Published online Oct 16, 2024. doi: 10.12998/wjcc.v12.i29.6314
Revised: July 27, 2024
Accepted: August 8, 2024
Published online: October 16, 2024
Processing time: 116 Days and 6.2 Hours
In general, venous aneurysm associated with dural arteriovenous fistula (dAVF) is considered to be developed under long standing venous hypertension and manifested as venous ectasia of draining vein itself. However, discrete saccular shaped venous aneurysm without angiographic evidence of venous hypertension arising from the draining vein, like cerebral arterial aneurysm, is quite rare and its pathomechanism remains unclear in patients with dAVF.
In this report, we present two cases of ruptured saccular venous aneurysms associated with dAVF without venous hypertension or venous ectasia. In both cases, significant curve or stenosis is observed in draining vein, which is located in just distal portion of the venous aneurysms. These aneurysms were successfully treated with a transarterial embolization. Underlying mechanism of venous aneurysms in these cases is discussed.
Although there is little doubt that hemodynamic stress has a critical role in the development of venous aneurysms in patients with dAVF, preceding venous hypertension or venous ectasia is not necessary for development and enlargement of venous aneurysms. Considering the significant risk of rupture, a careful review of draining vein features including tortuosity or stenosis is needed, especially in venous aneurysms without evidence of venous hypertension.
Core Tip: Venous aneurysms associated with dural arteriovenous fistula (dAVF) have been generally considered to be developed in the setting of venous hypertension or venous ectasia. However, saccular venous aneurysm in patients with dAVF could develop without preceding venous ectasia or venous hypertension and this situation is very rare. So, its pathomechanism remains unclear in patients with dAVF. In this report, we present rare cases of venous aneurysms in the absence of venous hypertension, and describe underlying unique angiographic features; presence of curved or stenotic portion within long and tortuous draining vein.
- Citation: Kim YS, Yoon W, Baek BH, Kim SK, Joo SP, Kim TS. Ruptured venous aneurysm associated with a dural arteriovenous fistula: Two case reports. World J Clin Cases 2024; 12(29): 6314-6319
- URL: https://www.wjgnet.com/2307-8960/full/v12/i29/6314.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i29.6314
There has been an established consensus that treating intracranial dural arteriovenous fistula (dAVF) with cortical venous drainage (CVD) reduces the risk of intracranial hemorrhage (ICH), that is associated with increased mortality as high as 10.4%[1].
However, clinicians often face dilemma when dAVFs are detected incidentally or presented with benign symptoms without CVD. Moreover, when angiogram reveals several unique radiologic features such as deep vein drainage, long tortuous drainage vein, or venous aneurysm in asymptomatic patients with dAVF, it is difficult to establish treatment plan.
Venous aneurysms seem to be developed from ectatic drainage vein under long standing venous hypertension and are significantly related to an increased risk of ICH[2,3]. However, discrete saccular venous aneurysm arising from the draining vein, like cerebral arterial aneurysm, without angiographic evidence of venous hypertension or venous ectasia is quite rare and little is known about its natural course and pathomechanism.
In this report, we present rare cases of ruptured venous aneurysm associated with dAVF without venous hypertension, successfully treated with transarterial embolization, and we discuss the underlying angiographic features.
Case 1: A 65- year-old female was emergently transferred to our hospital due to a sudden onset of headache and dizziness.
Case 2: A 58- year-old male was admitted to our hospital due to a persistent headache that had lasted for several months.
Case 1: A 65- year-old female was emergently transferred to our hospital due to a sudden onset of headache and dizziness.
Case 2: The 18 months ago, he had detected an ICH in the right occipital lobe accompanied by an aneurysmal lesion. However, he received conservative treatment without further examination at that time.
Case 1: She had a medical history of hypertension and diabetes mellitus.
Case 2: He had no previous medical history.
There were no relevant personal and family history.
Case 1: Upon arrival, her Glasgow Coma Scale score was 14 (E3, V5, M6).
Case 2: There was no significant any neurological deficits in the physical examination.
Platelet counts and coagulation profile were normal at the time of admission.
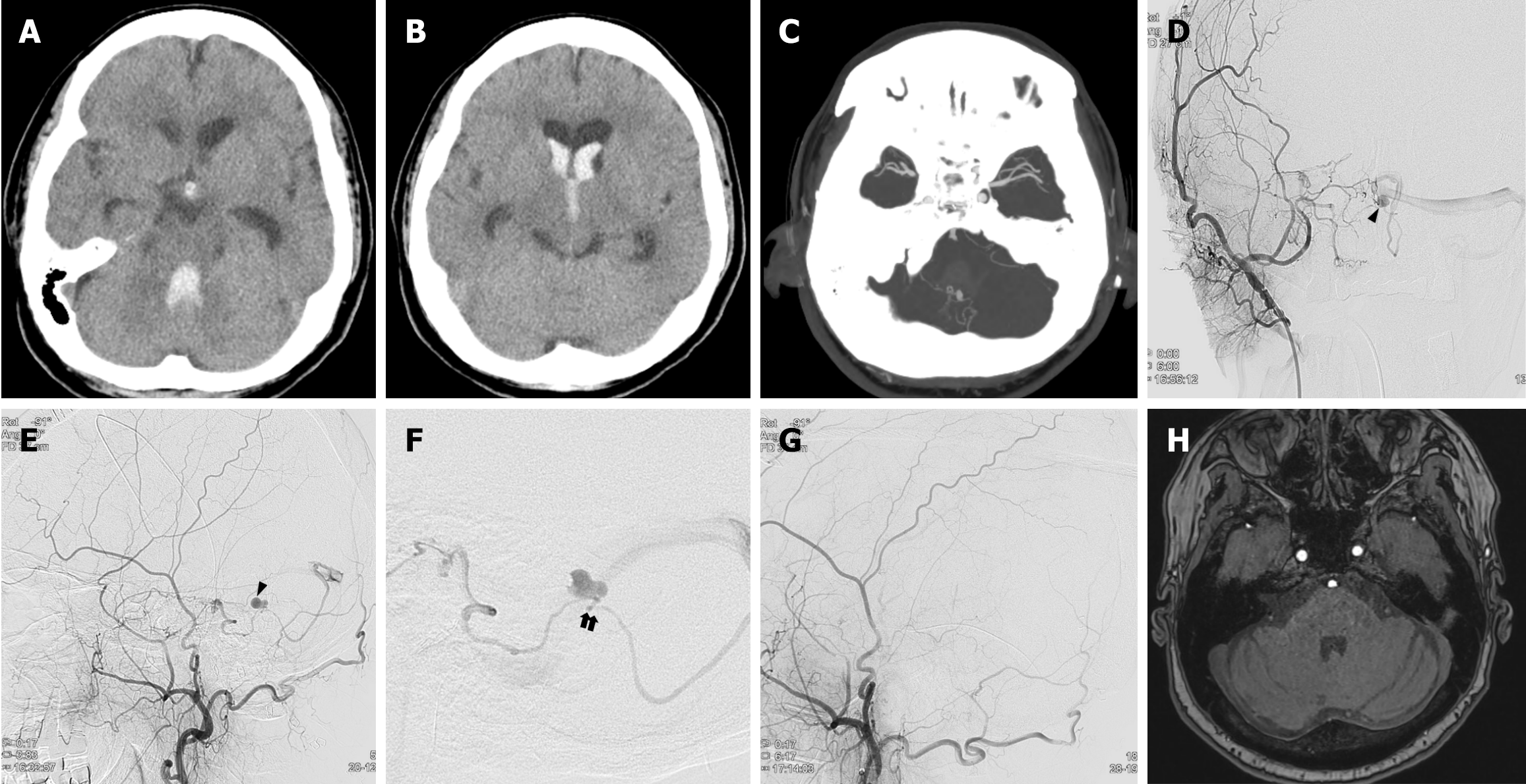
Case 1: A computed tomography (CT) scan revealed an intraventricular hemorrhage (IVH), particularly in the 4th ventricle (Figure 1A and B). In addition, CT angiography (CTA) showed an aneurysmal lesion with hemorrhage in the 4th ventricle (Figure 1C). Initially, we suspected that this hemorrhage was due to a ruptured PICA aneurysm. However, digital subtraction angiography (DSA) revealed dAVF at the posterior cranial fossa supplied by the petrosal branch of the right middle meningeal artery and drained into the left transverse sinus. The dilated pouch through the course of the draining vein suggested a venous aneurysm, which is consistent with CTA findings (Figure 1D and E). A significant stenotic segment of the draining vein is detected immediately distal to the aneurysm (Figure 1F).
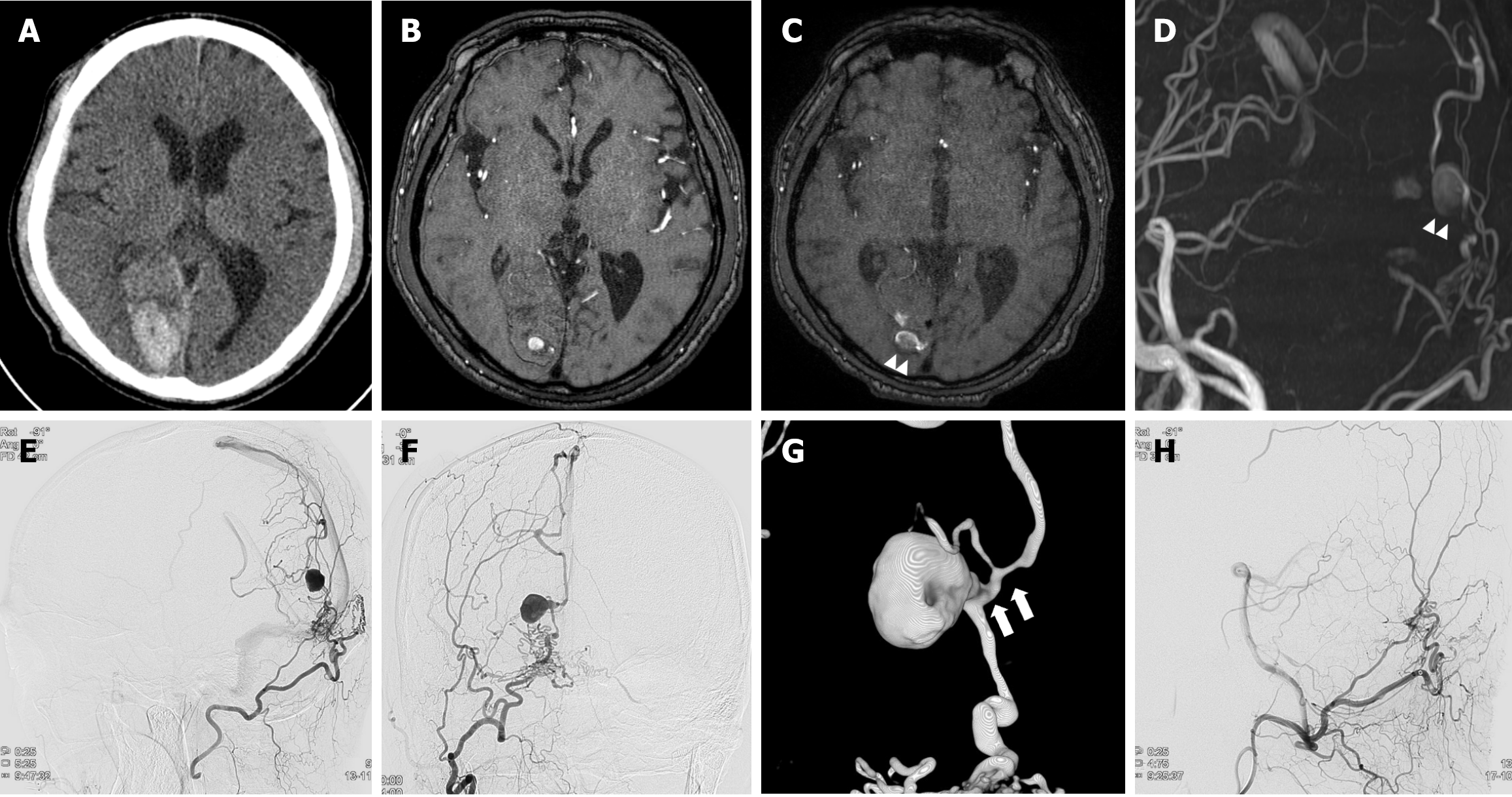
Case 2: Previously, CT, magnetic resonance imaging (MRI), and magnetic resonance angiography (MRA) scans from another hospital 18 months ago had detected an ICH in the right occipital lobe accompanied by an aneurysmal lesion (Figure 2A and B). The MRI and MRA scans at admission revealed an enlarged previous aneurysmal lesion within encephalomalacic change resulting from a previous hemorrhage (Figure 2C and D). This lesion was supplied by the right occipital artery and drained into the dilated internal cerebral vein, suggesting dAVF. Subsequently, DSA was performed, revealing a dAVF supplied by the right occipital artery and drained into the superior sagittal sinus and internal cerebral vein. The dilated pouch through the course of draining the vein suggested venous aneurysm was noted, which is consistent with MRA findings (Figure 2E and F). An obvious curve and stenosis were observed at the immediately distal segment of the aneurysm within the draining vein (Figure 2G).
Ruptured venous aneurysm associated with dAVF.
Considering the risk of recurrent bleeding, transfemoral arterial embolization with histoacryl (mixed with lipiodol) was performed. Immediately after embolization, DSA showed a complete obliteration of the fistula (Figure 1G).
Considering the history of ICH associated with this lesion, transfemoral arterial embolization was performed using histoacryl (mixed with lipiodol). Immediately after embolization, DSA showed a complete obliteration of the fistula (Figure 2H).
A follow-up CTA and DSA obtained 2 weeks after embolization showed disappearance of the venous aneurysm. Throughout her admission, the patient gradually recovered to baseline neurologic condition without any complications. A follow-up MRA at 1 year after embolization showed no evidence of recurrence (Figure 1H).
He was discharged without any complications.
Clinical symptoms of dAVF range from asymptomatic to severe symptoms such as ICH or non-hemorrhagic neurologic deficits, including seizures, parkinsonism, and cerebellar symptoms[4,5]. These symptoms almost always correlate with the pattern of venous drainage and the anatomic location of the fistula[6,7].
ICH has been reported in 35%-42% of dAVF cases, and it is generally accepted that the venous drainage pattern is the most important factor in predicting ICH[1,8]. Several studies have reported that the presence of CVD further increases the risk of hemorrhage, especially when coupled with cortical venous ectasia or aneurysm formation[9,10]. Cognard et al[11] reported that hemorrhage was present in 40% of direct CVD cases and in 65% of direct CVD cases with venous ectasia. In addition, sinus outflow thrombosis or obstruction may accelerate the formation and progression of venous ectasia, potentially leading to rupture.
However, although most venous ectasia or venous aneurysms tend to develop and progress in scenarios involving venous hypertension, such as venous outlet thrombosis, they have also been observed to develop or enlarge without any associated venous hypertension or changes in the venous outlet[2,12,13]. Hashiguchi et al[2] reported ruptured venous aneurysm associated with a dAVF of anterior cranial fossa. In this report, venous aneurysm had developed at the branching site of the two major venous drainers, which may cause increased hemodynamic stress without venous hypertension. Im et al[12] also concluded that venous aneurysm associated with a dAVF of anterior cranial fossa may result from increased hemodynamic stress in the absence of changes in the venous outlet.
In our cases, the venous aneurysms were located in the stenotic, a significantly curved section within long and tortuous draining veins, just distal to the aneurysm. There was no clear sign of venous ectasia or occlusion of the venous outlet. Thus, we could postulate that preceding venous hypertension is not necessarily required to develop a venous aneurysm. Similar to arterial aneurysms, venous aneurysms may develop in the areas with a significant curve or stenosis in the draining veins where hemodynamic stress occurs in patients with dAVF. Increased localized intravenous pressure in these areas may contribute to the development and enlargement of aneurysm.
In the histopathological study, the venous aneurysms presenting with ICH differed from those developed under venous hypertensive conditions in patients with dAVF[6]. Degenerative changes, including irregular wall thickness, loss of internal elastic lamina, and decreased smooth muscle cells, were more severe in venous aneurysms presenting with ICH than in those with venous hypertension. This result also supported that patients with venous aneurysms presenting with ICH do not necessarily manifest venous hypertension before the insult, and thus it remains unclear whether venous aneurysms presenting with ICH accompanied by venous hypertension progress to venous aneurysms.
Hemodynamic stress undoubtedly plays a major role in the formation of venous and enlargement of venous aneurysms may be part of the natural course of dAVF leading to ICH. However, more complex interactions between hemodynamic stress, degenerative factors, and anatomical factors may seem to be involved in the formation of venous aneurysms. These factors may include location of dAVF, degenerative factors leading to histological changes in drainage vein, and factors related to drainage vein, which is most important, such as existence of stenotic and curved portion, or branching site within the drainage vein, in the setting of long and tortuous drainage course. Further study is needed to fully understand venous aneurysms in patients with dAVF.
Although rare, venous aneurysms associated with dAVF could develop without preceding venous ectasia or venous hypertension. When dealing with these lesions, care should be taken not to overlook the radiologic features of draining veins as seen in our cases predicting enlargement and rupture of venous aneurysm.
| 1. | van Dijk JM, terBrugge KG, Willinsky RA, Wallace MC. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke. 2002;33:1233-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 396] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 2. | Hashiguchi A, Mimata C, Ichimura H, Morioka M, Kuratsu J. Venous aneurysm development associated with a dural arteriovenous fistula of the anterior cranial fossa with devastating hemorrhage--case report. Neurol Med Chir (Tokyo). 2007;47:70-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Song W, Sun H, Liu J, Liu L, Liu J. Spontaneous Resolution of Venous Aneurysms After Transarterial Embolization of a Variant Superior Sagittal Sinus Dural Arteriovenous Fistula: Case Report and Literature Review. Neurologist. 2017;22:186-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Liu JK, Dogan A, Ellegala DB, Carlson J, Nesbit GM, Barnwell SL, Delashaw JB. The role of surgery for high-grade intracranial dural arteriovenous fistulas: importance of obliteration of venous outflow. J Neurosurg. 2009;110:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Ma C, Lu Q, Shi W, Su Z, Zhao Y, Li C, Liu Z. Diagnosis and treatment of a dural arteriovenous fistula presenting with progressive parkinsonism and dementia: A case report and literature review. Exp Ther Med. 2015;9:523-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Hamada J, Yano S, Kai Y, Koga K, Morioka M, Ishimaru Y, Ushio Y. Histopathological study of venous aneurysms in patients with dural arteriovenous fistulas. J Neurosurg. 2000;92:1023-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Chung SJ, Kim JS, Kim JC, Lee SK, Kwon SU, Lee MC, Suh DC. Intracranial dural arteriovenous fistulas: analysis of 60 patients. Cerebrovasc Dis. 2002;13:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Della Pepa GM, Parente P, D'Argento F, Pedicelli A, Sturiale CL, Sabatino G, Albanese A, Puca A, Fernandez E, Olivi A, Marchese E. Angio-Architectural Features of High-Grade Intracranial Dural Arteriovenous Fistulas: Correlation With Aggressive Clinical Presentation and Hemorrhagic Risk. Neurosurgery. 2017;81:315-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Awad IA, Little JR, Akarawi WP, Ahl J. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg. 1990;72:839-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 525] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Brown RD Jr, Wiebers DO, Nichols DA. Intracranial dural arteriovenous fistulae: angiographic predictors of intracranial hemorrhage and clinical outcome in nonsurgical patients. J Neurosurg. 1994;81:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 193] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, Chiras J, Merland JJ. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1044] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 12. | Im SH, Oh CW, Han DH. Surgical management of an unruptured dural arteriovenous fistula of the anterior cranial fossa: natural history for 7 years. Surg Neurol. 2004;62:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Kim DK, Eskridge J, Mayberg MR. Progressive aneurysmal degeneration of cortical venous drainage of dural arteriovenous malformations: case report. Neurosurgery. 1997;41:673-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |