Published online Jan 26, 2024. doi: 10.12998/wjcc.v12.i3.538
Peer-review started: October 24, 2023
First decision: November 22, 2023
Revised: December 10, 2023
Accepted: December 28, 2023
Article in press: December 28, 2023
Published online: January 26, 2024
Processing time: 86 Days and 5.5 Hours
The incidence of chronic kidney disease among patients with diabetes mellitus (DM) remains a global concern. Long-term obesity is known to possibly influence the development of type 2 diabetes mellitus. However, no previous meta-analysis has assessed the effects of body mass index (BMI) on adverse kidney events in patients with DM.
To determine the impact of BMI on adverse kidney events in patients with DM.
A systematic literature search was performed on the PubMed, ISI Web of Science, Scopus, Ovid, Google Scholar, EMBASE, and BMJ databases. We included trials with the following characteristics: (1) Type of study: Prospective, retrospective, randomized, and non-randomized in design; (2) participants: Restricted to patients with DM aged ≥ 18 years; (3) intervention: No intervention; and (4) kidney adverse events: Onset of diabetic kidney disease [estimated glomerular filtration rate (eGFR) of < 60 mL/min/1.73 m2 and/or microalbuminuria value of ≥ 30 mg/g Cr], serum creatinine increase of more than double the baseline or end-stage renal disease (eGFR < 15 mL/min/1.73 m2 or dialysis), or death.
Overall, 11 studies involving 801 patients with DM were included. High BMI (≥ 25 kg/m2) was significantly associated with higher blood pressure (BP) [systolic BP by 0.20, 95% confidence interval (CI): 0.15–0.25, P < 0.00001; diastolic BP by 0.21 mmHg, 95%CI: 0.04–0.37, P = 0.010], serum albumin, triglycerides [standard mean difference (SMD) = 0.35, 95%CI: 0.29–0.41, P < 0.00001], low-density lipoprotein (SMD = 0.12, 95%CI: 0.04–0.20, P = 0.030), and lower high-density lipoprotein (SMD = –0.36, 95%CI: –0.51 to –0.21, P < 0.00001) in patients with DM compared with those with low BMIs (< 25 kg/m2). Our analysis showed that high BMI was associated with a higher risk ratio of adverse kidney events than low BMI (RR: 1.22, 95%CI: 1.01–1.43, P = 0.036).
The present analysis suggested that high BMI was a risk factor for adverse kidney events in patients with DM.
Core Tip: The effect of body mass index (BMI) on adverse kidney events in patients with diabetes mellitus (DM) remains unclear. Our meta-analysis showed that patients with DM with higher BMIs had higher blood pressures and serum albumin levels, as well as worse lipid profiles. High BMI was found to be a risk factor contributing to adverse kidney events in patients with DM.
- Citation: Wan JF, Chen Y, Yao TH, Wu YZ, Dai HZ. Impact of body mass index on adverse kidney events in diabetes mellitus patients: A systematic-review and meta-analysis. World J Clin Cases 2024; 12(3): 538-550
- URL: https://www.wjgnet.com/2307-8960/full/v12/i3/538.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i3.538
According to the 2020 Hemodialysis Annual Report[1], approximately 700,000 patients with end-stage renal disease (ESRD) require maintenance hemodialysis. The incidence of chronic kidney disease (CKD) among patients with diabetes mellitus (DM) was noted to be as high as 64.0 cases per 1000 person-years (95%CI, 62.2–65.9)[2].
Patients with DM and CKD have a substantially increased risk of all-cause mortality, cardiovascular mortality, and ESRD[3]. Obesity is a major contributor to the development of diabetes[4]. A historical cohort study[5] showed that a higher baseline body mass index (BMI) was an independent predictor of ESRD when compared to individuals with normal weight in the normal range (BMI: 18.5–24.9 kg/m2), and the adjusted relative risk of ESRD was 1.87 (95%CI, 1.64–2.14) for those who were overweight (BMI: 25–29.9 kg/m2). Long-term obesity has the potential to influence the development of type 2 diabetes mellitus and has significant effects on the kidneys that can include changes to intraglomerular hemodynamics, increased sympathetic activity, hypertension, systemic inflammation, endothelial dysfunction, expression of growth factors, and compression associated with visceral adiposity[6-8].
Some studies have found that obesity and DM exert a synergic effect on decreases in estimated glomerular filtration rate (eGFR)[5,9-11]. However, the influence of BMI on adverse kidney events in patients with DM remains unclear, and some studies have shown that BMI is positively correlated with diabetic kidney disease (DKD)[12-14]. Obesity has also been associated with an increased risk of decreased renal function in patients with DM[15-17]. Other studies have suggested that BMI is a protective factor against renal function deterioration[18-21] or that declines in renal parameters are not influenced by BMI[22,23].
To the best of our knowledge, no previous meta-analysis has evaluated the effects of BMI on adverse kidney events in patients with DM. Therefore, this study aimed to evaluate the effects of BMI on adverse kidney events in patients with DM.
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines[24]. Two reviewers independently identified relevant studies in the PubMed, ISI Web of Science, Scopus, Ovid, Google Scholar, EMBASE, and BMJ databases from their respective inceptions until December 2022. We restricted the included studies to those published in English. The following terms were used in our search: diabetes mellitus, diabetic kidney disease, diabetic nephropathy, DM, BMI, obesity, excessive body weight, overweight, and underweight.
We included trials with the following characteristics: (1) Type of study: Prospective, retrospective, randomized, and non-randomized in design; (2) participants: restricted to patients with DM aged ≥ 18 years; (3) intervention: No intervention; and (4) kidney adverse events: Onset of (DKD; eGFR of < 60 mL/min/1.73 m2 and/or microalbuminuria value of ≥ 30 mg/g. Cr), serum creatinine increase of more than double from baseline levels, ESRD (eGFR < 15 mL/min/1.73 m2, or need for dialysis), or death. Studies were excluded if: (1) They involved particular niche-group populations, such as pregnant or lactating women; (2) they were meta-analyses, case studies, duplicates, repetitive results, or reviews; or (3) they were preclinical studies that used animal models. The following data were extracted: The study characteristics, baseline data, clinical comorbidities, and clinical outcomes.
Various BMI classifications were observed in the selected studies. According to the World Health Organization, BMI values of > 30, 25–30, and < 25 kg/m2 have been defined in the Western population as obesity, overweight, and normal, respectively. In the Asian population, the recommended cut-off values of BMI corresponding to these three categories are > 25, 23–25, and < 23 kg/m2. Because of the variations in BMI classifications used in the studies we included, we simplified these classifications to ≥ 25 kg/m2 or < 25 kg/m2 to define high and low BMI, respectively.
We evaluated the quality of the cohort studies using the Newcastle–Ottawa Scale (NOS)[25]. The NOS evaluates the quality of articles using three levels, with eight items. The three major aspects include the selection of study groups, the comparability of the groups, and the ascertainment of either the exposure or outcome of interest for case-control or cohort studies. A star rating system was used to assess the quality of the literature through a semi-quantitative principle, with the highest score being nine stars.
The reliability of the eligible studies was assessed using the quality assessment forms recommended by the United States Agency for Healthcare Research and Quality[26]. This checklist comprises 11 items. Each item was coded with a “yes/no/unclear”: “no” or “unclear” was scored “0,” and “yes” was scored “1.” Quality scoring was performed out of 11, with scores of 8-11, 4-7, and < 3 indicating high, medium, and low quality, respectively.
Quality assessments demonstrated moderate to high quality for all included studies (Supplementary Tables 1 and 2).
Between-study heterogeneity was estimated using Cochran’s Chi-squared (χ2)-based Q statistical test and the I-squared
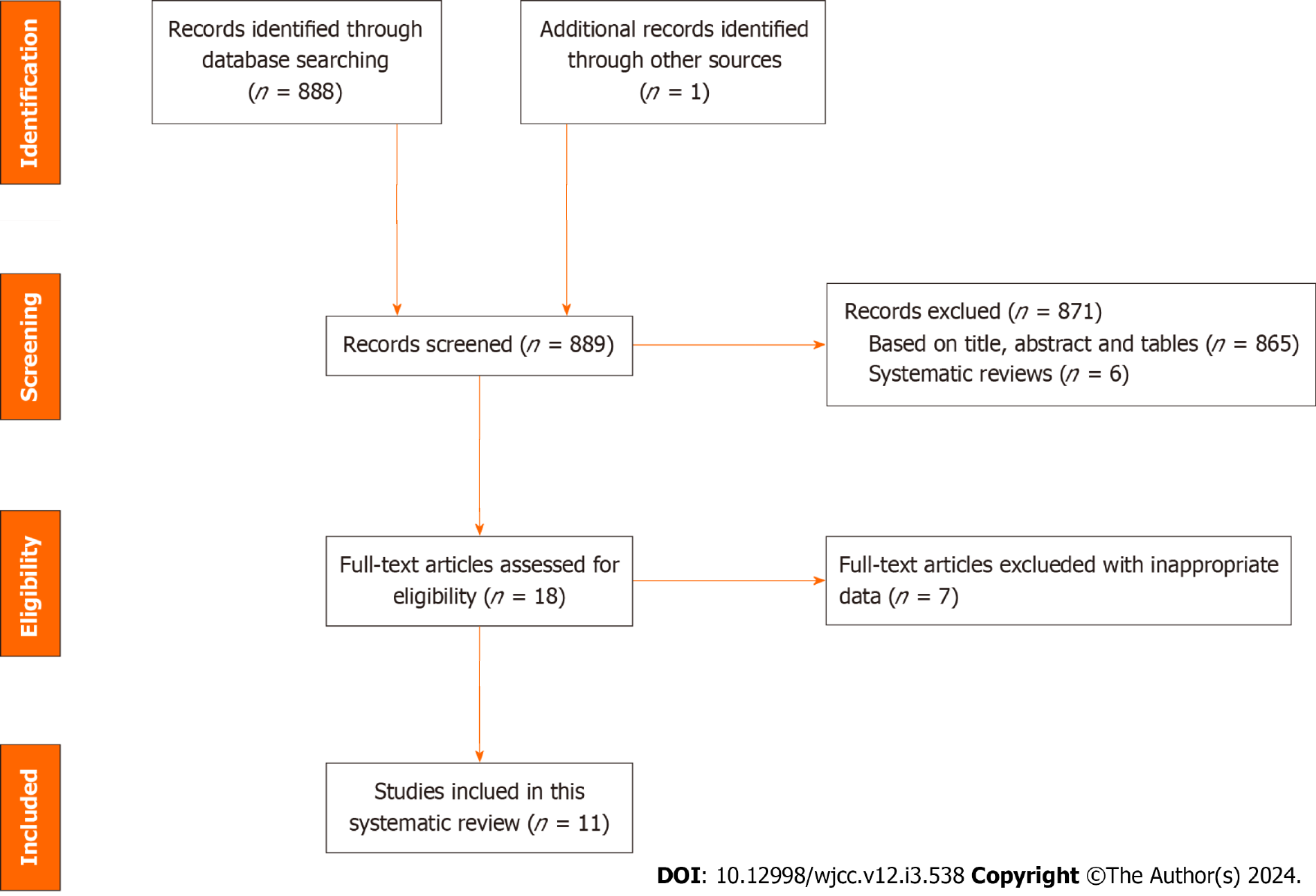
A flowchart of the article selection process is shown in Figure 1. We identified 889 articles through a literature search. After excluding systematic reviews and duplicate publications, as well as after reviewing the titles and abstracts, 18 studies remained. We further examined the full texts of these 18 studies and excluded incomplete data or data that could not be obtained through calculations. Ultimately, 11 studies[12,13,15-23] were included in our meta-analysis.
The included studies are summarized in Table 1. They were published between 1999 and 2022; of the 11 single-center studies, 5 were prospective, and 6 were retrospective. Data from 8011 patients were included, and the sample size varied from 49 (in the study by Kanauchi et al[21]) to 3224 (in the study by Zhang et al[13]). Three studies reported renal outcomes[19,22,23], one study evaluated mortality rates and renal endpoints[18], six studies included Asian Pacific populations [Chinese (n = 3)[13,18,19], Japanese (n = 2)[12,21], and Korean (n = 1)[17]], whereas five included Afro-European populations (African (n = 1)[22], Dutch (n = 1)[20], German (n = 1)[14], British (n = 1)[23], and French (n = 1)[16].
| Ref. | Huang etal[18], 2014 | Bentata etal[22], 2014 | Chen etal[19], 2013 | Drion etal[20], 2011 | Haupt etal[14], 1999 | Mohsen etal[23], 2012 | Zhang etal[13],2019 | Nakanishi etal[12], 2019 | Kanauchi etal[21], 2003 | Kim etal[17], 2021 | Belhatem etal[16], 2015 | ||
| Country | Chinese | African | Chinese | Dutchman | Germany | British | Chinese | Japanese | Japanese | Korean | France | ||
| Study design | Prospective | Prospective | Retrospective | Retrospective | Prospective | Prospective | Retrospective | Retrospective | Retrospective | Prospective | Retrospective | ||
| Institution | Single-center | Single-center | Single-center | Single-center | Single-center | Single-center | Single-center | Single-center | Single-center | Single-center | Single-center | ||
| BMI Categories Reported | 18.5-22.9: 23-24.9: ≥ 25 | 18.5–24.9: 25–29.9: ≥ 30 | < 25: 25-28: ≥ 28 | 18-24.9: 25-29.9: ≥ 30 | < 25: 25-30: 30–35: > 35 | < 30: ≥ 30 | < 21.62: 21.62–23.50: 23.51–25.16: 25.17–27.33: > 27.33 | Controlled HbA1c non-overweight BMI < 25: controlled HbA1c overweight BMI ≥ 25): UNCONTROLLEd HbA1c non-overweight BMI < 25: uncontrolled HbA1c overweight BMI ≥ 25 | ≤ 25: > 25 | < 23: 23- 25: ≥ 25 | < 25: 25-30: 30-40: > 40 | ||
| BMI Groups | 18.5-25: ≥ 25 | 18.5-25: ≥ 25 | < 25: ≥ 25 | 18-25: ≥ 25 | < 25: ≥ 25 | < 30: ≥ 30 | < 25.17: ≥ 25.17 | < 25: ≥ 25 | < 25: ≥ 25 | < 25: ≥ 25 | < 25: ≥ 25 | ||
| Patient characteristics by BMI groups | |||||||||||||
| Sample size | 105 | 292 | 264 | 844 | 698 | 229 | 3224 | 2306 | 49 | 1060 | 855 | ||
| Age (yr) | 61.98 ± 9.27: 60.60 ± 10.37 | 61.00 ± 11.00: 59.15 ± 9.28 | 54.10 ± 9.34: 53.63 ± 8.81 | 61.84 ± 18.64: 63.18 ± 12.29 | 46.00 ± 8.40: 45.11 ± 8.93 | 71.00 ± 9.90: 68.00 ± 9.40 | 60.72 ± 1.98: 62.18 ± 11.16 | 64.2 ± 10.4: 57.4 ± 12.9 | 55.7 ± 9.0: 53.2 ± 9.1 | 56.48 ± 8.44: 54.10 ± 8.70 | 60 ± 12: 60.22 ± 10.03 | ||
| Gender (Male %) | 0.6476 | 0.3801 | 0.5833 | 0.5403 | 0.692 | 0.655 | 0.507 | 0.6015 | 0.673 | 0.4557 | 0.6023 | ||
| BMI value | 22.53 ± 1.36: 22.76 ± 2.09 | 23.06 ± 1.68: 3.19 ± 3.97 | 22.60 ± 1.59: 28.44 ± 2.79 | 23.00 ± 1.49: 30.58.00 ± 54.76 | 25.90 ± 2.60: 35.00 ± 4.90 | 21.70 ± 2.30: 28.70 ± 3.40 | 21.70 ± 2.50: 27.50 ± 2.30 | 22.65 ± 1.83: 29.4 ± 2.7 | 22.70 ± 2.00: 32.21 ± 5.88 | ||||
| RAS | 51:53:00 | 64:217 | NR | NR | NR | NR | 216:243 | NR | NR | NR | NR | ||
| Hypertension | 50:51:00 | NR | NR | NR | NR | NR | 1130:946 | NR | NR | 370:344 | NR | ||
| Follow-up (m) | 24 | 40.51 ± 14.00: 44.85 ± 11.21 | The median follow-up time was 39.0 0months (range 0–87.00) | NR | NR | 31.00 ± 19.50: 31.00 ± 19.40 | NR | NR | NR | NR | NR | ||
| Baseline renal characteristics | |||||||||||||
| Serum creatinine (mg/dL) | 0.95 ± 0.43: 2.03 ± 0.47 | NR | 2.39 ± 2.27: 1.72 ± 1.76 | 0.89 ± 0.23: 0.94 ± 0.27 | 0.8 ± 0.2: 0.85 ± 0.34 | NR | 0.69 ± 0.21: 0.73 ± 0.26 | 0.74 ± 0.28: 0.62 ± 0.15 | NR | NR | 0.89 ± 0.25: 0.92 ± 0.32 | ||
| eGFR (ml/min/1.73 m2) | 38.83 ± 8.36: 38.33 ± 9.34 | 85.59 ± 40.93: 80.67 ± 38.19 | 81.60 ± 51.00: 111.92 ± 60.52 | NR | NR | NR | 114.01 ± 36.71: 104.35 ± 35.08 | NR | 108.00 ± 41.00: 134.00 ± 37.00 | 74.93 ± 12.41: 70.50 ± 13.00 | 87.00 ± 9: 83.89 ± 30.48 | ||
| Proteinuria | Daily urinary protein (g/24 h): 3.09 ± 2.62: 2.90 ± 2.35 | Urine protein excretion (g/24 h): 3.19 ± 2.19: 3.02 ± 2.55 | Albumin (mg in 24-h urine): 64.4 ± 294.9: 170.69 ± 776 | Urine protein (g/24 h): 72 (30-266): 67 (30-210) | |||||||||
| Follow-up end kidney adverse events | Serum creatinine ≥ 2-fold vs Baseline, Dialysis, or Death | ESRD. ESRD was defined by eGFR < 15 ml/min/1.73 m2 and/or initiation of dialysis. | ESRD. ESRD was defined as the requirement for permanent renal replacement therapy or serum creatinine exceeding 6.0 mg/dL for more than 1 mo without other causes of renal dysfunction | NR | NR | Deceased or commenced on renal replacement therapy | NR | eGFR of < 30 mL/min/1.73 m2 and/or microalbuminuria value of ≥ 30 mg/gCr | NR | eGFR < 60 mL/min/1.73 m2, or albumin/creatinine ratio in spot urine of 30–300 mg/g | NR | ||
| The risk of kidney adverse events | RR: 2.88 (95%CI: 1.24-6.66) | RR: 1.26 (95%CI: 0.72-2.22) | OR: 1.32 (95%CI: 1.00-1.71) | HR: 1.03 (95%CI: 1.01-1.07) | OR: 1.40 (95%CI: 1.08-2.04 ) | OR: 1.34 (95%CI: 1.03-1.43) | |||||||
The results of our pooled analysis revealed a significant positive association between high BMI and the incidence of hypertension (OR: 1.640, 95%CI: 1.17–2.29, P = 0.004; I2 = 68%; Supplementary Figure 1A)[13,17,18], without publication bias (P = 0.378, Egger). High-BMI patients with DM were also associated with a low prevalence of Renin-angiotensin system blockers (RAS) (OR: 0.690, 95%CI: 0.57–0.84, P = 0.0002; I2 = 0%; Supplementary Figure 1B)[13,18,22], without publication bias (P = 0.178, Egger). Using the random-effects model, we found a higher proportion of males in the high BMI group (OR: 1.33, 95%CI: 1.10–1. 61, P < 0.003), without publication bias (P = 0.087, Egger), but with higher heterogeneity (P = 0.0003, I2 = 70%; Supplementary Figure 1C). The study by Haupt et al[14] contributed to most of the heterogeneity. Compared to patients with low BMIs, high-BMI patients showed significantly higher systolic blood pressure (SBP) levels, by 0.20 mmHg (SMD: 0.20, 95%CI: 0.15–0.25, P < 0.00001; I2 = 0%; P = 0.895, Egger; Supple
Our meta-analysis showed no significant increases in cholesterol levels in the high BMI group (SMD = 0.07, 95%CI:
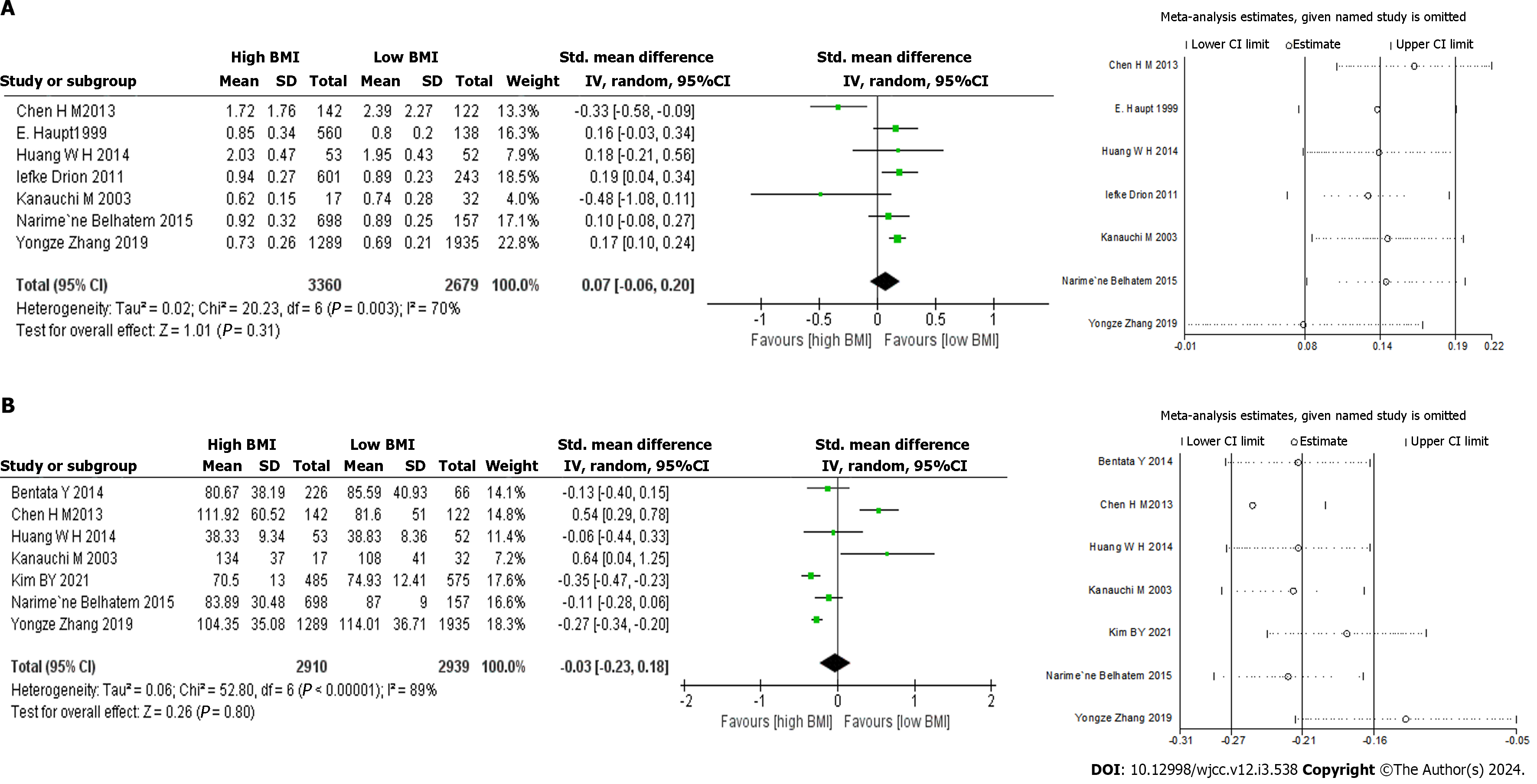
The association between BMI and urine protein level was reported in four studies. Two assessed 24-h proteinuria[18,19], and two measured 24-h urinary albumin[14,21]. No significant increase in urine protein levels was found in the high BMI group via random effects analyses. There was also no significant difference in baseline serum creatinine levels (SMD: 0.07, 95%CI -0.06–0.20, P = 0.310; I² = 70%; Figure 2A)[13,14,16,18-21], without publication bias (P = 0.141, Egger). Our subgroup analysis showed that the serum creatinine levels in the high BMI group were significantly higher compared to those of the low BMI group in the European population for those aged > 60 (sample size > 500; Table 2). When the study by Chen et al[19] was excluded, heterogeneity decreased significantly (I2 = 6%, P = 0.380).
| Serum creatinine | eGFR | |||||||||||||
| Subgrouped | By No. of trials | SMD | 95%CI | P value | I2 (%) | P for heterogeneity | By No. of trials | SMD | 95%CI | P value | I2 (%) | P for heterogeneity | ||
| Country | ||||||||||||||
| Asian | 4 | -0.08 | -0.41 | 0.26 | 0.66 | 84.60 | < 0.001 | 6 | -0.01 | -0.24 | 0.23 | 0.96 | 90 | < 0.00001 |
| European | 3 | 0.15 | 0.06 | 0.25 | < 0.001 | 0.00 | 0.71 | 1 | -0.11 | -0.28 | 0.06 | 0.12 | - | - |
| Age | ||||||||||||||
| ≤ 60 | 3 | -0.18 | -0.59 | 0.24 | 0.41 | 83.40 | < 0.001 | 3 | -0.32 | -0.86 | 0.21 | 0.23 | 90.50 | < 0.001 |
| > 60 | 4 | 0.17 | 0.11 | 0.23 | < 0.001 | 0.00 | 0.86 | 4 | 0.27 | 0.19 | 0.35 | < 0.001 | 22.00 | 0.28 |
| Subject type | ||||||||||||||
| Retrospective | 5 | 0.02 | -0.16 | 0.20 | 0.81 | 0.80 | < 0.001 | 5 | -0.01 | -0.29 | 0.27 | 0.92 | 0.92 | < 0.001 |
| Prospective | 2 | 0.16 | -0.01 | 0.33 | 0.06 | 0.00 | 0.93 | 2 | 0.10 | -0.06 | 0.26 | 0.20 | 0.00 | 0.80 |
| Sample size | ||||||||||||||
| ≤ 500 | 3 | -0.20 | -0.57 | 0.18 | 0.31 | 0.65 | 0.06 | 4 | -0.22 | -0.63 | 0.18 | 0.28 | 86.20 | < 0.001 |
| > 500 | 4 | 0.17 | 0.11 | 0.22 | < 0.001 | 0.00 | 0.86 | 3 | 0.28 | 0.21 | 0.36 | < 0.001 | 21.70 | 0.28 |
No significant difference was found in baseline eGFR between the two groups (SMD = -0.03, 95%CI: -0.23–0.18, P = 0.800; I2 = 89%; Figure 2B)[13,16-19,21,22], with no significant publication bias (P = 0.085, Egger). The study by Chen et al[19] contributed the greatest source of heterogeneity; when this article was removed, the heterogeneity decreased significantly (I2 = 68%, P = 0.009). Our results showed a significant decrease in eGFR in our subgroup analysis based on age > 60 and study sample size > 500 in the high BMI group (Table 2).
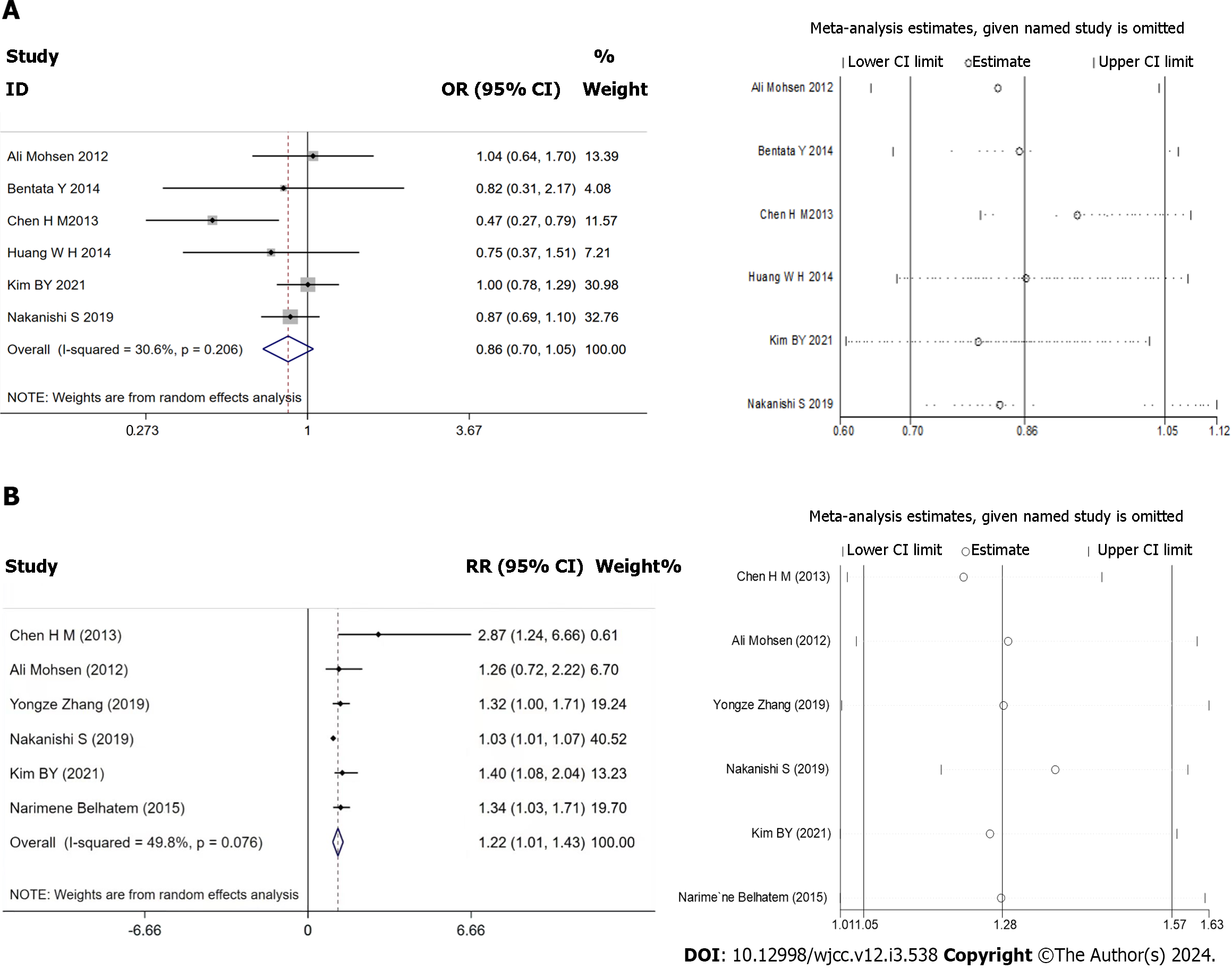
Six of the included studies reported adverse kidney events, but each reported different endpoints, and the definitions of these endpoints were inconsistent[12,17-19,22,23]. These endpoints were combined to perform a meta-analysis. The random-effects model was used owing to the obvious heterogeneity (I2 = 64%, P = 0.02). No significant difference was found between the two groups in terms of adverse kidney events (OR: 0.86, 95%CI: 0.70–1.05, P = 0.132; Figure 3A)[19,22,23], without publication bias (P = 0.389, Egger). The study by Chen et al[19] was found to be the most significant source of heterogeneity, and six of the studies[12,13,16,17,19,23] evaluated the risk of adverse kidney events. Various statistical indicators were pooled in this meta-analysis. The ORs and RRs were combined, which resulted in our meta-analysis suggesting that high BMI was associated with a higher RR than lower BMI (RR: 1.22, 95%CI: 1.01–1.43, P = 0.036; Figure 3B), without publication bias (P = 0.389, Egger).
To the best of our knowledge, this is the first meta-analysis to assess the role that BMI plays in the renal prognoses of patients with DM. Our analysis showed that the Serum creatinine of the high BMI group was significantly higher in the older European population. Compared with patients with low BMIs, those with high BMIs showed significantly higher levels of blood pressure (BP), serum albumin, TG, LDL, and significantly lower levels of HDL. High BMI was also associated with higher RR in adverse kidney events. These results have important clinical implications for intervention and risk stratification.
Obesity is a global issue. According to an international epidemiologic study[28], the global prevalence of obesity will reach 18% in men and surpass 21% in women by 2025. Obesity is regarded as an overnutrition-induced state, with hypertension and diabetes representing its three most common comorbidities. We found higher levels of serum albumin and BP and rate of male sex in patients with DM with higher BMIs. These findings are consistent with those of other studies[29,30].
Obesity and DM lead to elevated serum uric acid levels[31], and we observed lower serum uric acid levels in patients with DM with higher BMIs; however, the data supporting this notion were provided by only two studies. Thus, more well-designed studies with larger sample sizes are warranted to confirm the relationship between BMI and serum uric acid levels in patients with DM. High BMI in patients with DM was associated with a low rate of RAS use; however, only three articles provided limited data on this matter. Therefore, more evidence is necessary to draw a clear relationship between RAS, BMI, and adverse kidney events in patients with DM.
Owing to factors such as ethnic differences and dietary habits, the median BMI is higher in the European population than in Asian ones[13,23,32]. For example, the rate of high BMI (≥ 25 kg/m2) varies from 34.7% in Japan[21] to 71.21% in the Netherlands[20]. BMI is associated with new-onset CKD, CKD progression, and end-stage renal failure[33]. Kidney complications are highly prevalent in patients with DM[8]. The results of our study were similar to those of previous ones, with the scores of the high-BMI group being significantly higher in the European population aged > 60 years (sample size > 500). Obesity is the main cause of the worldwide DM epidemic[34]. Studies have found that both DM and obesity may play key roles in the pathophysiology of CKD[32,35]. However, other studies have suggested that BMI is a renal protective factor in patients with DM[18-21]. Our study has answered this question by demonstrating that high BMI is associated with a higher RR of adverse kidney events than lower BMI (RR: 1.22, 95%CI: 1.01–1.43, P = 0.036).
Hypertension was found to be the most common comorbidity associated with obesity in patients with DM. Our study showed that high BMI was associated with significantly higher levels of BP (SBP by 0.20, 95%CI: 0.15–0.25, P < 0.00001; DBP by 0.21 mmHg, 95%CI: 0.04–0.37, P = 0.01). Tightening of the afferent arterioles in patients with hypertension may cause partial ischemia of the glomerulus with varying degrees of capillary collapse and tuft retraction; however, the ischemic glomerulosclerosis and nephron loss that occur over time are usually not sufficient to result in ESRD[36]. Patients with hypertension progress to ESRD at a faster speed if their conditions are further complicated by obesity and DM[37].
Obesity and DM are also the most common causes of dyslipidemia[38-40], and our findings support this notion as well. Abnormal lipid homeostasis (biosynthesis, lipid transport, and degradation) was observed at a higher prevalence in patients with both obesity and DM[41], likely because these conditions produce local inflammation and oxidative stress that promote atherogenicity and the progression of kidney damage[42].
Two articles reported on adverse kidney events in patients with diabetic nephropathy (DN), but their results regarding the role of BMI were conflicting. Chen et al[19] reported that the lean phenotype (BMI < 25 kg/m2) was associated with the development of ESRD, particularly in the later stages, while Bentata et al[22] claimed that eGFR declines observed in patients with DN are not directly influenced by BMI. Thus, we did not examine the relationship between BMI and the development of CKD in this study owing to a lack of sufficient usable data.
Our study has a few limitations worth noting. First, varying classifications of BMI were observed among the selected studies. Therefore, we divided the participants into high- and low-BMI groups according to a cut-off value of 25 kg/m2. Second, we did not account for changes in BMI values during follow-ups, which may have influenced occurrences of adverse kidney events. Third, the use of varying eGFR calculation formulas means that final eGFR results may not always reflect actual renal status. Drion et al[20] found that all equations used to predict renal function (including the Modification of Diet in Renal Disease formula and the Chronic Kidney Disease Epidemiology Collaboration equation) are biased when used in populations with DM who are overweight or obese but have preserved renal function. In these cases, the Cockcroft-Gault equation provides the best estimate of kidney function. Fourth, most of the included studies did not report renal endpoint events or mortality; therefore, we could not exclude the potential effects of survival bias and competing risks. Finally, high-quality and rigorously controlled observational studies were lacking from our pool of studies; currently, evidence to conclusively evaluate the effects of BMI on the long-term outcomes of patients with DM is insufficient.
Patients with DM and higher BMIs had higher BP and serum albumin levels, as well as worse lipid profiles. We demonstrated that high BMI was a risk factor that contributed to the development of adverse kidney events in patients with DM. Further studies that focus on the optimal weight range for patients with DM would be beneficial to this field of study.
Obesity and diabetes are global public health concerns. Poor control of weight or blood sugar may lead to damage to multiple organs, including the kidneys.
The effect of obesity on adverse renal effects in patients with diabetes remains unclear.
This study aimed to explore the impact of body mass index (BMI) on adverse kidney events in patients with diabetes mellitus (DM).
A systematic literature search was performed of the PubMed, ISI Web of Science, Scopus, Ovid, Google Scholar, EMBASE, and BMJ databases. We included trials with the following characteristics: (1) Type of study: Prospective, retrospective, randomized, and non-randomized in design; (2) participants: Restricted to patients with DM aged ≥ 18 years; (3) intervention: No intervention; (4) kidney adverse events: onset of diabetic kidney disease [estimated glomerular filtration rate (eGFR) of < 60 mL/min/1.73 m2 and/or microalbuminuria value of ≥ 30 mg/g Cr], serum creatinine increase of more than double the baseline or end stage renal disease (eGFR < 15 mL/min/1.73 m2 or dialysis), or death.
High BMI (≥ 25 kg/m2) was significantly associated with higher blood pressure, serum albumin, triglycerides, low-density lipoprotein cholesterol, and lower high-density lipoprotein cholesterol levels in patients with DM than in those with low BMIs (< 25 kg/m2). Our analysis showed that a high BMI was associated with a higher risk ratio of adverse kidney events than a low BMI.
High BMI was identified as a risk factor contributing to adverse kidney events in patients with DM.
A larger sample size and higher quality studies are warranted to corroborate the findings of this meta-analysis, and future studies focusing on the optimal weight range for patients with DM would also be beneficial.
We appreciate the statistical support from the Teaching and Research Office of the Statistics of the Army Medical University.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology & nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eiras S, Spain S-Editor: Liu JH L-Editor: A P-Editor: Chen YX
| 1. | Tuttle KR, Jones CR, Daratha KB, Koyama AK, Nicholas SB, Alicic RZ, Duru OK, Neumiller JJ, Norris KC, Ríos Burrows N, Pavkov ME. Incidence of Chronic Kidney Disease among Adults with Diabetes, 2015-2020. N Engl J Med. 2022;387:1430-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 2. | Ye N, Yang L, Wang G, Bian W, Xu F, Ma C, Zhao D, Liu J, Hao Y, Yang N, Cheng H; CCC-ACS. Admission fasting plasma glucose is associated with in-hospital outcomes in patients with acute coronary syndrome and diabetes: findings from the improving Care for Cardiovascular Disease in China - Acute Coronary Syndrome (CCC-ACS) project. BMC Cardiovasc Disord. 2020;20:380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Rasines-Perea Z, Teissedre PL. Grape Polyphenols' Effects in Human Cardiovascular Diseases and Diabetes. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 4. | Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 985] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 5. | D'Agati VD, Chagnac A, de Vries AP, Levi M, Porrini E, Herman-Edelstein M, Praga M. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12:453-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 488] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 6. | Forst T, Mathieu C, Giorgino F, Wheeler DC, Papanas N, Schmieder RE, Halabi A, Schnell O, Streckbein M, Tuttle KR. New strategies to improve clinical outcomes for diabetic kidney disease. BMC Med. 2022;20:337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016;12:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 483] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 8. | Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U. Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol. 2000;278:F817-F822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 424] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Ribstein J, du Cailar G, Mimran A. Combined renal effects of overweight and hypertension. Hypertension. 1995;26:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 215] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Aksoy N, Şelimen D. Investigation of the Causes and Risk Factors of Previous End-Stage Renal Disease in Kidney Transplant Recipients. Transplant Proc. 2020;52:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Nakanishi S, Hirukawa H, Shimoda M, Tatsumi F, Kohara K, Obata A, Okauchi S, Katakura Y, Sanada J, Fushimi Y, Kan Y, Tomita A, Isobe H, Iwamoto H, Takahashi K, Mune T, Kaku K, Kaneto H. Comparison of HbA1c levels and body mass index for prevention of diabetic kidney disease: A retrospective longitudinal study using outpatient clinical data in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2019;155:107807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Zhang Y, Guo Y, Shen X, Zhao F, Yan S. Lower body mass index is not of more benefit for diabetic complications. J Diabetes Investig. 2019;10:1307-1317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Haupt E, Benecke A, Haupt A, Herrmann R, Vogel H, Walter C. The KID Study VI: diabetic complications and associated diseases in younger type 2 diabetics still performing a profession. Prevalence and correlation with duration of diabetic state, BMI and C-peptide. Exp Clin Endocrinol Diabetes. 1999;107:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Kutyrina IM, Savel'eva SA, Kriachkova AA, Shestakova MV. [Contribution of obesity to renal lesions in patients with type 2 diabetes mellitus]. Ter Arkh. 2010;82:21-25. [PubMed] |
| 15. | Belhatem N, Mohammedi K, Rouzet F, Matallah N, Al Baloshi A, Travert F, Velho G, Roussel R, Le Guludec D, Marre M, Hansel B. Impact of morbid obesity on the kidney function of patients with type 2 diabetes. Diabetes Res Clin Pract. 2015;108:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Kim BY, Choi DH, Jung CH, Mok JO, Kim CH. Associations between obesity, weight change and decreased renal function in Korean type 2 diabetic patients: a longitudinal follow-up study. BMC Endocr Disord. 2021;21:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Huang WH, Chen CY, Lin JL, Lin-Tan DT, Hsu CW, Yen TH. High body mass index reduces glomerular filtration rate decline in type II diabetes mellitus patients with stage 3 or 4 chronic kidney disease. Medicine (Baltimore). 2014;93:e41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Chen HM, Shen WW, Ge YC, Zhang YD, Xie HL, Liu ZH. The relationship between obesity and diabetic nephropathy in China. BMC Nephrol. 2013;14:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Drion I, Joosten H, Santing L, Logtenberg SJ, Groenier KH, Lieverse AG, Kleefstra N, Bilo HJ. The Cockcroft-Gault: a better predictor of renal function in an overweight and obese diabetic population. Obes Facts. 2011;4:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Kanauchi M. Comparison in renal histology between Japanese obese and non-obese microalbuminuric type 2 diabetic patients. Nephrol Dial Transplant. 2003;18:849-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Bentata Y, Latrech H, Abouqal R. Does body mass index influence the decline of glomerular filtration rate in diabetic type 2 patients with diabetic nephropathy in a developing country? Ren Fail. 2014;36:838-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Mohsen A, Brown R, Hoefield R, Kalra PA, O'Donoghue D, Middleton R, New D. Body mass index has no effect on rate of progression of chronic kidney disease in subjects with type 2 diabetes mellitus. J Nephrol. 2012;25:384-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13347] [Article Influence: 834.2] [Reference Citation Analysis (0)] |
| 24. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12641] [Article Influence: 842.7] [Reference Citation Analysis (0)] |
| 25. | Rostom ADCCA. 2004. Appendix D quality assessment forms. Agency for Healthcare Research and Quality, 2004. Available from: https://www.ncbi.nlm.nih.gov/books/NBK35156/. |
| 26. | Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2650] [Cited by in RCA: 2991] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 27. | NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3952] [Cited by in RCA: 3518] [Article Influence: 390.9] [Reference Citation Analysis (0)] |
| 28. | Kambham N, Markowitz GS, Valeri AM, Lin J, D'Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 855] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 29. | Regensteiner JG, Reusch JEB. Sex Differences in Cardiovascular Consequences of Hypertension, Obesity, and Diabetes: JACC Focus Seminar 4/7. J Am Coll Cardiol. 2022;79:1492-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 30. | Ismail L, Materwala H, Al Kaabi J. Association of risk factors with type 2 diabetes: A systematic review. Comput Struct Biotechnol J. 2021;19:1759-1785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 31. | Lu JL, Molnar MZ, Naseer A, Mikkelsen MK, Kalantar-Zadeh K, Kovesdy CP. Association of age and BMI with kidney function and mortality: a cohort study. Lancet Diabetes Endocrinol. 2015;3:704-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 32. | Wang HY, Shi WR, Yi X, Wang SZ, Luan SY, Sun YX. Value of reduced glomerular filtration rate assessment with cardiometabolic index: insights from a population-based Chinese cohort. BMC Nephrol. 2018;19:294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1234] [Cited by in RCA: 1702] [Article Influence: 212.8] [Reference Citation Analysis (1)] |
| 34. | Man REK, Gan ATL, Fenwick EK, Gupta P, Wong MYZ, Wong TY, Tan GSW, Teo BW, Sabanayagam C, Lamoureux EL. The Relationship between Generalized and Abdominal Obesity with Diabetic Kidney Disease in Type 2 Diabetes: A Multiethnic Asian Study and Meta-Analysis. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Hill GS. Hypertensive nephrosclerosis. Curr Opin Nephrol Hypertens. 2008;17:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 36. | Costantino VV, Gil Lorenzo AF, Bocanegra V, Vallés PG. Molecular Mechanisms of Hypertensive Nephropathy: Renoprotective Effect of Losartan through Hsp70. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 37. | Uno K, Yamada T, Ishigaki Y, Imai J, Hasegawa Y, Sawada S, Kaneko K, Ono H, Asano T, Oka Y, Katagiri H. A hepatic amino acid/mTOR/S6K-dependent signalling pathway modulates systemic lipid metabolism via neuronal signals. Nat Commun. 2015;6:7940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Wu D, Yi Y, Sun F, Zhou L, Yang F, Wang H, Zhang G, Zhang YA, Yue F. Effects of age and sex on the hematology and blood chemistry of Tibetan macaques (Macaca thibetana). J Am Assoc Lab Anim Sci. 2014;53:12-17. [PubMed] |
| 39. | Lumu W, Kampiire L, Akabwai GP, Ssekitoleko R, Kiggundu DS, Kibirige D. Dyslipidaemia in a Black African diabetic population: burden, pattern and predictors. BMC Res Notes. 2017;10:587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Opazo-Ríos L, Mas S, Marín-Royo G, Mezzano S, Gómez-Guerrero C, Moreno JA, Egido J. Lipotoxicity and Diabetic Nephropathy: Novel Mechanistic Insights and Therapeutic Opportunities. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 41. | Kramer HJ, Saranathan A, Luke A, Durazo-Arvizu RA, Guichan C, Hou S, Cooper R. Increasing body mass index and obesity in the incident ESRD population. J Am Soc Nephrol. 2006;17:1453-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |