Published online Oct 16, 2024. doi: 10.12998/wjcc.v12.i29.6307
Revised: June 4, 2024
Accepted: August 1, 2024
Published online: October 16, 2024
Processing time: 117 Days and 14.5 Hours
Telitacicept reduces B cell activation and abnormal immunoglobulin A (IgA) antibody production by inhibiting the activity of B lymphocyte stimulator (BLyS) and a proliferation-inducing ligand (APRIL), thereby decreasing IgA deposition in the glomeruli and local inflammatory response. This ultimately protects the kidneys from damage. This mechanism suggests that Telitacicept has potential efficacy in the treatment of IgA nephropathy.
We present the case of a 24-year-old female who was diagnosed with IgA nephropathy due to significant proteinuria and mild renal impairment. Pathologically, she exhibited focal proliferative glomerulonephritis. Treatment with angiotensin II receptor blocker, hormones, and mycophenolate mofetil did not lead to a significant improvement in her condition. However, upon the addition of telitacicept, the patient’s renal function recovered and her proteinuria rapidly reduced. Hormones were swiftly tapered and discontinued, with no occurrence of severe infections or related complications.
Telitacicept combined with hormones and mycophenolate mofetil may be a safe and effective induction therapy for IgA nephropathy.
Core Tip: Telitacicept reduces B cell activation and abnormal immunoglobulin A (IgA) antibody production by inhibiting the activity of B lymphocyte stimulator and a proliferation-inducing ligand, thereby decreasing IgA deposition in the glomeruli and local inflammatory response. This mechanism suggests that Telitacicept has potential efficacy in the treatment of IgA nephropathy. This paper reports a successful clinical case of using Telitacetin to treat IgA nephropathy.
- Citation: Shen Y, Yuan J, Chen S, Zhang YF, Yin L, Hong Q, Zha Y. Combination treatment with telitacicept, mycophenolate mofetil and glucocorticoids for immunoglobulin A nephropathy: A case report. World J Clin Cases 2024; 12(29): 6307-6313
- URL: https://www.wjgnet.com/2307-8960/full/v12/i29/6307.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i29.6307
Immunoglobulin A (IgA) nephropathy is one of the most common primary glomerulonephritides worldwide and is characterized by the deposition of IgA in the glomerulus, the filtering unit of the kidney. Consequently, the filtration function of the kidney is compromised, allowing larger molecules such as proteins to pass from the blood into the urine. Microscopic hematuria and proteinuria are the most common clinical manifestations of IgA nephropathy[1]. Up to 20%-40% of patients gradually progress to end-stage kidney disease within 20 years after onset. The pathogenesis of IgA nephropathy is believed to be closely associated with the immune system[2].
IgA is an important subtype of antibody that plays a crucial role in mucosal immunity[3]. Human IgA consists of two subtypes, namely, IgA1 and IgA2[3]. IgA1 primarily exists on mucosal surfaces and in the circulation, while IgA2 is predominantly found on mucosal surfaces[3]. The difference between these two IgA subtypes lies in the variation of N-linked carbohydrates on their heavy and light chains. In comparison to IgA2, IgA1 tends to incorporate additional O-linked glycans consisting of N-acetylgalactosamine, galactose, and sialic acid[4]. Notably, the O-linked glycans formed by IgA1 are unstable and incomplete, leading to the production of abnormally glycosylated IgA1, known as galactose-deficient IgA1 (Gd-IgA1)[5]. B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) are crucial factors in maintaining B-cell levels and humoral immunity and are involved in various autoimmune diseases, including IgA nephropathy[6]. BAFF sustains cell levels, while APRIL regulates B-cell function[7]. A study showed elevated levels of BAFF and APRIL in IgA nephropathy patients, which were positively correlated with Gd-IgA1 Levels and disease severity[8]. Compared with healthy individuals, IgA nephropathy patients exhibit increased APRIL-stimulating lymphocytes and generate more Gd-IgA1[5]. Recently, a study involving 44 IgA nephropathy patients, 22 non-IgA nephropathy kidney disease patients, and 23 healthy individuals demonstrated increased levels of BAFF and APRIL in IgA nephropathy patients. Additionally, circulating B-cell levels were also elevated[9]. Telitacicept is a novel fully human TACI-Fc recombinant fusion protein targeting B-cell stimulating factors[10]. Telitacicept can block B lymphocyte stimulator (BLyS) to inhibit abnormal B-cell development and maturity and impede APRIL to inhibit abnormal plasma cell antibody production[10].
As reported below, we observed that the combination of telitacicept with conventional therapy (steroids, MMF) exhibited good safety and efficacy in treating a patient with IgA nephropathy.
A 24-year-old female was admitted to the hospital due to bilateral lower limb edema for more than one month.
Over a month ago, the patient developed symmetrical edema in both lower limbs without any obvious triggers. She did not experience fever, rash, joint pain, or other discomfort.
Her past medical history was unremarkable.
Her personal history and family history were unremarkable.
On admission, her vital signs were normal, her blood pressure was 16.5/10.1 kPa, and no rashes or bleeding points were observed on her skin or mucous membranes. No apparent abnormalities were noted in the cardiovascular, respiratory, or abdominal examinations, except for mild symmetrical pitting edema of both lower limbs.
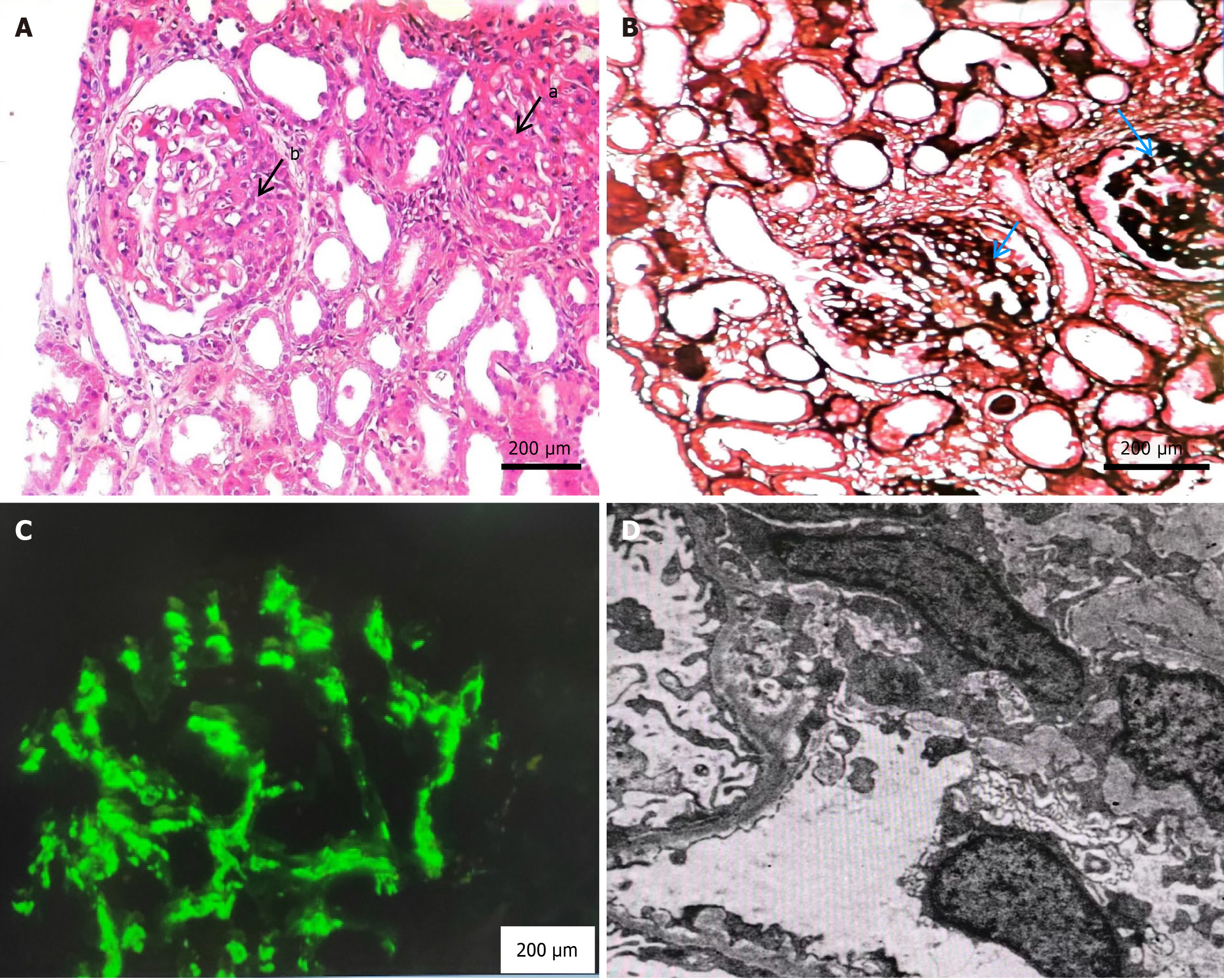
On admission, laboratory examination results were as follows: Urine red blood cells, 2 +; 90% of the urine red blood cells were abnormal in shape, and wrinkled red blood cells, small red blood cells, and ghost cell were observed. Blood examination revealed a white blood cell count of 5.66 × 109/L (reference range 3.5-9.5 × 109/L) and a hemoglobin concentration of 129 g/L (reference range 130-175 g/L). 24-hours urinary protein quantification, 8.87 g/24 hours (reference range 0-150 mg/24 hours); Biochemistry indicators were as follows: Creatinine, 382 mmol/L (reference range 57-97 μmol/L), and albumin, 23.0 g/L (reference range 40-55 g/L). Antinuclear antibody spectrum, ANCA combination, immunofixation electrophoresis, immunoglobulin tests, and complement tests showed no abnormalities. No abnormalities in routine stool parameters, C-reactive protein, erythrocyte sedimentation rate, B-type natriuretic peptide, coagulation function parameters, blood lipids, thyroid function parameters, infectious disease pathogens, or the PLA2R receptor were observed. T-SPOT.TB, electrocardiography, abdominal color Doppler ultrasound, echocardiography, and pulmonary computed tomography showed no abnormalities. Light microscopy examination of renal perforation pathology revealed 21 glomeruli, with 4 showing ischemic sclerosis. The basement membranes of the rest of the glomerular capillaries displayed widespread vacuolar degeneration. There was mild diffuse proliferation of mesangial cells and matrix, focal segmental moderate exacerbation with endothelial cell proliferation. The tubular epithelial cells exhibited vacuolation and granular degeneration, focal small atrophy, interstitial fibrous tissue proliferation, and focal lymphocytic infiltration. The small arteries showed no significant lesions. Immunofluorescence examination revealed IgG-, IgA + + +, IgM + +, C3 + +, and C1q, along with clustered deposits along the mesangial area. Electron microscopy revealed changes in 3 glomeruli, including mesangial cell and matrix proliferation, along with electron-dense deposits in the mesangial area. The lesion was consistent with IgA nephropathy (focal proliferative glomerulonephritis) according to the Oxford classification: M0E1S1T0C1 (Figure 1).
No abnormalities.
IgA nephropathy (focal proliferative glomerulonephritis).
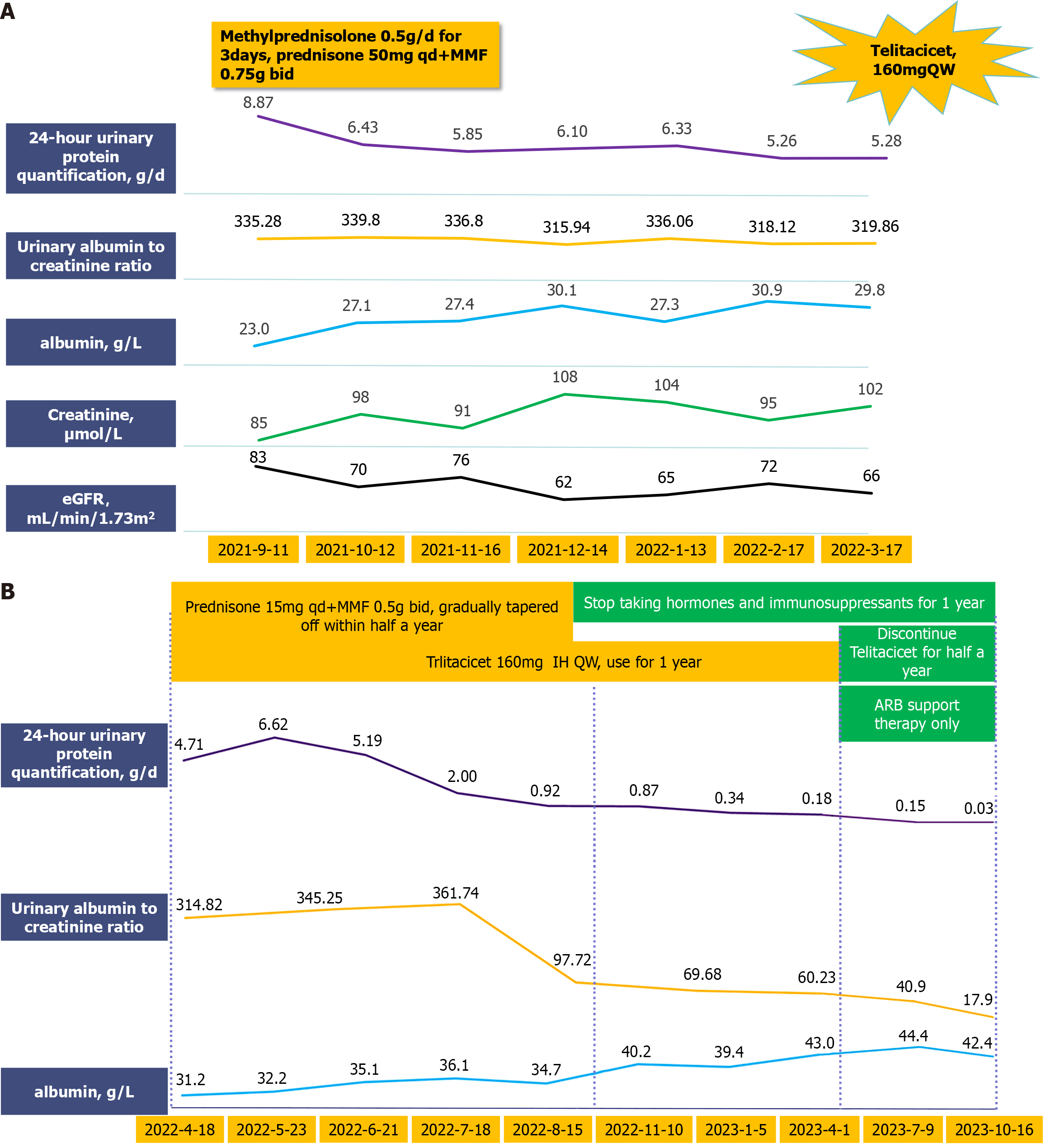
The patient received routine treatment upon admission, including sodium restriction, weight maintenance, appropriate exercise, and proteinuria reduction through the addition of telmisartan, which was gradually titrated to a tolerable dose. After thorough discussion, both the patient and family agreed to the use of steroids and immunosuppressants. Methylprednisolone was administered at 0.5 g/d for 3 days, followed by prednisone 50 mg qd + mycophenolate mofetil (MMF) 750 mg bid for 6 months.
There was no significant improvement in the patient's condition, with 24-hours urine protein quantification remaining at approximately 5 g/d. After multiple discussions and thorough communication with the patient and family, a decision was made to attempt treatment with biologics.
On April 18, 2022, 160 mg telitacicept was administered subcutaneously qw, the MMF dose was gradually reduced to discontinuation (after 2 months), and the prednisone dosage was gradually reduced to 15 mg qd. After adjusting the treatment plan, the 24-hours proteinuria decreased rapidly and was < 1 g/d after 4 months of treatment, and renal function improved significantly. In October 2022, the patient’s urine protein level was < 0.5 g/d, and her serum creatinine level was normal. Telitacicept was discontinued, and the hormone dose was reduced to 5 mg/day. The patient is currently in ongoing follow-up. Hormone therapy has been discontinued, urine protein has been measured at 0.20 g/day, renal function has stabilized, and no obvious adverse drug reactions have occurred. The patient's quality of life has significantly improved, and she returned to normal work and activities of daily living without signs of recurrence or progression (Figure 2).
IgA nephropathy is a glomerular disease characterized by the deposition of IgA or IgA-dominant immune complexes in the mesangial area of the kidney, leading to glomerular inflammation and varying degrees of renal impairment[2]. First described by French scholars in 1968, IgA nephropathy is considered the most common primary glomerular disease worldwide. The incidence of adult IgA nephropathy is much greater than that of other primary glomerulonephritides. In China, IgA nephropathy confirmed by renal biopsy accounts for more than 45% of primary glomerular diseases, with 20% to 40% of patients progressing to end-stage kidney disease within 20 years after biopsy-confirmed diagnosis[11]. The current cornerstone of therapy for primary IgA nephropathy is maximal supportive care.
As recommended by the 2021 KDIGO guidelines, steroid therapy may be considered when proteinuria persists despite maximal supportive therapy. However, due to the numerous adverse effects associated with steroids, their use in treating primary IgA nephropathy in high-risk patients with progressive disease remains controversial[12]. The largest clinical trial in the field of international glomerular disease research, the TESTING study, published midterm results in JAMA in 2017. These results suggested that the full-dose corticosteroid therapy recommended by current clinical practice guidelines for IgA nephropathy might pose a significant risk of adverse events for patients. The subsequent continuation of this study with lower doses ultimately revealed that, over 2.5 years, steroid dose reduction decreased the incidence of major endpoint events by 17 individuals per 100 treated, yet the incidence of severe side effects still increased by 2.4 individuals per 100 treated[13]. Furthermore, the 10-year follow-up results of the notable STOP study confirmed that the addition of immunosuppressive agents to supportive therapy for IgA nephropathy patients did not yield statistically significant differences in outcomes[14]. Hence, there is an urgent need for new approaches to treat IgA nephropathy.
Immunoglobulin A nephropathy (IgAN) is an immune-mediated kidney disease characterized by IgA deposition in the glomeruli. BAFF is closely related to the pathogenesis of IgAN because it plays a key role in B cell survival, differentiation, and antibody production. Overexpression of BAFF can lead to B cell proliferation and the production of abnormal IgA antibodies, which may deposit in the glomeruli, causing inflammation and damage. BAFF's pathogenicity mainly lies in promoting B cell survival and antibody production. Blocking BAFF alone may not fully inhibit B cell survival, as APRIL can still provide survival signals. By simultaneously blocking APRIL, dual inhibitors can more comprehensively cut off B cell survival and function signals, reducing abnormal B cell proliferation and IgA antibody production. Therefore, dual inhibitors show higher efficacy in treating IgAN.
The relationship between BLyS, BAFF, and APRIL is crucial in understanding the pathogenesis of IgAN and the rationale for using a dual blocker. While BLyS and BAFF are often used interchangeably, BAFF is actually a member of the TNF family that plays a significant role in B cell survival and maturation, which is pivotal in the pathogenesis of IgAN. BAFF binds to three receptors: B-Cell Activating Factor Receptor (BAFF-R), Transmembrane Activator and CAML Interactor (TACI), and B-Cell Maturation Antigen (BCMA), with BAFF-R being the primary receptor mediating B cell survival and proliferation. BLyS, on the other hand, is another term for BAFF, highlighting its function as a B lymphocyte stimulator.
APRIL shares receptors with BAFF, particularly TACI and BCMA, and also contributes to B cell survival and antibody class switching. Elevated levels of BAFF and APRIL have been implicated in the pathogenesis of autoimmune diseases, including IgAN, by promoting the survival and activation of autoreactive B cells. The dual blockade of BAFF and APRIL is effective because it simultaneously inhibits two pathways that contribute to B cell proliferation and survival. This approach not only reduces the overall B cell population but also disrupts the survival signals that these cytokines provide, potentially leading to a more significant reduction in pathogenic autoantibody production.
The effectiveness of the dual blocker can be attributed to its comprehensive inhibition of these overlapping yet distinct pathways. By targeting both BAFF and APRIL, the dual blocker ensures that compensatory mechanisms do not undermine the therapeutic effect, thus providing a more robust and sustained suppression of pathogenic B cell activity. This explains why a dual blockade might be more effective than targeting BAFF or APRIL alone, as it addresses the redundancy and compensatory feedback mechanisms within the cytokine signaling network.
Telitacicept, a "dual-target" biological agent, can block both BLyS and APRIL. The results of the phase II clinical trial of telitacicept for the treatment of IgA nephropathy were presented in poster format during the latest clinical trial update at the Renal Week of the American Society of Nephrology in 2021[15]. Led by the Nephrology Department of Peking University First Hospital, this study is a multicenter, randomized, double-blind, placebo-controlled phase II clinical trial that preliminarily assessed the efficacy and safety of telitacicept for the treatment of IgA nephropathy. The study enrolled a total of 44 IgA nephropathy patients with persistent proteinuria despite optimized supportive therapy. The patients were randomly assigned in a 1:1:1 ratio to receive telitacicept at doses of 240 mg, 160 mg, or placebo. The results indicated that after 24 weeks of treatment, patients in the telitacicept 240 mg group exhibited a significant reduction in urinary protein levels compared to baseline, with a 49% decrease in mean 24-hour urinary protein levels relative to baseline, showing statistical significance when compared to the placebo group. Additionally, telitacicept treatment did not cause significant adverse events, demonstrating good safety. Consequently, telitacicept reduced proteinuria in high-risk IgA nephropathy patients, effectively decreasing the risk of IgA nephropathy progression while maintaining safety and tolerance. Specifically, telitacicept demonstrated excellent efficacy by rapidly reducing urinary protein levels after two months of treatment in patients for whom proteinuria remained high despite steroid treatment for more than six months. This effect was significant, and the patients experienced no adverse reactions during the tapering of steroids.
In summary, telitacicept, as the world's first and pioneering fusion protein targeting both BLyS and APRIL, holds promise as an innovative therapy for IgA nephropathy. By targeting two critical factors involved in B-cell survival and activity, BLyS and APRIL, telitacicept inhibits the maturation of B cells and antibody secretion. This novel drug is a potential new option for the treatment of IgA nephropathy, marking the beginning of a new era in targeted biologic therapy for IgA nephropathy.
I would like to express my heartfelt thanks to all those who have supported and contributed to this project. First and foremost, I thank Dr. Zha for their invaluable guidance, insightful feedback, and unwavering support. I am also deeply grateful to my colleague, Jing Yuan, for their encouragement and collaborative spirit.
| 1. | Lee M, Suzuki H, Nihei Y, Matsuzaki K, Suzuki Y. Ethnicity and IgA nephropathy: worldwide differences in epidemiology, timing of diagnosis, clinical manifestations, management and prognosis. Clin Kidney J. 2023;16:ii1-ii8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 2. | Zhang Q, Huang Y, Ren X, Yang X, Mei X, Bi L, Li J, Zhai W, Ding Y. Characteristics of innate and adaptive immune disorder in IgA nephropathy based on integrated bioinformatics. Clin Nephrol. 2024;101:109-122. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Meng MJ, Hu L, Fan Y, Gao H, Chen HZ, Chen CM, Qi Z, Liu B. Efficacy of prednisone combined with mycophenolate mofetil for immunoglobulin A nephropathy with moderate-to-severe renal dysfunction. World J Clin Cases. 2023;11:8300-8309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Conca W, Saleh SM, Al-Rabiah R, Parhar RS, Abd-Elnaeim M, Al-Hindas H, Tinson A, Kroell KB, Liedl KR, Collison K, Kishore U, Al-Mohanna F. The immunoglobulin A isotype of the Arabian camel (Camelus dromedarius) preserves the dualistic structure of unconventional single-domain and canonical heavy chains. Front Immunol. 2023;14:1289769. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Yeo SC, Barratt J. The contribution of a proliferation-inducing ligand (APRIL) and other TNF superfamily members in pathogenesis and progression of IgA nephropathy. Clin Kidney J. 2023;16:ii9-ii18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Asplund Högelin K, Isac B, Khademi M, Al Nimer F. B cell activating factor levels are linked to distinct B cell markers in multiple sclerosis and following B cell depletion and repopulation. Clin Immunol. 2024;258:109870. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Klocperk A, Bloomfield M, Parackova Z, Aillot L, Fremuth J, Sasek L, David J, Fencl F, Skotnicova A, Rejlova K, Magner M, Hrusak O, Sediva A. B cell phenotype and serum levels of interferons, BAFF, and APRIL in multisystem inflammatory syndrome in children associated with COVID-19 (MIS-C). Mol Cell Pediatr. 2023;10:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Maixnerova D, Tesar V. Emerging role of monoclonal antibodies in the treatment of IgA nephropathy. Expert Opin Biol Ther. 2023;23:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Evans LS, Lewis KE, DeMonte D, Bhandari JG, Garrett LB, Kuijper JL, Ardourel D, Wolfson MF, Debrot S, Mudri S, Kleist K, Griffin LL, Hebb L, Sanderson RJ, Wang N, Seaberg M, Chunyk AG, Yang J, Hong Y, Maria Z, Messenheimer DJ, Holland PM, Peng SL, Rixon MW, Dillon SR. Povetacicept, an Enhanced Dual APRIL/BAFF Antagonist That Modulates B Lymphocytes and Pathogenic Autoantibodies for the Treatment of Lupus and Other B Cell-Related Autoimmune Diseases. Arthritis Rheumatol. 2023;75:1187-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Fan Y, Gao D, Zhang Z. Telitacicept, a novel humanized, recombinant TACI-Fc fusion protein, for the treatment of systemic lupus erythematosus. Drugs Today (Barc). 2022;58:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | Sun M, Shi G, Zhang X, Kan C, Xie S, Peng W, Liu W, Wang P, Zhang R. Deciphering roles of protein post-translational modifications in IgA nephropathy progression and potential therapy. Aging (Albany NY). 2024;16:964-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 12. | Beck LH Jr, Ayoub I, Caster D, Choi MJ, Cobb J, Geetha D, Rheault MN, Wadhwani S, Yau T, Whittier WL. KDOQI US Commentary on the 2021 KDIGO Clinical Practice Guideline for the Management of Glomerular Diseases. Am J Kidney Dis. 2023;82:121-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, Monaghan H, Zhao M, Barbour S, Reich H, Cattran D, Glassock R, Levin A, Wheeler D, Woodward M, Billot L, Chan TM, Liu ZH, Johnson DW, Cass A, Feehally J, Floege J, Remuzzi G, Wu Y, Agarwal R, Wang HY, Perkovic V; TESTING Study Group. Effect of Oral Methylprednisolone on Clinical Outcomes in Patients With IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA. 2017;318:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 372] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 14. | Rauen T, Wied S, Fitzner C, Eitner F, Sommerer C, Zeier M, Otte B, Panzer U, Budde K, Benck U, Mertens PR, Kuhlmann U, Witzke O, Gross O, Vielhauer V, Mann JFE, Hilgers RD, Floege J; STOP-IgAN Investigators. After ten years of follow-up, no difference between supportive care plus immunosuppression and supportive care alone in IgA nephropathy. Kidney Int. 2020;98:1044-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 15. | Lv J, Liu L, Hao C, Li G, Fu P, Xing G, Zheng H, Chen N, Wang C, Luo P, Xie D, Zuo L, Li R, Mao Y, Dong S, Zhang P, Zheng H, Wang Y, Qin W, Wang W, Li L, Jiao W, Fang J, Zhang H. Randomized Phase 2 Trial of Telitacicept in Patients With IgA Nephropathy With Persistent Proteinuria. Kidney Int Rep. 2023;8:499-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 84] [Reference Citation Analysis (0)] |