for a broad clinical application.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American association for cancer research, 461256.
Specialty type: Emergency medicine
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Li C, China; Yin C, China S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Cai YX
| 1. | Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156:254-272.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1072] [Article Influence: 178.7] [Reference Citation Analysis (1)] |
| 2. | Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, Petrov MS. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 470] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 3. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-e15.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1028] [Article Influence: 85.7] [Reference Citation Analysis (6)] |
| 4. | Bakker OJ, van Brunschot S, van Santvoort HC, Besselink MG, Bollen TL, Boermeester MA, Dejong CH, van Goor H, Bosscha K, Ahmed Ali U, Bouwense S, van Grevenstein WM, Heisterkamp J, Houdijk AP, Jansen JM, Karsten TM, Manusama ER, Nieuwenhuijs VB, Schaapherder AF, van der Schelling GP, Schwartz MP, Spanier BW, Tan A, Vecht J, Weusten BL, Witteman BJ, Akkermans LM, Bruno MJ, Dijkgraaf MG, van Ramshorst B, Gooszen HG; Dutch Pancreatitis Study Group. Early versus on-demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med. 2014;371:1983-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 197] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 5. | Dutta AK, Goel A, Kirubakaran R, Chacko A, Tharyan P. Nasogastric versus nasojejunal tube feeding for severe acute pancreatitis. Cochrane Database Syst Rev. 2020;3:CD010582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (2)] |
| 6. | Kashintsev AA, Rusanov DS, Antipova MV, Anisimov SV, Granstrem OK, Kokhanenko NY, Medvedev KV, Kutumov EB, Nadeeva AA, Proutski V. Hemostasis of massive bleeding from esophageal tumor: A case report. World J Gastrointest Endosc. 2022;14:636-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Kashintsev AA, Proutski VYu, Anisimov SV, Granstrem OK. Catheter and method for isolating a region in a hollow organ of a mammal, and system based on the catheter, and use of the catheter. WO/2021/137739. 2021. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Jamison RN, Gracely RH, Raymond SA, Levine JG, Marino B, Herrmann TJ, Daly M, Fram D, Katz NP. Comparative study of electronic vs. paper VAS ratings: a randomized, crossover trial using healthy volunteers. Pain. 2002;99:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Mikó A, Vigh É, Mátrai P, Soós A, Garami A, Balaskó M, Czakó L, Mosdósi B, Sarlós P, Erőss B, Tenk J, Rostás I, Hegyi P. Computed Tomography Severity Index vs. Other Indices in the Prediction of Severity and Mortality in Acute Pancreatitis: A Predictive Accuracy Meta-analysis. Front Physiol. 2019;10:1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | MDCALC application. Accessed August 14, 2023. Available from: https://www.mdcalc.com. |
| 11. | Thrower E, Husain S, Gorelick F. Molecular basis for pancreatitis. Curr Opin Gastroenterol. 2008;24:580-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Hammer HF. An update on pancreatic pathophysiology (do we have to rewrite pancreatic pathophysiology?). Wien Med Wochenschr. 2014;164:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Barreto SG, Habtezion A, Gukovskaya A, Lugea A, Jeon C, Yadav D, Hegyi P, Venglovecz V, Sutton R, Pandol SJ. Critical thresholds: key to unlocking the door to the prevention and specific treatments for acute pancreatitis. Gut. 2021;70:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 14. | Swain SM, Romac JM, Shahid RA, Pandol SJ, Liedtke W, Vigna SR, Liddle RA. TRPV4 channel opening mediates pressure-induced pancreatitis initiated by Piezo1 activation. J Clin Invest. 2020;130:2527-2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 15. | Chandra R, Liddle RA. Regulation of pancreatic secretion. In: Gorelick FS, Williams JA. eds. The Pancreas: Biology and physiology. Michigan Publishing Manufactured. 2021;221-250. [DOI] [Full Text] |
| 16. | de Oliveira C, Khatua B, Bag A, El-Kurdi B, Patel K, Mishra V, Navina S, Singh VP. Multimodal Transgastric Local Pancreatic Hypothermia Reduces Severity of Acute Pancreatitis in Rats and Increases Survival. Gastroenterology. 2019;156:735-747.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |