Published online Jan 26, 2024. doi: 10.12998/wjcc.v12.i3.582
Peer-review started: September 19, 2023
First decision: December 5, 2023
Revised: December 17, 2023
Accepted: January 4, 2024
Article in press: January 4, 2024
Published online: January 26, 2024
Processing time: 118 Days and 19 Hours
Rhabdomyosarcoma is a tumor of mesenchymal origin. Secondary leukemia is a complication of previous transformation to other hematologic disorders or is a treatment-related acute myeloid leukemia secondary to cytotoxic chemotherapy or radiation therapy for other malignancies.
We present the case of a 36-year-old female patient who was diagnosed with rhabdomyosarcoma and acute myeloid leukemia. Further disease progression was observed after multiline chemotherapy. Eventually, the patient suffered cerebral hemorrhage, which resulted in death.
The incidence of rhabdomyosarcoma in adults is extremely low, and secondary leukemia caused by rhabdomyosarcoma is even rarer. Secondary leukemia has a very poor prognosis and a low overall survival rate.
Core Tip: This article reports on a patient with rhabdomyosarcoma who developed acute myeloid leukemia (AML-M5a) after 11 chemotherapy sessions. Eventually, the patient suffered cerebral hemorrhage, which resulted in death. The incidence of rhabdomyosarcoma in adults is extremely low, and secondary leukemia caused by rhabdomyosarcoma is even rarer. Secondary leukemia has a very poor prognosis and a low overall survival rate.
- Citation: Zheng L, Zhang FJ. Adult rhabdomyosarcoma combined with acute myeloid leukemia: A case report. World J Clin Cases 2024; 12(3): 582-586
- URL: https://www.wjgnet.com/2307-8960/full/v12/i3/582.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i3.582
Rhabdomyosarcoma is a tumor of mesenchymal origin. It occurs most frequently in children and adolescents and has a very low incidence in adults[1]. The main treatments for rhabdomyosarcoma are surgery, chemotherapy and radio
A 36-year-old female patient visited our hospital on October 30, 2019, due to right cheek swelling for 1 month.
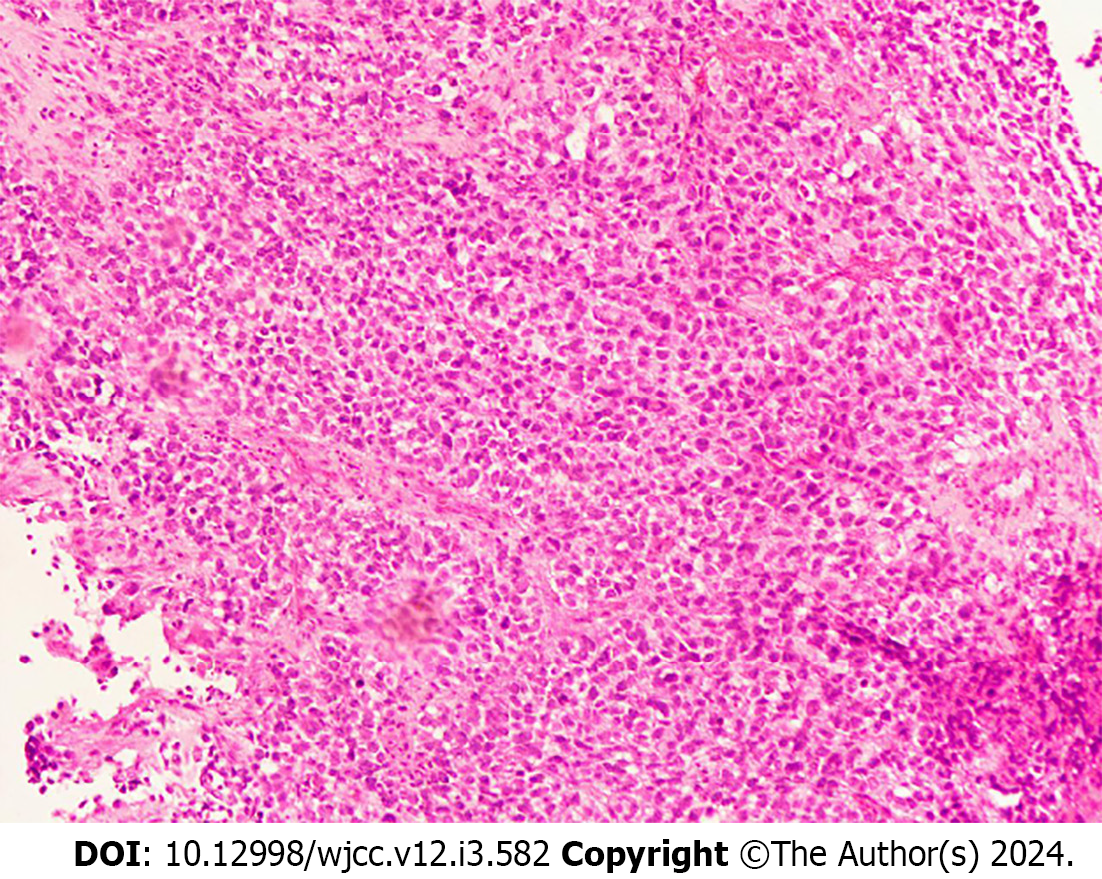
The patient was admitted with a mass on the left cheek. Physical examination revealed a mass approximately 3.4 cm in size on the right cheek. The peripheral blood and biochemical parameters (liver and renal function and serum lactate dehydrogenase level) were within normal limits. Pathology of the right buccal mass puncture showed spindle cell rhabdomyosarcoma. Immunohistochemistry (Figure 1) showed the following: ALK IA4 (portion+), Caldesmon (-), Desmin (+), H3K27ME3 (excalation), MyoD1 (+), Myogenin (+), S-100 (portion+), and SMA (-). She was diagnosed with spindle cell rhabdomyosarcoma. After the diagnosis, the patient underwent surgical treatment. The postoperative pathologic diagnosis was consistent with spindle cell rhabdomyosarcoma. The patient was subsequently given a 12-cycle VAC/IA regimen (VAC; Vinorelbine, 38 mg; cyclophosphamide, 1.4 mg; Epirus; IA: isocyclophosphamide 2.5 g 1-5 d, etoposide 0.1 g 1-5 d). Her disease went into remission after treatment. On April 28, 2021, the patient's repeat blood test showed a white blood cell count of 115.4 × 109/L, a hemoglobin level of 112 g/L, a blood platelet count of 144 × 109/L, and a primary cell count of 49%.
The patient had no family history or specific social history.
No personal or family history was available.
Physical examination revealed no palpable lymph nodes, organomegaly, or cutaneous lesions.
Her bone marrow morphology showed active cell hyperplasia, accounting for 77%. For these types of cells, the cytoplasm was 12-25 μm in size, the shape was round, oval or irregular, and the cytoplasmic volume was rich and colored dark blue. Part of the cell cytoplasm could be seen as tiny purplish-red particles, and the cytosolic nuclei were round and oval. The nuclear chromatin was meticulously granular, with 1 to several nucleoli that were not clear and seemed to be monophyletic. POX (+), butyric acid (+) and NaF (inhibition). The bone marrow picture was consistent with acute nonlymphocytic leukemia, with cytomorphology resembling M5. Immunohistochemistry revealed the following results: 87.57% of the primary cells predominantly expressed CD33, CD38, CD13, HLA-DR, CD11b, CD36, and CD64, while CD34, CD117, CD14, and cMPO were not expressed. The karyotype was 45, XX, -7. The leukemia genetic testing reported that the mutated genes are NRAS, CUX1, LIG4, MYH1, KMT2A, CTC1, ANKRD26, BCOR, KAT6A, GFI1 and MLLT3.
The patient did not have an imaging examination.
Based on the above findings, the final diagnosis was acute myeloid leukemia (AML-M5a).
On May 5, 2021, the patient was treated with a VA program (venetoclax 400 mg for 28 d and azacitidine for 7 d). Repeat bone marrow cytology suggested a state of disease remission. On June 25, 2021, the patient relapsed with acute myeloid leukemia. Multiple but ineffective platelet transfusions were performed.
On July 10, 2021, the patient suffered cerebral hemorrhage, which resulted in death.
In 1992, fusiform rhabdomyosarcoma was first discovered by Cavazzana et al[4]. Rhabdomyosarcoma is a malignant tumor arising from embryonic mesenchymal tissue that occurs most commonly in children and adolescents and rarely in adults and accounts for 2%-5% of all tumors. When it is present in the head and neck, the prognosis is often poor, and the clinical manifestations are more malignant[5]. According to the WHO classification, rhabdomyosarcoma is divided into embryonal rhabdomyosarcoma, acinar rhabdomyosarcoma, polymorphic rhabdomyosarcoma and spindle cell/sclerosing rhabdomyosarcoma. Rhabdomyosarcoma has a very low incidence in adults, accounting for less than 1% of all adult malignancies and 3% of adult soft tissue sarcomas[6]. Rhabdomyosarcoma generally has a soft morphology, and its gross appearance is mainly a cauliflower-like or polypoid mass that can invade surrounding tissues, cause tissue adhesion, and subsequently invade bone[7]. Microscopically, the tumors were diverse and mainly composed of small round cells. After HE staining, the rhabdomyosarcoma cells showed small, elongated nuclei with frequent mitotic activity, a reddish cytoplasm, and a clear stroma. Immunohistochemistry revealed SMA (+), MyoD1 (+), myogenin (+), CD99 (+), and vimentin (+), with MyoD1 and vimentin being specific markers of striated muscle.
The immunophenotype of the patient in this case was MyoD1 (+) and Myogenin(+), which was consistent with the findings of other reports[8]. The optimal treatment method for rhabdomyosarcoma has not yet been determined, and there is no standardized systematic treatment method. Currently, local extensive resection combined with postoperative chemotherapy is the main treatment method.
The causes of secondary leukemia include chemotherapy, radiotherapy, genetics, and other factors, and secondary leukemia caused by chemotherapy drugs ranks first. The main chemical drugs that can cause secondary leukemia are alkylating agents (such as cyclophosphamide, mefalam, nitrogen mustard, etc.), topoisomerase II inhibitors (etoposide, doxorubicin, mitoxantrone, etc.), dioxyphrazine drugs, etc. The disease progression of leukemia caused by alkylating agents is gradual and is usually accompanied by myelodysplastic syndrome, with a median latency of 5 yr (range 1-14 yr)[9]. The median latency of leukemia caused by topoisomerase II inhibitors was 1.5 yr[9]. The patient in this case had a history of rhabdomyosarcoma and was cured after multiple rounds of chemotherapy. Multiple chemotherapy cycles may also cause secondary leukemia, and the duration from chemotherapy to secondary leukemia was 1.5 yr. Studies have shown that the incidence of secondary leukemia increases 7-fold when etoposide and/or cyclophosphamide are used[10].
Compared with those of primary leukemia, secondary leukemia often has worse clinical outcomes, including a significantly worse complete remission (CR) rate, relapse-free survival rate and overall survival rate[11]. This may be related to the older age and worse organ function of patients with secondary leukemia. In addition, these patients may have persistent malignant disease or experience recurrence of primary malignancies. Previous chemotherapy or radiotherapy leads to hematopoietic failure and prolongs bone marrow suppression after treatment for acute myeloid leukemia, which can easily cause more serious treatment-related complications.
The median survival time of patients with secondary leukemia is 6-12 mo, and the main cause of death is the tumor itself[12]. Molecular mutations and cytogenetic abnormalities, such as TP53 mutations, may be more common in patients with secondary leukemia and are associated with resistance to conventional chemotherapy[13]. The median survival time of patients with secondary leukemia is 6-12 mo, and the main cause of death is the tumor itself[12]. However, molecular mutations and cytogenetic abnormalities, such as TP53 mutations, which are associated with resistance to conventional chemotherapy[13], may be more common in patients with secondary leukemia.
With the progress of medical technology, there have been obvious breakthroughs in understanding the etiology and pathogenesis of secondary leukemia and in its treatment. At present, the best method for treating secondary leukemia is still unclear, and intensive chemical treatment is still one of the main methods for treating secondary leukemia. Godley et al[12] conducted a study on 32 patients with secondary leukemia. After treatment with high-dose cytarabine + mitoxantrone, the complete response rate was 66%, the partial response rate was 16%, and the overall response rate was 82%. The mortality rate after treatment on the 30th day was 9%. At present, a variety of small molecule inhibitors targeting Bcl-2 have been marketed, among which venetoclax has been widely used. Venetoclax is an oral, potent, selective Bcl-2 inhibitor. Several studies have reported clinical trials of venetoclax in elderly AML patients who cannot tolerate chemotherapy. The results showed that the median CR rate was 62%, the median response time was 1 mo, the median survival time was 18.4 mo, and the 1-yr OS was 70.4%[14]. Venetoclax in combination with the hypomethylating agents azacitidine and decitabine is a new treatment for refractory relapsed leukemia. At present, venetoclax is mainly used in elderly patients with acute leukemia and recurrent/refractory acute leukemia, and it is still rarely used for the treatment of primary drug-related secondary leukemia.
In this case, the patient had received multiple rounds of chemotherapy for the treatment of spindle rhabdomyosarcoma and was physiologically resistant to chemotherapy. Therefore, she first received 100 mg azacitidine on days 1 to 7 and 400 mg venetoclax once daily for 28 d. She achieved CR, but the remission time was not long. Finally, the complications of cerebral hemorrhage led to death. It was also suggested that the prognosis of secondary leukemia patients was very poor, which was basically consistent with reports in the literature. In this case, the patient had received multiple rounds of chemotherapy for the treatment of spindle rhabdomyosarcoma and was physiologically resistant to chemotherapy. Therefore, she first received 100 mg azacitidine for days 1 to 7 and 400 mg venetoclax once daily for days. She achieved CR, but the remission time was not long. Ultimately, cerebral hemorrhage complications led to death. These findings also suggest that the prognosis of secondary leukemia patients is extremely poor, which is basically consistent with reports in the literature.
In summary, the incidence of rhabdomyosarcoma in adults is extremely low, and secondary leukemia caused by rhabdomyosarcoma is even rarer. Secondary leukemia has a very poor prognosis and a low overall survival rate. Despite the development of various new treatment technologies in recent years, we still need to continue to explore treatment options.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sultana N, Bangladesh S-Editor: Gao CC L-Editor: A P-Editor: Zheng XM
| 1. | Akki AS, Harrell DK, Weaver KD, Esnakula AK, Shenoy A. Rare case of spindle cell/sclerosing rhabdomyosarcoma in adult liver. Pathology. 2019;51:745-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Cheung E, Perissinotti AJ, Bixby DL, Burke PW, Pettit KM, Benitez LL, Brown J, Scappaticci GB, Marini BL. The leukemia strikes back: a review of pathogenesis and treatment of secondary AML. Ann Hematol. 2019;98:541-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Micallef IN, Lillington DM, Apostolidis J, Amess JA, Neat M, Matthews J, Clark T, Foran JM, Salam A, Lister TA, Rohatiner AZ. Therapy-related myelodysplasia and secondary acute myelogenous leukemia after high-dose therapy with autologous hematopoietic progenitor-cell support for lymphoid malignancies. J Clin Oncol. 2000;18:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 144] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Cavazzana AO, Schmidt D, Ninfo V, Harms D, Tollot M, Carli M, Treuner J, Betto R, Salviati G. Spindle cell rhabdomyosarcoma. A prognostically favorable variant of rhabdomyosarcoma. Am J Surg Pathol. 1992;16:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 149] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Owosho AA B Ch D, Huang SC Md, Chen S Mbbs, Kashikar S Dds, Estilo CL Dmd, Wolden SL Md, Wexler LH Md, Huryn JM Dds, Antonescu CR Md. A clinicopathologic study of head and neck rhabdomyosarcomas showing FOXO1 fusion-positive alveolar and MYOD1-mutant sclerosing are associated with unfavorable outcome. Oral Oncol. 2016;61:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Kayser S, Döhner K, Krauter J, Köhne CH, Horst HA, Held G, von Lilienfeld-Toal M, Wilhelm S, Kündgen A, Götze K, Rummel M, Nachbaur D, Schlegelberger B, Göhring G, Späth D, Morlok C, Zucknick M, Ganser A, Döhner H, Schlenk RF; German-Austrian AMLSG. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117:2137-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 343] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 7. | Ciammella P, Galeandro M, D'Abbiero N, Palmieri T, Donini E, Iotti C. Prostate embryonal rhabdomyosarcoma in adults: Case report and review of literature. Rep Pract Oncol Radiother. 2013;18:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Yasui N, Yoshida A, Kawamoto H, Yonemori K, Hosono A, Kawai A. Clinicopathologic analysis of spindle cell/sclerosing rhabdomyosarcoma. Pediatr Blood Cancer. 2015;62:1011-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Mauritzson N, Albin M, Rylander L, Billström R, Ahlgren T, Mikoczy Z, Björk J, Strömberg U, Nilsson PG, Mitelman F, Hagmar L, Johansson B. Pooled analysis of clinical and cytogenetic features in treatment-related and de novo adult acute myeloid leukemia and myelodysplastic syndromes based on a consecutive series of 761 patients analyzed 1976-1993 and on 5098 unselected cases reported in the literature 1974-2001. Leukemia. 2002;16:2366-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 185] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Heyn R, Khan F, Ensign LG, Donaldson SS, Ruymann F, Smith MA, Vietti T, Maurer HM. Acute myeloid leukemia in patients treated for rhabdomyosarcoma with cyclophosphamide and low-dose etoposide on Intergroup Rhabdomyosarcoma Study III: an interim report. Med Pediatr Oncol. 1994;23:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Borthakur G, Lin E, Jain N, Estey EE, Cortes JE, O'Brien S, Faderl S, Ravandi F, Pierce S, Kantarjian H. Survival is poorer in patients with secondary core-binding factor acute myelogenous leukemia compared with de novo core-binding factor leukemia. Cancer. 2009;115:3217-3221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Godley LA, Njiaju UO, Green M, Weiner H, Lin S, Odenike O, Rich ES, Artz A, Van Besien K, Daugherty CK, Zhang Y, Le Beau MM, Stock W, Larson RA. Treatment of therapy-related myeloid neoplasms with high-dose cytarabine/mitoxantrone followed by hematopoietic stem cell transplant. Leuk Lymphoma. 2010;51:995-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Yang D, Fu X, Zhang X, Li W, Zhang M. Therapy-related acute myeloid leukemia in patients with lymphoma: A report of four cases and review of the literature. Oncol Lett. 2015;10:3261-3265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Bedikian AY, Garbe C, Conry R, Lebbe C, Grob JJ; Genasense Melanoma Study Group. Dacarbazine with or without oblimersen (a Bcl-2 antisense oligonucleotide) in chemotherapy-naive patients with advanced melanoma and low-normal serum lactate dehydrogenase: 'The AGENDA trial'. Melanoma Res. 2014;24:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |