Published online Jan 26, 2024. doi: 10.12998/wjcc.v12.i3.630
Peer-review started: November 6, 2023
First decision: November 22, 2023
Revised: December 3, 2023
Accepted: January 2, 2024
Article in press: January 2, 2024
Published online: January 26, 2024
Processing time: 72 Days and 19.3 Hours
Esophageal adenoid cystic carcinoma (EACC) is an exceedingly rare malignant tumor of the esophagus, posing significant challenges in the clinic.
This report detailed the case of a 72-year-old male whose diagnosis of EACC was confirmed through postoperative histopathological examination. The patient underwent thoracoscopy-assisted radical resection of the esophageal tumor, coupled with lymph node dissection. Pathological findings revealed an adenoid cystic carcinoma infiltrating the entire layer of the muscularis propria, locally extending into the outer membrane of the esophageal fiber, involving the cardia and exhibiting no lymph node metastasis. The patient’s condition was classified as primary EACC, T3N0M0, per the American Joint Committee on Cancer (2017; 8th edition). One month after surgery, the patient received postoperative adjuvant radiation therapy.
In addressing the rarity and high potential for biopsy misdiagnosis of EACC, this study delved into its diagnostic methods and treatment.
Core Tip: This manuscript discussed the rarity of esophageal adenoid cystic carcinoma, a malignancy accounting for only 0.1% of esophageal cancers. The case report highlighted a 72-year-old male with dysphagia, initially misdiagnosed as squamous cell carcinoma. The patient underwent radical resection and adjuvant radiotherapy. The discussion emphasized the challenges in diagnosing esophageal adenoid cystic carcinoma, its histological features, and controversies regarding its origin and prognosis. Limited data and a lack of standardized treatment protocols underscore the need for further research to determine optimal management and prognosis for this rare malignancy.
- Citation: Geng LD, Li J, Yuan L, Du XB. Rare esophageal carcinoma-primary adenoid cystic carcinoma of the esophagus: A case report. World J Clin Cases 2024; 12(3): 630-636
- URL: https://www.wjgnet.com/2307-8960/full/v12/i3/630.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i3.630
Adenoid cystic carcinoma (ACC) is an epithelial tumor that primarily manifests in the salivary glands, with additional occurrences documented in the tracheobronchial tree, breast, skin, lacrimal gland, and female genital tract. ACC, characterized by its slow- growth, constitutes approximately 18.1% of all salivary gland neoplasms[1], with 60% of cases originating in the minor salivary glands. Esophageal ACC (EACC) represents 0.1% of esophageal malignancies[2]. EACC is a rare clinical entity that mostly consists of individual reports or small sample cases in the literature, approximately half of which are from Asian populations. It is a malignant epithelial tumor of the esophagus that differentiates into epithelial cells. The arrangement of epithelial and myoepithelial cells forms true and false glandular lumens, exhibiting cribriform, tubular, or solid structures. Regional lymph node metastasis is rare in patients with EACC, but metastasis to the lungs and bones is common[3]. The most predominant symptom is dysphagia. Diagnostic confirmation and disease extent assessment rely on endoscopy and endoscopic ultrasonography, revealing protrusions, bulges, or ulcerative lesions. Radiological assessment utilizing computed tomography (CT), magnetic resonance imaging, or positron emission tomography scans further elucidates the clinical manifestations of the tumor. The prognosis is contingent on the pathological stage. Prior research has yielded conflicting findings regarding the prognostic indicators for EACC. Some studies assert that a higher incidence of lymph node metastasis and vascular invasion is associated with a less favorable prognosis compared to that of squamous cell carcinoma (SCC)[4,5]. Conversely, recent studies revealed that lymph node metastasis is less prevalent at an early stage, leading to a superior prognosis to that of SCC. Hiromichi et al[6] reported that the average overall survival of patients with EACC was 25 mo with tumors having no mixture of SCC or basal cell carcinoma (BSC) components.
A 72-year-old male presented to the hospital with a complaint of dysphagia.
The patient developed dysphagia 5 mo ago with no obvious cause, which was evident when eating rough food, no poststernal burning pain, no back pain, no nausea, vomiting, or fever, and no hoarseness. Three months prior, the patient was admitted to our hospital. The gastroscopy showed that a new protrusion-type tumor could be seen about 35 cm away from the incisor, and the examination indicated poorly differentiated cancer. The chest enhanced CT showed that the lower thoracic segment of the esophagus and the wall of the cardiac tube were irregularly thickened, which indicated the possibility of esophageal cancer. Small nodules in the apex segment of the upper lobe of the right lung were considered chronic inflammation. Calcification in the dorsal segment of the lower lobe of the right lung and scattered interstitial changes in both lungs were observed.
The patient had no history of hypertension or diabetes.
The patient had a history of smoking and drinking for > 40 years.
On physical examination, the vital signs were as follows: body temperature, 36.4 °C; heart rate, 62 beats per min; respiration, 20 breaths per min; blood pressure, 125/79 mmHg; and Eastern Cooperative Oncology Group score, 0 points. The superficial lymph nodes of the whole body were not enlarged. Lung sounds were normal, the heart boundary was not enlarged, the membrane was flat and soft, and there was no tenderness or rebound pain. Physiological reflex existed, and pathological reflex did not arch out.
Blood analysis, liver function, kidney function, coagulation function, and urine and stool analysis showed no abnormalities.
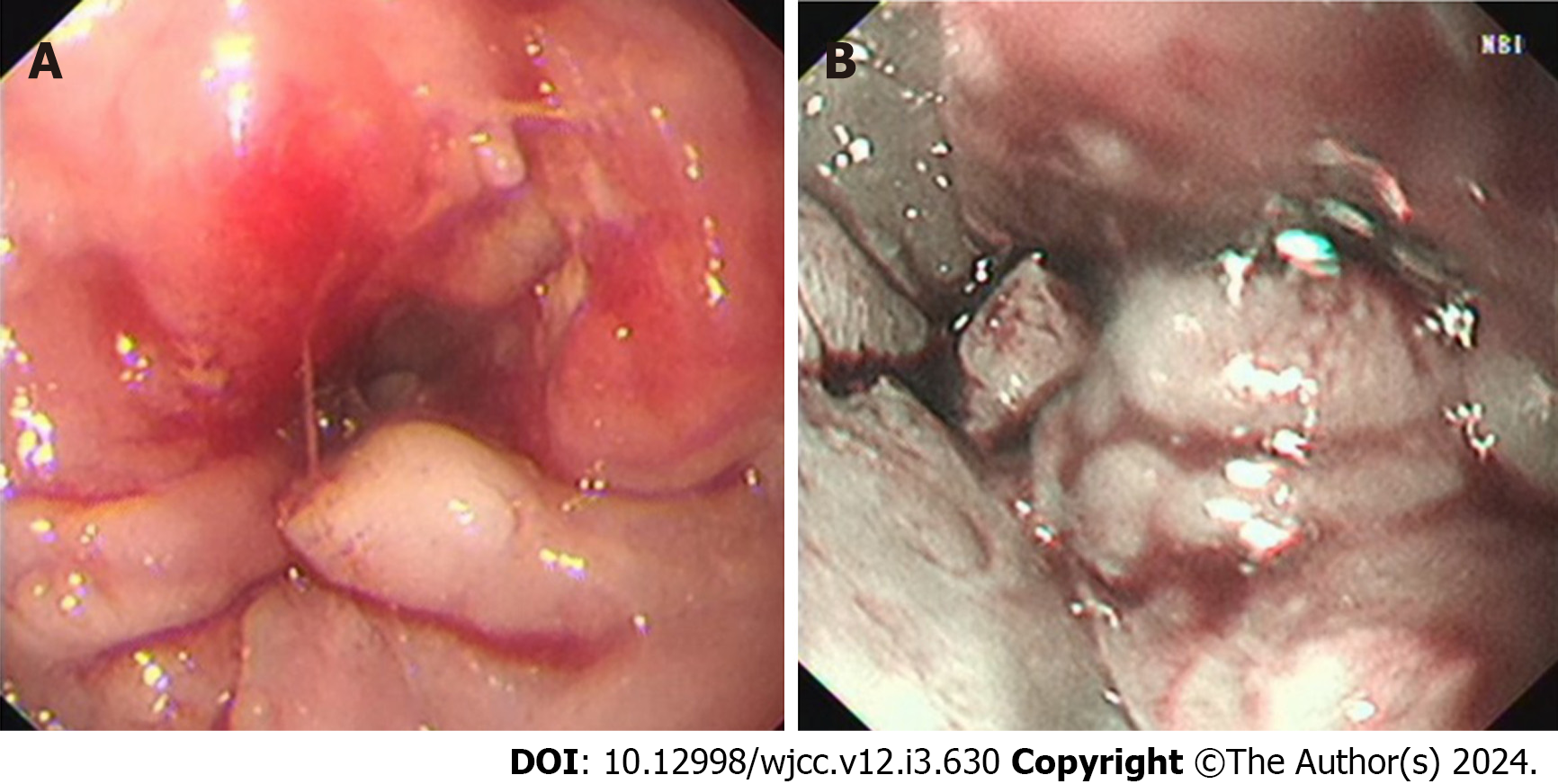
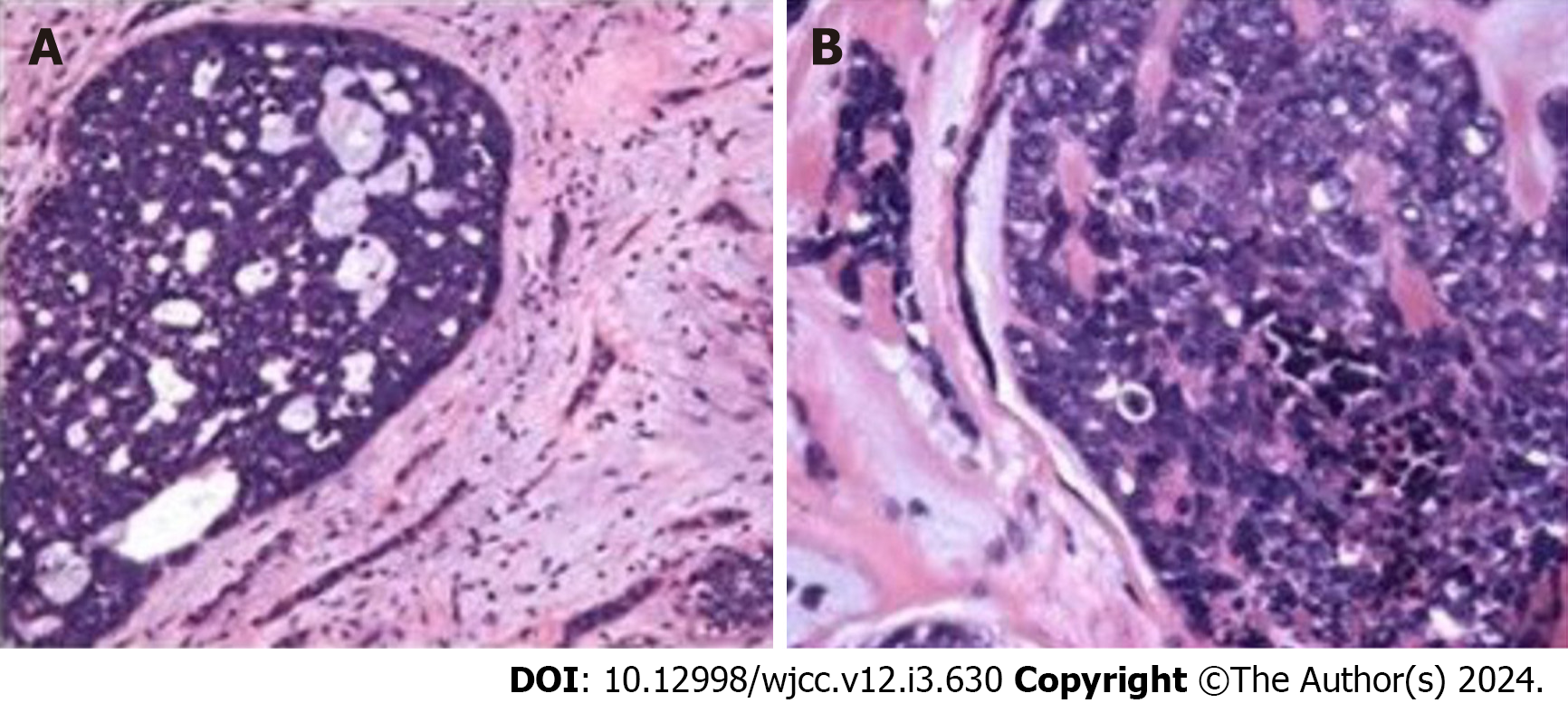
Narrow band imaging revealed vascular texture, and biopsy indicated soft texture (Figure 1). Pathological section findings were observed by microscopy (Figure 2).
The patient underwent enhanced chest CT before surgery, which indicated that the tumor was in the lower esophagus without distant metastasis. The Department of Cardiothoracic Surgery recommended surgery for the patient, and the postoperative pathological examination of the patient indicated ACC. Cancer invasion was found around the nerve, and cancer embolus was found inside the vessel. The Department of Radiation Oncology recommended postoperative adjuvant radiotherapy.
The final diagnosis was ACC of the lower esophagus (Staging: T3N0M0, IIB).
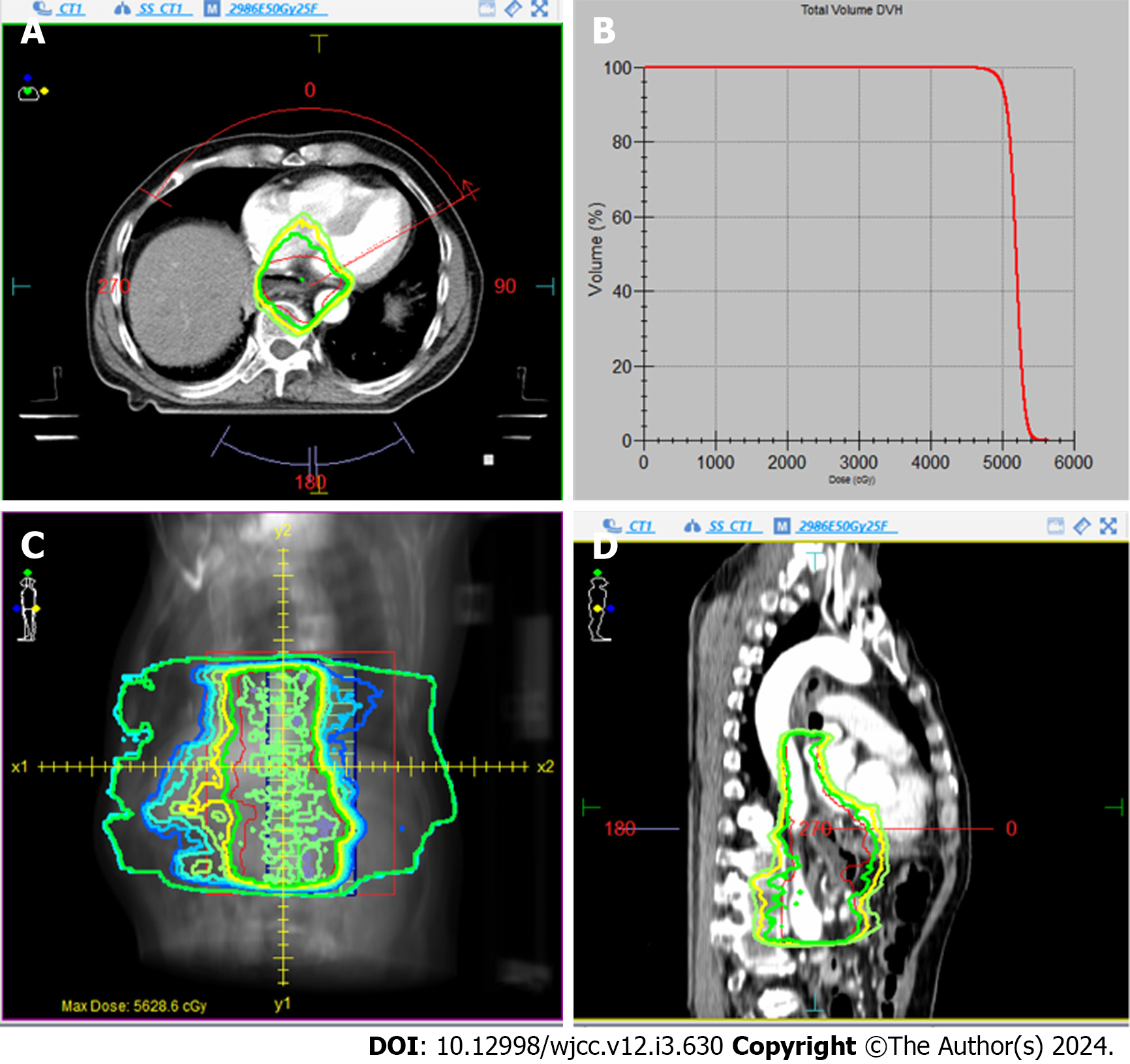
One month after surgery, the patient received adjuvant radiotherapy with a dosage of 50 Gy. The radiotherapy targeted part of the abdominal plexus and the primary tumor site in the esophagus. The radiation dose-distribution diagram and dose-volume histogram are illustrated in Figure 3.
The patient successfully completed the radiotherapy plan. Six months after the end of radiotherapy, chest enhanced CT and gastroscopy were reviewed, and no tumor recurrence was found.
ACC is rare in the digestive system. It represents only 0.1% of esophageal malignancies[7]. Due to the limited number of reported cases, the clinical behavior of EACC remains poorly understood. Patients with EACC, on average, are 65-years-old, and the incidence is higher in males than in females. Most patients will have difficulty swallowing[8-10]. ACCs are frequently located in the middle third of the esophagus (63%), less commonly in the lower third (30%), and rarely in the upper third (7%). Clinical progress is slow, with frequent growth along the nerve with vascular invasion. This is similar to esophageal SCC[11]. The origin of EACC is controversial, with most studies suggesting submucosal glandular origin, while a few suggest basal cells of the epithelium of the esophagus or remnants of the trachea and bronchus. Mucinous gland transition to cancer can be observed under the esophageal mucosa, supporting the view that EACC originates from the submucosal mucous glands of the esophagus. Studies have reported higher rates of reflux esophagitis, a recognized risk factor for EACC, in patients with hiatal hernias, obesity, smoking, and alcohol abuse[12].
The diagnosis of EACC primarily relies on the patient’s symptoms, signs, imaging, and endoscopy. Ultimately, confirmation is obtained through a histopathological examination of biopsy specimens. The clinical manifestations and imaging examinations of EACC often lack specificity, leading to potential misdiagnosis as esophageal SCC and other conditions. The main symptoms associated with EACC include upper abdominal pain or discomfort following meals and progressive dysphagia. Common endoscopic findings include a fungating or polypoid mass rather than an ulcerative or invasive growth pattern[8].
Histologically, ACCs of the salivary gland can exhibit three distinct growth patterns: cribriform; tubular; and solid. The cribriform pattern, which is most frequently observed, is characterized by the presence of nests of cells with cylindromatous microcystic spaces. In the tubular form, well-formed ducts and tubules with central lumina are lined by inner epithelial and outer myoepithelial cells. Small true ducts are invariably present in cribriform and solid variants but may not be immediately apparent[13,14]. The ACC classification method, aligned with the World Health Organization (2005) standard, classifies tumors based on their organizational type. Tumors with a tubular structure accompanied by a sieve structure but lacking a solid structure are designated as level 1. Those with a pure sieve structure or < 30% mixed solid structure are categorized as level 2, and those with ≥ 30% solid structure are classified as level 3.
The immunophenotype of EACC aligns with other types of ACC. Currently, the key immunohistochemical detection indices include CK, P63, Vim, SMA, S-100, GFAP, and CD117. Electron microscopy has shown that basophilic substances are glycosaminoglycans, whereas eosinophilic substances are basal membrane substances[12]. Basal-like SCC may be considered in differential diagnosis due to its pseudoglandular pattern akin to ACC. However, ACC typically lacks squamous cells, central necrosis, or prominent mitotic images. Basal-like SCCs are negative for SMA and S100. EACC has a unique histological pattern, often necessitating a definitive diagnosis through immunohistochemistry or rebiopsy.
Lymph node metastases are reportedly rare in patients with esophageal SCC when the tumor is confined to the mucosal layer. The incidence of metastasis is 9.3% when the tumor reaches the muscularis muscle plate, and it is accepted that the higher the degree of infiltration, the higher the probability of lymph node metastasis[15]. This was consistent with previous reports that concluded that EACC was derived from the esophageal glands, and ulceration was subse
It has been reported that endoscopic biopsy frequently fails to provide a correct preoperative diagnosis of EACC because of its similar growth pattern to submucosal tumors. Additionally, accurate diagnosis can become challenging when EACC contains SCC or BSC components[18]. Some cases of BSC and SCC may have been misdiagnosed as EACC. Larger studies are needed to clarify metastasis, prognosis, and the presence of other tumor components in EACC[19]. Our patient’s preoperative pathological examination differed from the postoperative findings. We attribute this disparity to the limited nature of the preoperative electronic gastroscopic biopsy samples, making it challenging to accurately depict the true characteristics of the tumor. This limitation increases the risk of misdiagnosing it as esophageal SCC.
Due to the rarity of EACC and the lack of a large sample and systematic data in the literature, only 18 cases have been reported in China since it was first documented by Gregg and Stamler[20] in 1954. Our case represents one of these instances. The clinical data of the 18 patients are summarized in Table 1. A review of this data revealed that the patients are aged 42-72 years (average age: 60.8 years), making our patient the oldest. The sex ratio was 14:4, and the middle third of the esophagus was the most affected region.
| Clinical features | Subcategory | Number |
| Age | 42-72 (60.8) | |
| Sex | Male | 14 |
| Female | 4 | |
| Location | Cervical | 1 |
| Upper thoracic | 0 | |
| Mid-thoracic | 9 | |
| Lower thoracic | 7 | |
| Esophagogastric junction | 1 | |
| Endoscopic presentation | Protruding | 13 |
| Ulcerative | 5 | |
| Treatment | Surgery (including ESD) | 17 |
| Chemoradiotherapy | 1 (metastasis) | |
| Depth of invasion (surgical cases) | Mucous layer | 10 |
| Muscularis propria | 2 | |
| Adventitia | 5 | |
| Metastasis to lymph nodes (Surgical cases) | LN positive | 0 |
| LN negative | 17 |
A total of 17 out of the 18 patients opted for surgery as their treatment choice. Currently, there is no standard treatment protocol for EACC, and radical resection is the treatment option. Postoperative radiotherapy is recommended in cases where surgical margins are positive. Chemotherapy is typically avoided due to a low response rate[19]. For patients with advanced inoperable conditions, the primary objective is to delay tumor progression as much as possible and enhance their quality of life. A previous case report described the use of chemotherapy, including doxorubicin, mitomycin C, and 5-fluorouracil, with local radiation[8], but chemotherapy is generally considered ineffective[19]. Whether it is adjuvant or primary chemotherapy remains unknown[21].
The 5-year survival rate of ACC is approximately 35%; however, the long-term survival rate is poor. About 80%-90% of the patients die of this disease within 10-15 years. Poor prognostic factors influencing survival include solid histological patterns, advanced clinical stage, and positive surgical margins[22]. However, the severity and prognosis of EACC remain controversial. Some suggest similarities with esophageal SCC, while others indicate a significantly worse prognosis than salivary gland ACC. Sawada et al[23] showed that the lymph node metastasis rate of EACC is 22.2%, and distant organ metastasis occurs more frequently than lymph node metastasis. The role of chemotherapy, through either adjuvant or primary chemotherapy, is not clear[24]. Postoperative radiotherapy may help reduce the progressive dys
In the present case, the tumor cells infiltrated the entire layer of the muscularis propria proper, locally infiltrated the outer fibrous membrane of the esophagus, and involved the cardia. Immunostaining confirmed myoepithelial differentiation, and the diagnosis of EACC was made. Characteristics such as ductal epithelium formation, the biphasic nature of the tumor, and the presence of an eosinophilic substance in the cell cytoplasm were consistent with ACC. Our patient showed no lymph node metastasis; however, nerve examination showed cancer invasion, and a cancer thrombus was found in the vascular canal. Therefore, we opted for postoperative adjuvant radiation therapy. There are no established guidelines for defining the target areas for radiotherapy in cases of EACC. To address this gap, we considered several key characteristics associated with ACC, including its high degree of malignancy, local invasion, strong invasiveness, and propensity to spread along nerve growth. As a result, our delineated target areas included the primary tumor area of the esophagus and some abdominal plexus disfigurement areas.
Herein, we presented a case of adjuvant radiotherapy following radical surgery in a patient diagnosed with EACC. Given the low incidence of EACC, it is important to accumulate and analyze more cases to elucidate the prognosis and determine the most suitable treatment for individuals experiencing this condition.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fabbri N, Italy S-Editor: Lin C L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Li LJ, Li Y, Wen YM, Liu H, Zhao HW. Clinical analysis of salivary gland tumor cases in West China in past 50 years. Oral Oncol. 2008;44:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Suzuki H, Nagayo T. Primary tumors of the esophagus other than squamous cell carcinoma--histologic classification and statistics in the surgical and autopsied materials in Japan. Int Adv Surg Oncol. 1980;3:73-109. [PubMed] |
| 3. | Bradley PJ. Adenoid cystic carcinoma of the head and neck: a review. Curr Opin Otolaryngol Head Neck Surg. 2004;12:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Matsushima S, Kawamoto M, Shioda M, Koizumi K, Tanaka S, Shoji T. An operated case of adenoid cystic carcinoma of the esophagus. J Japanese Pract Surg Soc. 2009;52:1276-1280. [DOI] [Full Text] |
| 5. | Ueda S, Hara Y, Kirishi R, Shiomi K, Mishima H, Kim YK, Yamaga K, Imai R. A case of adenoid cystic carcinoma of the esophagus. J Japanese Soc Clin Surg. 1995;56:1367-1372. [DOI] [Full Text] |
| 6. | Hiromichi I, Hiroshi S, Yasuhiro T, Hiroto I, Kimihide H. A case of adenoid cystic carcinoma of the esophagus with reference to immunohistochemical study. Japanese J Gastroenterol Surg. 2007;40:547-552. [DOI] [Full Text] |
| 7. | Karaoglanoglu N, Eroglu A, Turkyilmaz A, Gursan N. Oesophageal adenoid cystic carcinoma and its management options. Int J Clin Pract. 2005;59:1101-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Petursson SR. Adenoid cystic carcinoma of the esophagus. Complete response to combination chemotherapy. Cancer. 1986;57:1464-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Cerar A, Jutersek A, Vidmar S. Adenoid cystic carcinoma of the esophagus. A clinicopathologic study of three cases. Cancer. 1991;67:2159-2164. [PubMed] [DOI] [Full Text] |
| 10. | Sweeney EC, Cooney T. Adenoid cystic carcinoma of the esophagus: a light and electron microscopic study. Cancer. 1980;45:1516-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Guo XF, Mao T, Gu ZT, Fang WT, Chen WH, Shao JC. Adenoid cystic carcinoma of the esophagus: report of two cases and review of the Chinese literature. Diagn Pathol. 2012;7:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Sato-Kuwabara Y, Fregnani JH, Jampietro J, Carvalho KC, Franco CP, da Costa WL Jr, Coimbra FJ, Soares FA. Comparative analysis of basaloid and conventional squamous cell carcinomas of the esophagus: prognostic relevance of clinicopathological features and protein expression. Tumour Biol. 2016;37:6691-6699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Nascimento AG, Amaral AL, Prado LA, Kligerman J, Silveira TR. Adenoid cystic carcinoma of salivary glands. A study of 61 cases with clinicopathologic correlation. Cancer. 1986;57:312-319. [PubMed] [DOI] [Full Text] |
| 14. | Matsuba HM, Mauney M, Simpson JR, Thawley SE, Pikul FJ. Adenocarcinomas of major and minor salivary gland origin: a histopathologic review of treatment failure patterns. Laryngoscope. 1988;98:784-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Yoshikawa K, Kinoshita A, Hirose Y, Shibata K, Akasu T, Hagiwara N, Yokota T, Imai N, Iwaku A, Kobayashi G, Kobayashi H, Fushiya N, Kijima H, Koike K, Kaneyama H, Ikeda K, Saruta M. Endoscopic submucosal dissection in a patient with esophageal adenoid cystic carcinoma. World J Gastroenterol. 2017;23:8097-8103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Murao T, Yoshioka H, Sunami M, Saito Y, Fujita T. [A case of adenoid cystic carcinoma of the esophagus]. Gan No Rinsho. 1989;35:1759-1763. [PubMed] |
| 17. | Akiyama M, Nakajima H, Baba T, Munakata A, Yoshida Y, Kako N. Two cases of adenoid cystic carcinoma of the esophagus. Nihon Shokakibyo Gakkai Zasshi. 1994;91:180-187. [PubMed] [DOI] [Full Text] |
| 18. | Tsang WY, Chan JK, Lee KC, Leung AK, Fu YT. Basaloid-squamous carcinoma of the upper aerodigestive tract and so-called adenoid cystic carcinoma of the oesophagus: the same tumour type? Histopathology. 1991;19:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 81] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Triantafillidou K, Dimitrakopoulos J, Iordanidis F, Koufogiannis D. Management of adenoid cystic carcinoma of minor salivary glands. J Oral Maxillofac Surg. 2006;64:1114-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Gregg JB, Stamler FW. Unusual neoplasms of the esophagus: review of literature and report of a case. AMA Arch Otolaryngol. 1954;59:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Matsumoto M, Nomiyama T, Nakae J, Nishikawa H. Combination chemotherapy for a senile patient with adenoid cystic carcinoma of the esophagus: a case report. Jpn J Clin Oncol. 1993;23:258-262. [PubMed] |
| 22. | Perzin KH, Gullane P, Clairmont AC. Adenoid cystic carcinomas arising in salivary glands: a correlation of histologic features and clinical course. Cancer. 1978;42:265-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Sawada G, Moon J, Saito A, Odagiri K, Kimura Y, Takahashi G, Yamashita S, Inoue M, Irei T, Nakahira S, Shimizu Y, Tominaga H, Kuraoka K, Taniyama K, Hatanaka N. A case of adenoid cystic carcinoma of the esophagus. Surg Case Rep. 2015;1:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Morisaki Y, Yoshizumi Y, Hiroyasu S, Shibata H, Terahata S, Tamai S, Sugiura Y, Shima S, Tanaka S. Adenoid cystic carcinoma of the esophagus: report of a case and review of the Japanese literature. Surg Today. 1996;26:1006-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |