Published online Jan 26, 2024. doi: 10.12998/wjcc.v12.i3.587
Peer-review started: September 21, 2023
First decision: November 20, 2023
Revised: December 4, 2023
Accepted: January 4, 2024
Article in press: January 4, 2024
Published online: January 26, 2024
Processing time: 119 Days and 4.3 Hours
Paramyotonia congenita (PMC) stands as a rare sodium channelopaty of skeletal muscle, initially identified by Eulenburg. The identification of PMC often relies on electromyography (EMG), a diagnostic technique. The child’s needle EMG unveiled trains of myotonic discharges with notably giant amplitudes, alongside irregular wave trains of myotonic discharges. This distinctive observation had not surfaced in earlier studies.
We report the case of a 3-year-old female child with PMC, who exhibited la
In this case revealed the two types of myotonic discharges, and had not been documented in other studies. We underscore two distinctive features: Giant-amplitude potentials and irregular waves.
Core Tip: Paramyotonia congenita (PMC) is a rare sodium channelopathy of skeletal muscle, first identified by Eulenburg. The distinguishing feature of PMC is paradoxical myotonia, where myotonia worsens with cold and exercise. In instances where genetic testing is unavailable, electromyography (EMG) is a swift, cost-effective diagnostic and differential diagnostic method for PMC. This article elaborates on the EMG characteristics of a recently diagnosed PMC case at our hospital. In this particular case revealed the two types of myotonic discharges, and had not been documented in other studies. We underscore two distinctive features: Giant-amplitude potentials and irregular waves.
- Citation: Yi H, Liu CX, Ye SX, Liu YL. Special electromyographic features in a child with paramyotonia congenita: A case report and review of literature. World J Clin Cases 2024; 12(3): 587-595
- URL: https://www.wjgnet.com/2307-8960/full/v12/i3/587.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i3.587
Paramyotonia congenita (PMC) is a rare sodium channelopathy of skeletal muscle[1,2], first identified by Eulenburg[3]. This autosomal dominant condition stems from mutations in the SCN4A gene, which encodes the α-subunit of Nav1.4[4]. These mutations cause the sodium channel to stay open, leading to sustained muscle contractions and resulting in myotonia[5]. PMC becomes evident in individuals displaying cold-and exercise-triggered myotonia, alongside muscle weakness[6,7]. In children, additional symptoms like neonatal stridor, dysphagia, and respiratory compromise can arise[8]. The distinguishing feature of PMC is paradoxical myotonia, where myotonia worsens with cold and exercise. The global prevalence of PMC is approximately 1 in 200000[9]. Around 50 distinct mutation sites in the SCN4A gene have been documented, with the majority being missense mutations[10]. Diagnosis relies on patient history, examination, character of electromyography (EMG), and genetic verification. In instances where genetic testing is unavailable, certain studies[11,12] have proposed that EMG is a swift, cost-effective diagnostic and differential diagnostic method for PMC[13]. This article elaborates on the EMG characteristics of a recently diagnosed PMC case at our hospital. The case exhibited distinctive manifestations of various types of myotonia discharges, a feature previously unreported. By sharing this novel finding, this article aims to enhance the understanding of neurophysiologists, neurologists, and pediatricians regarding the EMG of PMC. This will ultimately enhance the diagnostic accuracy for patients with this condition, leading to improved treatment and relief of symptoms and quality of life.
A 3-year-old child presented to the rehabilitation department, Qilu Children’s Hospital of Shandong University with a complaint of inflexible physical activity for 3 years on July 16, 2022.
A 3-year-old child exhibited laryngeal stridor, muffled speech, and sporadic stridor from birth, a condition unaffected by positioning. This manifestation occurred after feeding and crying, leading to issues with water consumption, mild breathlessness, and occasional breath-holding. Severe cases brought about breathlessness, apnea, lip and facial cyanosis, and myotonia. Cold, exposure to cool water, crying, and physical activity exacerbated the myotonia, which was relieved in warmth, yet never normalized. At 6 mo, restricted upper limb movement was observed. The child managed independent standing and walking by 1 year and 3 mo, but upper limb inflexibility persisted. At 2 years and 8 mo, calf spasms, walking stiffness, knee-flexed lower limb posture abnormalities, and occasional limb rigidity after trips were noted. Instances of ptosis, difficulty in eye opening, and eyeball movement restriction following face washing were present. Myotonia symptoms remained unchanged after potassium-rich food consumption like bananas.
The patient underwent inguinal hernia repair (bilateral) in 2021 at Qilu Children’s Hospital of Shandong University.
Non-consanguineous parents and no family history of similar neurological disorders.
Physical traits encompassed a short neck, hunched back, limited bilateral eye abduction, motor skill delays, and firm muscles with evident muscle bulges. Percussion myotonia was observable in bilateral biceps.
Blood potassium remained within normal range (4.1-4.8 mmol/L), while creatine kinase showed slight elevation (279-771 U/L). Liver function, renal function, thyroid function and inorganic ions were normal.
Cranial magnetic resonance imaging yielded normal results.
Low-frequency (2 Hz, 3 Hz, 5 Hz) and high-frequency (10 Hz, 20 Hz, 30 Hz) repetitive frequency electrical stimulation exhibited normal outcomes. EMG indicated challenging motor unit action discharge (MUP) differentiation, with abundant myotonic discharges detected in muscles examined. Facial nerve motor conduction and blink reflex testing were unremarkable.
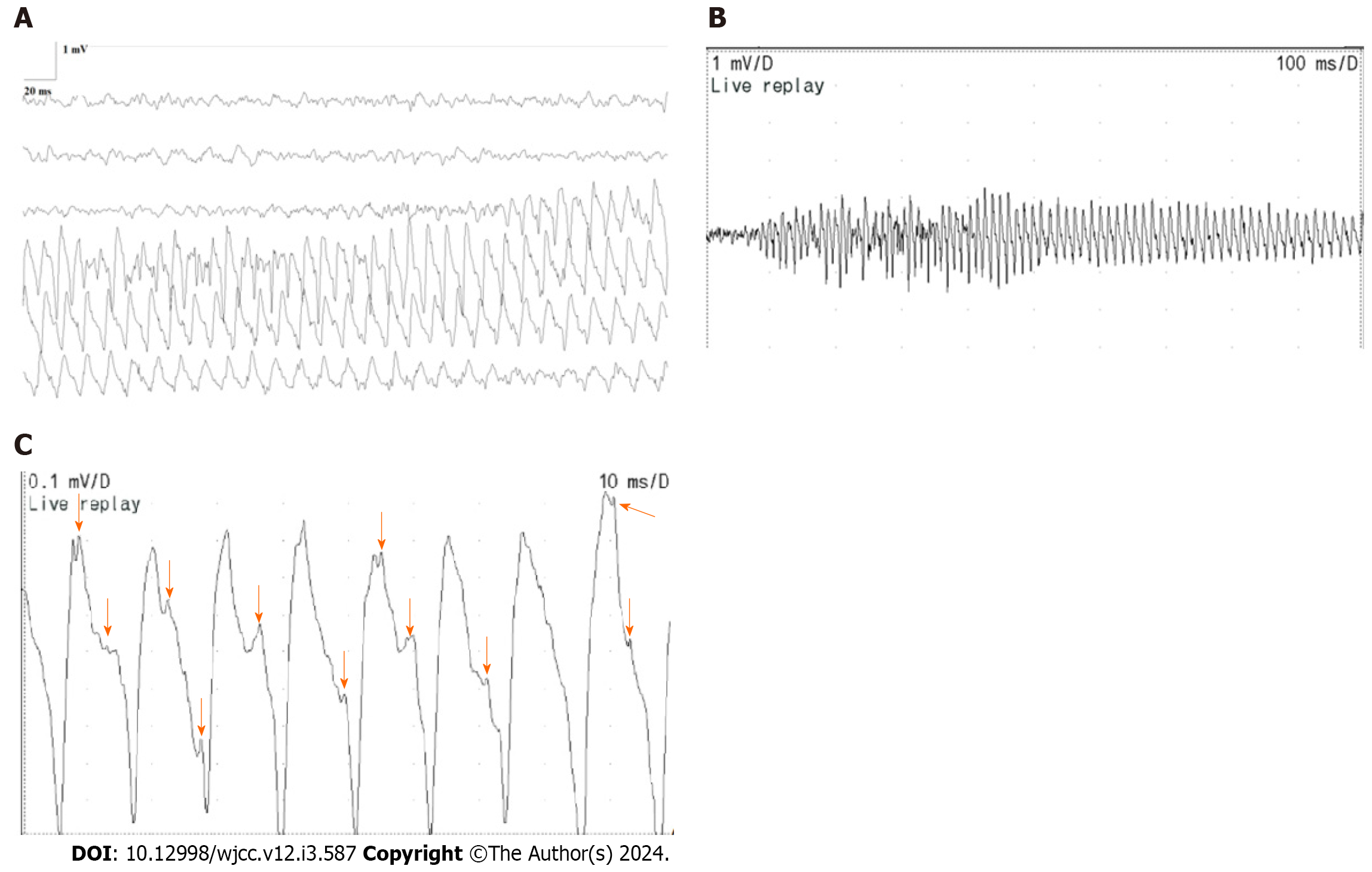
A notable quantity of myotonic discharges was documented in all muscles subjected to the needle EMG investigation. Particularly noteworthy were the discernment of trains of positive waves in the myotonic discharges, accompanied by a sound reminiscent of a motorcycle. This phenomenon is evident in Figure 1A-C. Each of these diagrams conveyed a specific muscle’s single discharge pattern. Figure 1A captured the complete progression of a train of positive waves within myotonic discharges, featuring gradual fluctuations in amplitude and frequency, spanning a frequency range of 70-150 Hz and an amplitude range of 0.5-3 mV. Figure 1B highlighted a section of the train of positive waves depicted in Figure 1A, revealing an oscillating pattern in both amplitude and frequency. This pattern exhibited an abrupt surge followed by a gradual decline. Figure 1C showcased a compact portion of the positive wave train featured in Figure 1B. This illustration elucidated the structure of a single positive wave, which originated as a positive wave and transitioned into a negative wave characterized by a prolonged duration. The negative wave exhibited the fusion of multiple negative wave forms, each distinguished by varying frequencies and morphologies (as indicated by the arrows in Figure 1C).
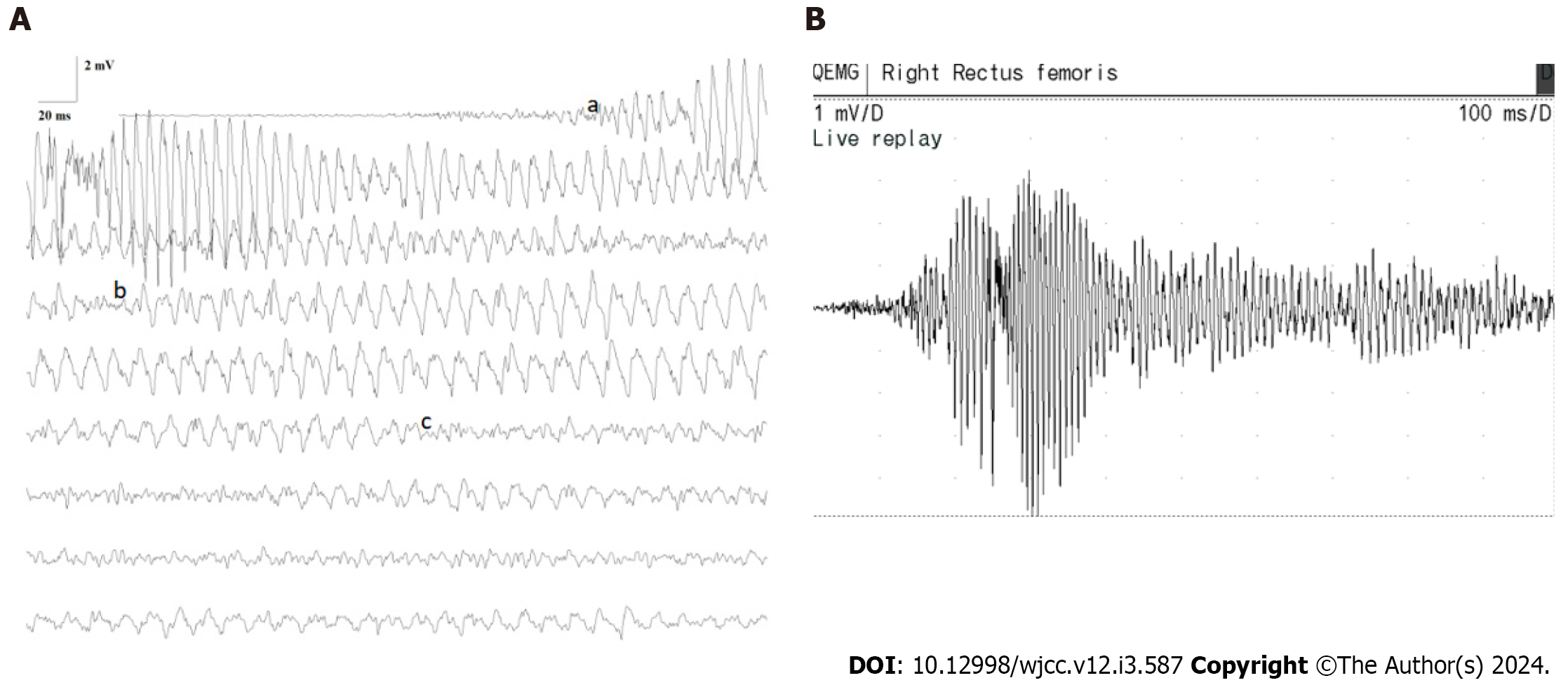
As depicted in Figure 2A and B, the child’s muscle needle EMG displayed abrupt and sudden trains of giant-amplitude within myotonic discharges (a: Frequency range: 100-150 Hz, amplitude range: 3-15 mV, span: 300-600 ms). These discharges exhibited a waxing-waning pattern in both amplitude and frequency, with a sudden onset and cessation. Subsequently, there was a gradual reduction in amplitude and frequency. This was succeeded by the appearance of the second form of discharge (b): A train of positive waves within myotonic discharge. This pattern initiated with a positive wave, followed by a negative wave, characterized by a frequency of 100-150 Hz, amplitude of 0.5-3 mV, and a duration of approximately 6-10 s. Over time, frequency and amplitude diminished. The third type of myotonic discharge (c) materialized: A train of irregular waves within myotonic discharge. The irregularities in frequency and waveforms were apparent, with an amplitude range of 0.2-2 mV. During periods of desynchronization, estimating the frequency proved challenging. The auditory accompaniment for all these myotonic discharges resembled that of a dive bomber aircraft or a motorcycle. It is important to note that these three forms of myotonic discharges might coexist within a single myotonic discharge, rather than manifesting in isolation.
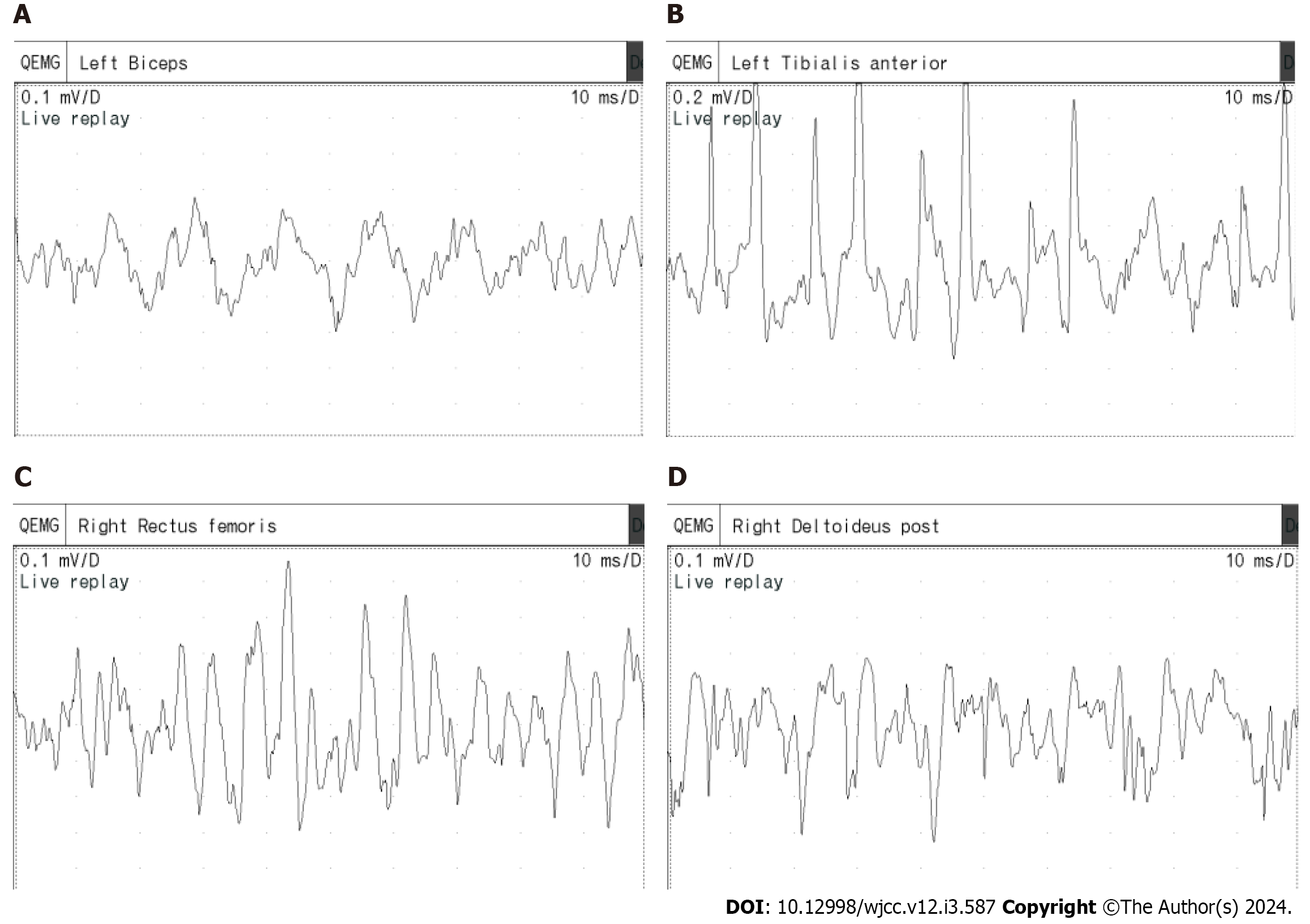
The most prevalent type observed within the needle EMG is the trains of irregular waves within myotonic discharge (Figure 3). This pattern comprises multiple biphasic spike myotonic discharges and positive wave myotonic discharges. Distinguishing irregular waves within myotonic discharge with varying shapes, frequencies, and amplitudes presents difficulties. Amplitude ranges from 50-1000 μV, while the presence of multiple irregular waves complicates frequency assessment. The extensive myotonic discharges rendered the analysis of MUPs during voluntary contractions challenging.
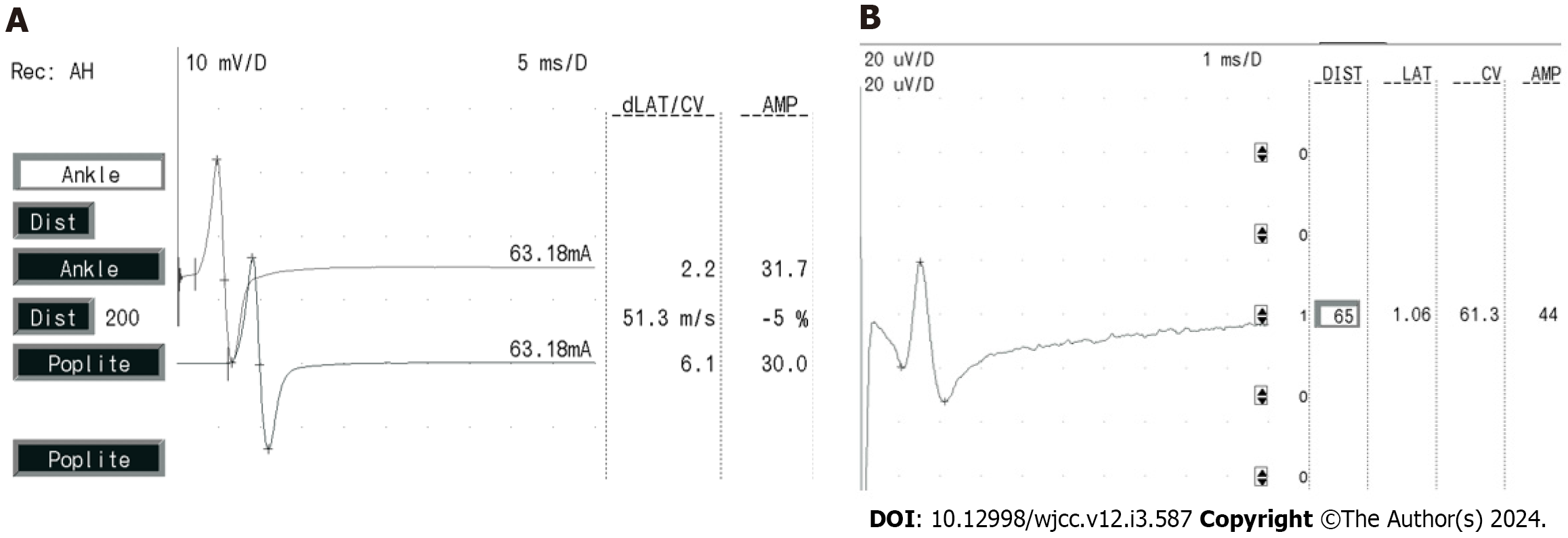
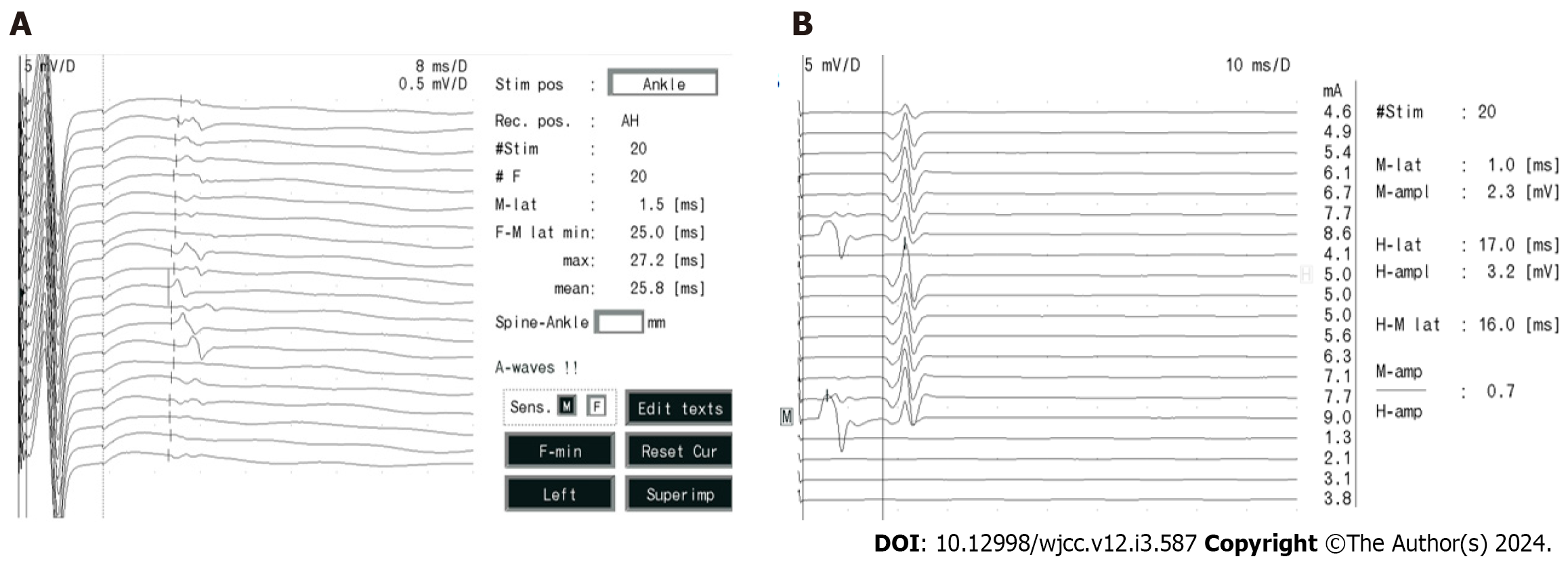
The measurements for her sensory conduction velocity (SCV), sensory nerve action potential (SNAP), motor nerve conduction velocity (MCV), and compound muscle action potential (CMAP) yielded normal results, illustrated in Figure 4A and B. Her F-wave occurrences and latency rates exhibited normal values, as depicted in Figure 5A, while the H-reflex showcased normal amplitude and latency, as displayed in Figure 5B.
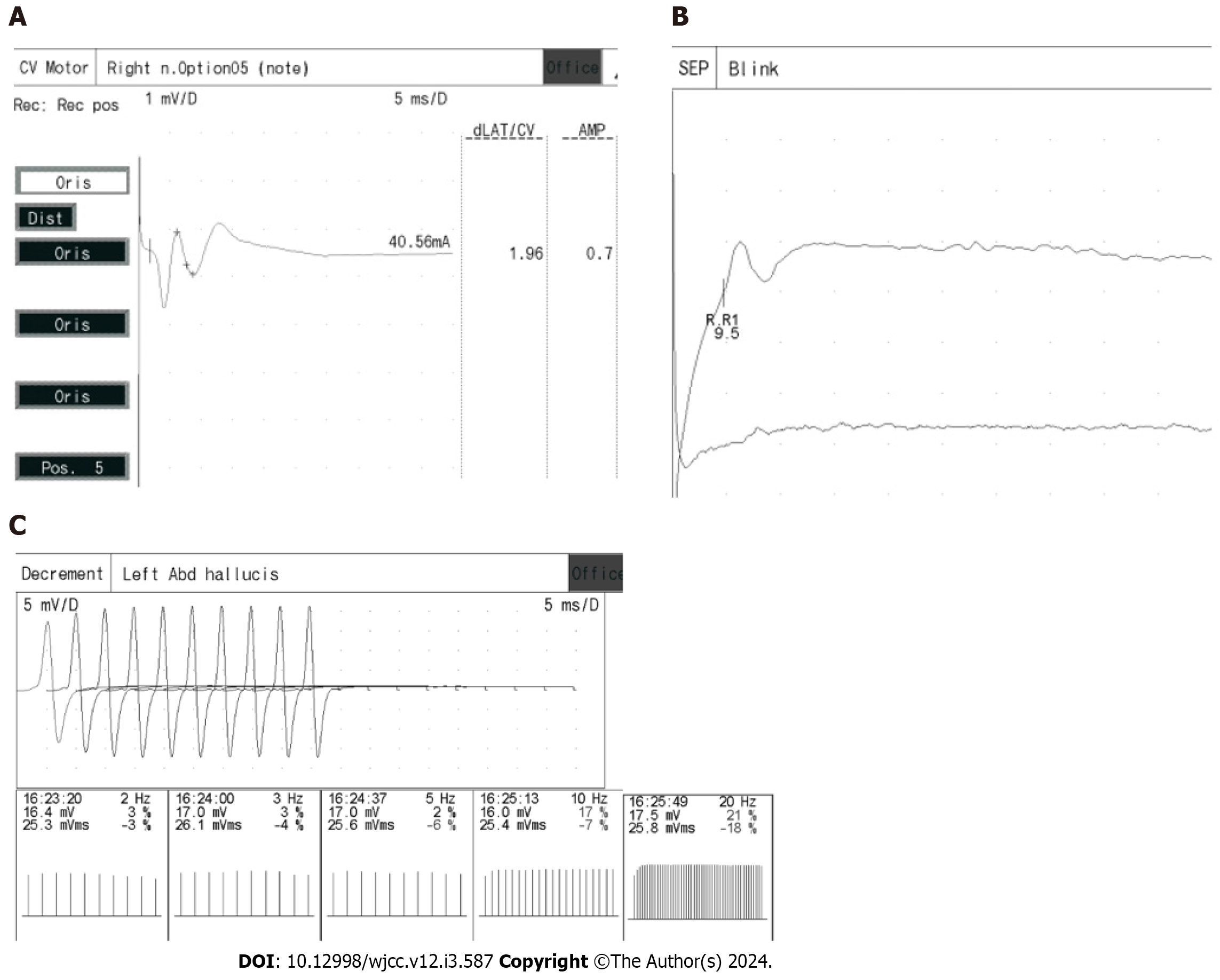
Regarding the facial nerve, both CMAP and MCV measurements were within the normal range, as shown in Figure 6A. Additionally, blink reflexes were elicited with standard latency and amplitude, presented in Figure 6B. Repetitive frequency electrical stimulation results indicated normalcy for various muscles, including bilateral orbicularis oculi, left trapezius, abductor hallucis, right abductor digitorum minimi low frequency (2 Hz, 3 Hz, 5 Hz), and right abductor digitorum minimi high frequency (30 Hz), left abductor hallucis high frequency (10 Hz, 20 Hz), as depicted in Figure 6C.
The diagnosis aligned with PMC, grounded in clinical features, lab tests, neurophysiology, and genetic evaluations[3,11-14,16]. Neither parent carried mutations in the gene, and familial history was absent, suggesting sporadic occurrence.
The child received mexiletine at an initial dose of 50 mg three times a day. The dose was increased to 75 mg three times a day after 2 wk, and to 100 mg three times a day after a month. In addition, the patients also participated in physical exercises for rehabilitation.
The frequency and severity after an eight-month mexiletine regimen, which affirmed the therapeutic effect.
In the context of PMC, the identification of myotonia is frequently more accurately achieved through needle EMG than neurological examination. In instances where genetic testing is unavailable, EMG can serve as a diagnostic and distinguishing tool for PMC[13]. Previous investigations have delineated two primary variants of myotonic discharge[15]: (1) Biphasic spike potentials, resembling fibrillation potentials, with a duration of less than 5 ms; and (2) Positive waves, akin to positive sharp waves, possessing a duration of 5-20 ms. A singular myotonic potential might visually and audibly resemble either a fibrillation potential or a positive sharp wave. The distinctive attribute of myotonic discharge lies in its waxing-waning pattern. Beyond the positive wave-like myotonic discharge, this particular case revealed the trains of giant-amplitude within myotonic discharges and the trains of irregular wave within myotonic discharges. Interestingly, these two types of myotonic discharges had not been documented in other studies.
In the child under consideration, spontaneous discharges manifested in all the examined muscle EMGs. The forms of myotonic discharge exhibited morphological resemblance or even identical traits across the non-endplate regions of over three muscles, coupled with a distinct auditory signature reminiscent of a dive bomber aircraft or a motorcycle[15].
All examined muscles subjected to needle EMG displayed trains of high-amplitude within myotonic discharges characterized by frequencies ranging from 70 to 150 Hz and amplitudes between 3 to 15 mV. These discharges were accompanied by an audible resemblance to a dive bomber aircraft. Existing literature has documented myotonic discharge frequencies of 20-150 Hz and amplitudes spanning 10-1000 μV, which produces a distinctive auditory feature often referred to as a dive bomber aircraft[13,16-18]. In this specific case, the amplitude of myotonic discharge waves exceeded typical levels, even surpassing 10 mV - an amplitude magnitude greater than tenfold of previously reported values. Typically, myotonic discharges result from the spontaneous firing of an individual muscle fiber. However, a single concentric needle electrode can capture discharges from multiple muscle fibers[19]. In this instance, the presence of trains of irregular waves within myotonic discharge comprised diverse waves exhibiting different frequencies and shapes. This observation suggests that high-amplitude potentials emerge through ephaptic transmission between numerous closely positioned muscle fibers[20]. Consequently, multiple muscle fibers participate in myotonic discharge, operating in synchronization[21]. Wave amplitudes accumulate over time, yielding “giant-amplitude potentials”. On the other hand, PMC arises due to a mutation in the sodium channel gene (SCN4A), impairing rapid sodium channel inactivation during repolarization. The influx of numerous Na+ ions through Nav1.4 elevates the equilibrium potential of Na+ and the magnitude of myocyte action potential depolarization[22,23]. Consequently, potential amplitudes rise, with these dual mechanisms synergistically generating giant-amplitude potentials. Distinguishing trains of giant-amplitude within myotonic discharges from neurological myotonia is imperative. Neurogenic myotonic discharge patterns entail synchronous contraction discharges across all muscle fibers within a motor unit. These discharges possess frequencies ranging from 100 to 300 Hz, protracted durations, gradual frequency and amplitude reduction spanning several minutes, yet lack the distinct “motorcycle start-up-like sound”[15].
The child also exhibited myotonic discharges resembling positive waves. Earlier investigations have typically noted that positive sharp wave-like myotonic discharges are generally characterized by amplitudes below 1 mV and durations spanning 5-20 ms[13,15,24]. However, a distinctive feature of the positive wave-like myotonic discharges identified in this particular case is their higher amplitude, ranging from 0.5-3 mV. Moreover, within these positive wave myotonic discharges, simultaneous occurrence of one or more negative-phase waves was observed. This occurrence contributed to the non-smooth morphology of the positive wave-like myotonic discharges, yielding a resemblance to a spike-like fusion. This particular discharge pattern also resulted from the synchronized discharge of a few closely spaced muscle fibers, which aggregated to produce high-amplitude positive-wave myotonic discharges. Meanwhile, the sluggish inactivation of sodium ion channels further amplifies the amplitude. Additionally, other muscle fibers produce negative-phase wave discharges characterized by diverse frequencies, which spatially combine to produce fused myotonic discharges. Notably, the asynchronous nature of this subset of muscle fiber discharges does not impact the positive wave-dominated myotonic discharge, preserving the morphological similarity to positive waves.
Prevalent in this child was the occurrence of trains of irregular waves within myotonic discharges. These waves exhibit an irregular shape and frequency, possessing amplitudes below 1 mV and frequencies challenging to estimate. Within these irregular waves, distinguishing positive waves and biphasic spike potentials proves complex. This observation strongly suggests the involvement of multiple muscle fibers in generating these waves, each contributing with distinct frequencies and morphologies. This phenomenon rules out the dominance of any single form of myotonic discharge characterized by synchronized discharges of several muscle fibers. Instead, the irregular waves generated by these diverse muscle fibers fuse both temporally and spatially in accordance with their individual firing frequencies. This fusion process results in an amalgamation of waves, losing the original attributes of fibrillation potentials and positive sharp waves.
In summary, a distinctive myotonic discharge profile was observed in this child, characterized by a train of giant-amplitude within myotonic discharges and trains of irregular waves within myotonic discharges, featuring multiple fused myotonic potentials. This attribute arises from the compromised inactivation of sodium channels, leading to increased sodium ion influx and augmented wave amplitude during depolarization. Simultaneously, multiple muscle fibers participate in generating myotonic potentials. When these fibers synchronize at a common frequency, a giant-amplitude potential emerges. As the involvement of synchronized muscle fibers diminishes, certain fibers engage in synchronized discharge while others exhibit distinct frequencies, resulting in the generation of positive wave myotonic discharges. This pattern is dominated by positive wave myotonic discharges while encompassing additional frequencies of myotonic discharges. In cases where all muscle fibers discharge at varying frequencies, an indistinguishable irregular wave emerges, constituting a fusion of biphasic spike potentials and positive wave myotonic discharges of varying frequencies.
The child’s facial nerve motor conduction, nerve conduction studies, and repetitive nerve stimulation (RNS) yielded normal results. It’s worth noting that PMC, as a form of sodium channelopathy affecting skeletal muscles, generally spares peripheral nerves and the neuromuscular junction[4]. Since RNS is primarily employed for detecting neuromuscular junction abnormalities[25], the unremarkable nerve conduction studies and RNS findings are consistent with the lack of involvement of these elements in PMC.
PMC is a sodium channelopathy of skeletal muscle caused by mutations in the SCN4A gene. EMG can confirm the presence, severity and distribution of myotonic discharge, which can support the diagnosis of PMC, and determine whether or not a patient has myopathy. Paradoxical myotonia is the typical feature of PMC. Nearly all patients’ EMG showing myotonic potentials in previous studies[26-28]. CMAP decreased following exposure to cold or cold water tests in some patients[29-31]. Additionally, some people may also show a decrease in CMAP after a short period of exercise[27]. According to a report in China, one patient with PMC had delayed nerve conduction velocity and low F-wave appearance in both lower limbs[32]. If patients with PMC has not yet experienced clinical symptoms, their EMG were normal[33]. According to the studies, EMG in some patients with PMC exhibited both myotonic and myopathic potentials[32,34]. In presenting this case of PMC with unique potentials identified via needle EMG, we underscore two distinctive features: High-amplitude potentials and irregular waves. These features are linked to the synchronous activation of multiple muscle fibers and the impairment of sodium channel inactivation. By sharing this case, our intent is to assist clinicians in distinguishing this type of myotonic discharge from neurological myotonia.
The special case revealed two types of myotonic discharges, which had never been documented until now. We underscore two distinctive features: Giant-amplitude potentials and irregular waves. These features are linked to the synchronous activation of multiple muscle fibers and the impairment of sodium channel inactivation. By sharing this case, our intent is to assist clinicians in distinguishing this type of myotonic discharge from neurological myotonia and widens the known the special feature of EMG in PMC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dauyey K, Kazakhstan S-Editor: Wang JJ L-Editor: A P-Editor: Zhao YQ
| 1. | Jarecki BW, Piekarz AD, Jackson JO 2nd, Cummins TR. Human voltage-gated sodium channel mutations that cause inherited neuronal and muscle channelopathies increase resurgent sodium currents. J Clin Invest. 2010;120:369-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Yoshinaga H, Sakoda S, Good JM, Takahashi MP, Kubota T, Arikawa-Hirasawa E, Nakata T, Ohno K, Kitamura T, Kobayashi K, Ohtsuka Y. A novel mutation in SCN4A causes severe myotonia and school-age-onset paralytic episodes. J Neurol Sci. 2012;315:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Eulenburg A, Landois L. On nerve regeneration with the application of the suture. 1865. J Mol Med (Berl). 2001;79:4-5. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Russell SH, Hirsch NP. Anaesthesia and myotonia. Br J Anaesth. 1994;72:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 81] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Bouhours M, Luce S, Sternberg D, Willer JC, Fontaine B, Tabti N. A1152D mutation of the Na+ channel causes paramyotonia congenita and emphasizes the role of DIII/S4-S5 linker in fast inactivation. J Physiol. 2005;565:415-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Palma C, Prior C, Gómez-González C, Rodríguez-Antolin C, Martínez-Montero P, Pérez de Ayala L, Pascual SI, Molano Mateos J. A SCN4A mutation causing paramyotonia congenita. Neuromuscul Disord. 2017;27:1123-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Matthews E, Tan SV, Fialho D, Sweeney MG, Sud R, Haworth A, Stanley E, Cea G, Davis MB, Hanna MG. What causes paramyotonia in the United Kingdom? Common and new SCN4A mutations revealed. Neurology. 2008;70:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 8. | Matthews E, Manzur AY, Sud R, Muntoni F, Hanna MG. Stridor as a neonatal presentation of skeletal muscle sodium channelopathy. Arch Neurol. 2011;68:127-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Lehmann-Horn F, Rüdel R. Channelopathies: the nondystrophic myotonias and periodic paralyses. Semin Pediatr Neurol. 1996;3:122-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Stenson PD, Mort M, Ball EV, Evans K, Hayden M, Heywood S, Hussain M, Phillips AD, Cooper DN. The Human Gene Mutation Database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum Genet. 2017;136:665-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 852] [Cited by in RCA: 986] [Article Influence: 123.3] [Reference Citation Analysis (0)] |
| 11. | Feng Y, Zhang Y, Liu ZL, Zhang CD. Exercise test on the patients with normokalaemic periodic paralysis from a Chinese family with a mutation in the SCN4A gene. Chin Med J (Engl). 2008;121:1915-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Cannon SC. Sodium Channelopathies of Skeletal Muscle. Handb Exp Pharmacol. 2018;246:309-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | van den Bersselaar LR, Heytens L, Silva HCA, Reimann J, Tasca G, Díaz-Cambronero Ó, Løkken N, Hellblom A, Hopkins PM, Rueffert H, Bastian B, Vilchez JJ, Gillies R, Johannsen S, Veyckemans F, Muenster T, Klein A, Litman R, Jungbluth H, Riazi S, Voermans NC, Snoeck MMJ. European Neuromuscular Centre consensus statement on anaesthesia in patients with neuromuscular disorders. Eur J Neurol. 2022;29:3486-3507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 14. | Becker PE. Genetic approaches to the nosology of muscular disease: myotonias and similar diseases. Birth Defects Orig Artic Ser. 1971;7:52-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Miller TM. Differential diagnosis of myotonic disorders. Muscle Nerve. 2008;37:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Electromyography and Neuromuscular Disorders: Clinical-Electrodiagnostic-Ultrasound Correlations, Fourth Edition. J Clin Neurophysiol. 2021;38:e19. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Sawlani K, Katirji B. Peripheral Nerve Hyperexcitability Syndromes. Continuum (Minneap Minn). 2017;23:1437-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Aminoff MJ. Electrodiagnosis in clinical neurology. 4th ed. New York: Churchill Livingstone, 1999: 257-263. |
| 19. | Nandedkar SD, Sanders DB, Stålberg EV. Selectivity of electromyographic recording techniques: a simulation study. Med Biol Eng Comput. 1985;23:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Trontelj J, Stålberg E. Bizarre repetitive discharges recorded with single fibre EMG. J Neurol Neurosurg Psychiatry. 1983;46:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 51] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Torbergsen T, Hødnebø A, Brautaset NJ, Løseth S, Stålberg E. A rare form of painful nondystrophic myotonia. Clin Neurophysiol. 2003;114:2347-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Heckman JD. Electrodiagnosis in diseases of nerve and muscle. Orthopedics. 1984;7:601-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Huang CW, Lai HJ, Lin PC, Lee MJ. Changes of Resurgent Na(+) Currents in the Na(v)1.4 Channel Resulting from an SCN4A Mutation Contributing to Sodium Channel Myotonia. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Hehir MK, Logigian EL. Electrodiagnosis of myotonic disorders. Phys Med Rehabil Clin N Am. 2013;24:209-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Osborne MT. Repetitive stimulation of muscle. Nature. 1958;181:1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 26. | Park JH, Lee YW, Park SA, Lee TK, Rho HJ, Sung KB. A case of paramyotonia congenita without periodic paralysis: electrophysiological and molecular genetic studies. Neurologist. 2010;16:203-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Hahn C, Salajegheh MK. Myotonic disorders: A review article. Iran J Neurol. 2016;15:46-53. [PubMed] |
| 28. | Brooks EK, Schweitzer D, Robinson HL. A case of paramyotonia congenita in pregnancy. Obstet Med. 2020;13:192-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 29. | Huang S, Zhang W, Chang X, Guo J. Overlap of periodic paralysis and paramyotonia congenita caused by SCN4A gene mutations two family reports and literature review. Channels (Austin). 2019;13:110-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | van Osch T, Stunnenberg BC, Sternberg D, Kerklaan BJ. Prolonged attacks of weakness with hypokalemia in SCN4A-related paramyotonia congenita. Muscle Nerve. 2018;58:E27-E28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Taminato T, Mori-Yoshimura M, Miki J, Sasaki R, Sato N, Oya Y, Nishino I, Takahashi Y. Paramyotonia Congenita with Persistent Distal and Facial Muscle Weakness: A Case Report with Literature Review. J Neuromuscul Dis. 2020;7:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Zhang S, Wu SW. [Clinical and genetic study of a family with paramyotonia congenital]. Chin J Neuromed. 2017;630-632. [DOI] [Full Text] |
| 33. | Koul R, Alfutaisi A, Hira M. Paramyotonia congenita in 22 members of an Arab (Omani) kindred. J Child Neurol. 2010;25:212-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Song J, Zhang JW, Fu J, Pang M, Li G, Ma MM. [Clinical, myopathological and genetic features of two Chinese families with paramyotonia congenita]. Zhonghua Nei Ke Za Zhi. 2020;59:535-539. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |