Published online Oct 16, 2024. doi: 10.12998/wjcc.v12.i29.6285
Revised: July 31, 2024
Accepted: August 14, 2024
Published online: October 16, 2024
Processing time: 51 Days and 6.9 Hours
Delayed post hypoxic leukoencephalopathy syndrome (DPHLS), also known as Grinker’s myelinopathy, is a rare but significant neurological condition that mani
To consolidate current knowledge on pathophysiology, clinical features, diag
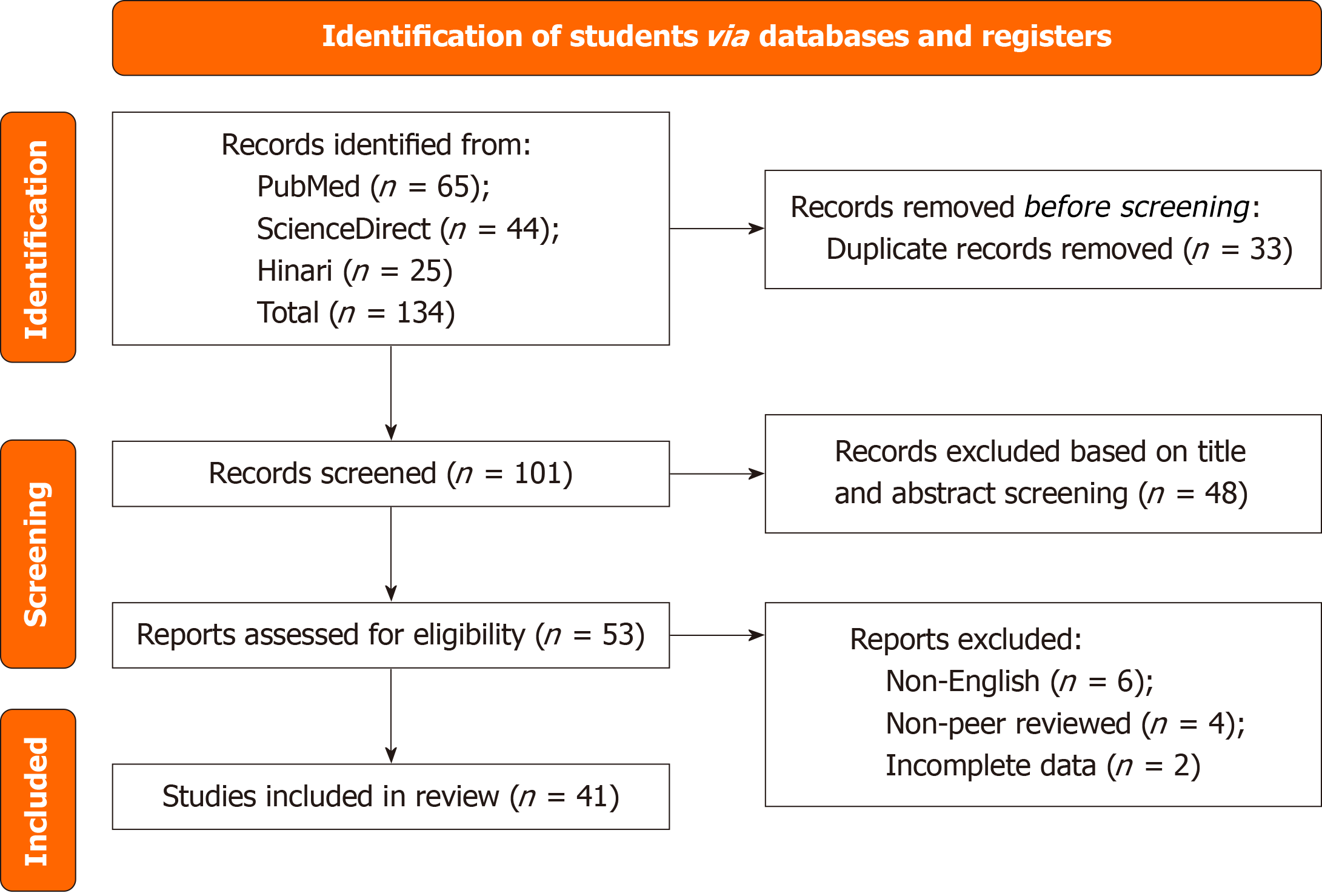
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes guidelines, we systematically searched PubMed, ScienceDirect and Hinari databases using terms related to delayed post-hypoxic leukoencepha
A total of 73 cases were reviewed. Common comorbidities included schizo
DPHLS remains a complex and multifaceted condition with various etiologies and clinical manifestations. Early recognition and appropriate management are crucial to improving patient outcomes. Future research should focus on standardizing diagnostic criteria, using advanced imaging techniques, and exploring therapeutic interventions to improve understanding and treatment of DPHLS. Conducting prospective cohort studies and developing bio
Core Tip: Delayed post hypoxic leukoencephalopathy syndrome (DPHLS) manifests days to weeks after a hypoxic event, presenting with neurological and cognitive deficits. This systematic review consolidates current knowledge on DPHLS, highlighting the complexity of its pathophysiology and the challenges in diagnosis and treatment. Common causes include benzodiazepine and opioid overdose, and carbon monoxide (CO) poisoning. Neuroimaging typically shows diffuse T2 hyperintensities in cerebral white matter sometimes involving subcortical structures such as the basal ganglia and thalamus. Early recognition and supportive management are crucial. Hyperbaric oxygen therapy may be beneficial in CO poisoning.
- Citation: Srichawla BS, Garcia-Dominguez MA. Spectrum of delayed post-hypoxic leukoencephalopathy syndrome: A systematic review. World J Clin Cases 2024; 12(29): 6285-6301
- URL: https://www.wjgnet.com/2307-8960/full/v12/i29/6285.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i29.6285
Delayed post hypoxic leukoencephalopathy syndrome (DPHLS), also known as Grinker's myelinopathy, is an intriguing and often under-recognized neurological condition that typically manifests after a latent period following a hypoxic event. This syndrome is characterized by delayed onset of neurological and cognitive deficits, often presenting several days to weeks after the initial hypoxic insult[1]. Despite its rarity, DPHLS poses significant diagnostic and therapeutic challenges due to its unpredictable course and diverse clinical manifestations[2]. Hypoxic events, such as cardiac arrest, carbon monoxide (CO) poisoning, and prolonged hypotension, can result in various forms of brain injury. Although the immediate consequences of such events are well documented, the delayed effects on the brain's white matter, leading to DPHLS, remain less understood. The pathophysiology of DPHLS involves a complex interplay of factors, including dem
The clinical spectrum of DPHLS is broad, ranging from mild cognitive impairment to severe neuropsychiatric disturbances and motor dysfunction[4]. This variability in presentation often leads to misdiagnosis or delayed diagnosis, complicating patient care and prognosis. Neuroimaging, particularly magnetic resonance imaging (MRI), plays a critical role in the identification and characterization of white matter changes associated with DPHLS, but there is a paucity of standardized diagnostic criteria[5]. Typically, confluent hyperintensities involving the centrum semiovale are observed in T2-weighted fast spin echo and T2-fluid attenuated inversion recovery (T2-FLAIR). Given the critical need for increased awareness and understanding of DPHLS, this Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) guideline directed systematic review aims to consolidate current knowledge on the pathophysiology, clinical characteristics, diagnostic approaches, and management strategies for DPHLS. By synthesizing data from various studies, we seek to provide a comprehensive overview of this syndrome, highlight gaps in the existing literature, and propose directions for future research. Through this review, we hope to enhance the recognition and treatment of DPHL, ulti
This systematic review will be conducted in accordance with the PRISMA guidelines[6]. The review protocol will be preregistered on the International Prospective Register of Systematic Reviews to ensure transparency and methodological rigor (CRD42024550991).
Three electronic databases will be systematically searched: PubMed, ScienceDirect, and Hinari. The search strategy will include terms related to delayed post-hypoxic leukoencephalopathy, hypoxic brain injury, and white matter damage. The following keywords and Medical Subject Headings terms will be used: 'Delayed post-hypoxic leukoencephalopathy', 'Grinker's myelinopathy', and 'delayed encephalopathy' (Table 1).
| Database | Search string |
| PubMed | ("Delayed post-hypoxic leukoencephalopathy" OR "delayed encephalopathy ") AND ("human" OR "case report" OR "case series") |
| ScienceDirect | ("Delayed post-hypoxic leukoencephalopathy" OR "delayed encephalopathy") AND ("human" OR "case report" OR "case series") |
| Hinari | ("Delayed post-hypoxic leukoencephalopathy" OR "delayed encephalopathy") AND ("human" OR "case report" OR "case series") |
Studies will be included if they meet the following criteria: (1) Original research articles, case reports, and case series; (2) Studies involving human subjects diagnosed delayed post-hypoxic leukoencephalopathy; (3) Articles published in Eng
Studies will be excluded if they meet the following criteria: (1) Reviews, editorials, conference abstracts, and animal studies; and (2) Studies not related to DPHL or without sufficient clinical or radiographic data (Figure 1).
Two independent reviewers (Srichawla and Garcia-Dominguez) will screen the titles and abstracts of all articles retrieved for relevance. Full-text articles will be obtained for all potentially eligible studies and will be assessed for inclusion based on the predefined criteria. Any discrepancies between the reviewers will be resolved through discussion and a third reviewer will be consulted if necessary.
A standardized data extraction form will be used to collect the following information from each included study: (1) Study characteristics (author, year, country, study design); (2) Characteristics of the participants (age, sex, underlying cause of hypoxia); (3) Clinical features (symptoms, time of onset); (4) Neuroimaging findings; (5) Pathological findings; and (6) Treatment and results.
The quality of the included studies will be assessed using the Joanna Briggs Institute (JBI) critical evaluation checklist for case reports and case series. Two reviewers (Srichawla and Garcia-Dominguez) will independently assess the quality of each study, and disagreements will be resolved through discussion.
Data will be synthesized descriptively due to the anticipated heterogeneity in study designs, patient populations, and outcome measures. A narrative synthesis will be provided, summarizing key findings related to the epidemiology, pathophysiology, clinical characteristics, diagnostic approaches, and DPHL management strategies. Where possible, data will be presented in tables and figures to facilitate comparison between studies.
As this study involves the synthesis of previously published data, no ethical approval is required. However, the review will be conducted in accordance with ethical guidelines for systematic reviews, ensuring transparency, objectivity, and reproducibility.
A total of 74 cases were procured and summarized in Table 2[7-55]. Of those with the gender recorded, 30 occurred in men and 15 in women. The most common reported comorbidities included schizoaffective disorder, bipolar disorder, depression, hypertension, hyperlipidemia, substance use disorder, and hepatitis C.
| Ref. | Year of publication | Age | Gender | Cause of hypoxia | Comorbidities | Symptomatology | Time to onset of symptoms | Neuroimaging findings | Other neurodiagnostics | Therapeutic interventions | Complications | Outcomes |
| Aljarallah and Al-Hussain[7] | 2015 | 19 | M | Benzodiazepine overdose | None | Comatose | 21 days | T2WI: Diffusely increased signal intensity in cerebral white matter. DWI/ADC: Diffuse and symmetric diffusion restriction within the subcortical cerebral white matter and the right globus pallidus. T1WI: Patchy enhancement within cerebral white matter | EEG: Diffuse slowing at 2-3 Hz | Osmolar therapy | Tonsillar herniation and brain death | Death (23rd day of hospitalization) |
| Arciniegas et al[8] | 2004 | 24 | M | Opioid and benzodiazepine overdose | NA | 21 days | Executive dysfunction | T2WI: Diffusely increased signal intensity in cerebral white matter | NA | Amantadine | NA | Did not return to baseline |
| Arimany et al[9] | 2017 | 43 | M | Heroin overdose | Schizoaffective disorder | Akinetic mutism and ataxia | 21 days | T2WI: Diffuse and symmetric increase in T2 signal | NA | Supportive | NA | Significant improvement at 16 weeks after overdose |
| Beeskow et al[10] | 2018 | 51 | F | Carbon monoxide poisoning | Hypertension, obesity, sleep apnea syndrome, and depression | Agitation, reduced psychomotor activity, strange behavior. Progressed to mutism | 21 days | T2WI: Diffusely increased the T2 signal of the bilateral cerebral hemispheres and the basal ganglia | NA | Supportive | NA | Discharged from neurological rehabilitation in 6 weeks. At nine months improvement in leukoencephalopathy with cerebral atrophy |
| Betts et al[11] | 2012 | 46-59 | -- | Benzodiazepine overdose, ETOH abuse | Cognitive decline, speech disturbance, and memory loss | 17 days, 24 days, and 5 days | T2WI: Diffusely increased T2 signal of the bilateral cerebral hemispheres, including the globus pallidi. MRS: Decreased NAA, increased Cho/Cr ratio | EEG: Diffuse slowing | Supportive | NA | Significant improvement in one patient. Persistent memory dysfunction in other two patients | |
| Brovelli et al[12] | 2022 | 55 | F | Opioid intoxication | None | Psychomotor slowing, apathy, cognitive decline, akinetic mutism | 14 days | T2WI: Diffusely increased T2 signal within the frontal regions. DWI/ADC: The corresponding diffusion restriction was confirmed on the ADC maps | EEG: Global slowing | Logopedic and physiotherapic treatment | None | Awake and collaborative, with mild hypomimia and decreased spontaneous speech upon discharge |
| Cardona Quiñones et al[13] | 2022 | 26 | M | Opioid intoxication with cardiac arrest | None | Anton-Babinski syndrome | Few days | T2WI: Bilateral cerebral hemisphere hyperintensities including corpus callosum | NA | Supportive | None | NA |
| Chachkhiani et al[14] | 2022 | 46 | M | Opioid intoxication with respiratory failure | Hepatitis C, substance use disorder, mesial temporal lobe epilepsy | Psychomotor agitation and abulia | 27 days | T2WI: Bilateral cerebral hemisphere hyperintensities | EEG: Diffuse polymorphic delta activity. CSF: Normal | High dose IVMP, and amantadine | None | Discharged on day 48 with mild abulia and day 138 with a normal clinical exam, except hyperreflexia. Radiographic resolution of cerebral white matter disease |
| Chen et al[15] | 2022 | 64 | M | Nitrite poisoning. Comatose on initial presentation | None | Cognitive decline and mental and behavioral abnormalities | 60 days | T2WI: Hyperintensities of the bilateral cerebral hemisphere, involving the basal ganglia and the thalamus. DWI/ADC: Corresponding diffusion restriction involving | NA | Supportive | None | Did not regain functional independence at 6-month follow-up |
| Choi et al[16] | 2013 | 37 | M | Traumatic cervical cord injury | None | Akinetic mutism | 7 days | T2WI: Bilateral fronto temporal and basal ganglia hyperintensities | NA | Supportive | None | At 2 months of follow-up, they continued to show cognitive disability and disorientation |
| Fong et al[17] | 2019 | 61 | F | Benzodiazepine overdose | None | Neuropsychiatric symptoms | 41 days | T2WI: Confluent cerebral white matter changes DWI/ADC: Associated diffusion restriction | EEG: Generalized slowing. CSF: Normal | Supportive | None | Clinical improvement at follow-up (MoCA: 26/30). Repeat neuroimaging at 3 months showed improvement |
| Garzón-Hernández et al[18] | 2022 | 68 | M | Severe acute respiratory syndrome-coronavirus 2related hypoxia | None | Unresponsiveness | 17 days | T2WI: Confluent cerebral white matter hyperintensities. SWI: Cerebral microbleeds | EEG: isolated polymorphic delta waves in the frontal region without epileptiform activity | Supportive | None | Discharged to rehab on day 30 of hospitalization |
| Geraldo et al[19] | 2014 | 61 | M | Carbon monoxide poisoning | None | Disorientation, incoherent speech, and behavior disturbances | 39 days | T2WI: Confluent cerebral white matter hyperintensities | CSF: Normal | Hyperbaric oxygen therapy (90 minutes daily sessions, 100 % oxygen at 2.5 atmospheres with a total of 40 sessions) | None | Mild to moderate improvement and discharged to a rehabilitation facility |
| Gottfried et al[20] | 1994 | 36 | M | Opioid overdose | NA | Quadriparesis, myoclonic jerks, encephalopathy, cognitive decline | 24 days | T2WI: Increased supratentorial white matter signal. Hyperintense foci within globus pallidi. MRS: Decreased NAA; elevated choline and elevated lactate | NA | NA | NA | Significant improvement |
| Hakamifard et al[21] | 2021 | 39 | M | Opioid (methadone) overdose | Substance use disorder | Aphasia and decreased level of consciousness | Approximately 30 days | T2WI: Confluent cerebral white matter hyperintensities | CSF: Normal | Vitamin E 400 mg/day, vitamin C 1000 mg/day, magnesium-sulfate 1000 mg/day and vitamin B complex | None | Significant improvement in two months |
| Hamlin et al[22] | 2020 | 29 | M | Opioid overdose | Substance use disorder | Malignant catatonia, paroxysmal sympathetic hyperactivity | Approximately 30 days | T1WI and T2WI: Confluent hyperintensities involving the bilateral centrum semiovale | EEG: No epileptiform discharges | Propranolol, clonidine, and lorazepam | Akinetic mutism and sympathetic hyperactivity after electroconvulsive therapy (ECT) | Moderate improvement in 30 days |
| Hori et al[23] | 1991 | 13 | Asphyxiation | NA | Pseudobulbar paralysis, choreoathestosis | 7 days | Lesion involving the putamen and caudate nuclei | NA | NA | NA | Significant improvement at 1.5 years | |
| Hsiao et al[24] | 2004 | 11-79 | Carbon monoxide poisoning | NA | Cognitive impairment, akinetic mutism, and parkinsonism | 14-45 days | T2WI: Increased signal within the subcortical white-matter, basal ganglia, and globus pallidus | NA | NA | NA | Moderate to considerable improvement | |
| Huarcaya-Victoria[25] | 2018 | 37 | F | Carbon monoxide poisoning | None | Progressive psychomotor agitation, catatonia, and cognitive decline | Approximately 30 days | T2WI: Confluent cerebral white matter hyperintensities | NA | Hyperbaric oxygen therapy (29 feet for one hour, 2.2 absolute atmospheres, 20 sessions). Aripiprazole and diazepam for the management of catatonia | None | Significant improvement and discharge to rehabilitation facility |
| Huisa et al[26] | 2013 | 19, 32 | -- | Opioid overdose | NA | Decreased level of arousal, and encephalopathy | 58 days and 112 days | T2WI: Confluent cerebral white matter hyperintensities. ADC: Diffusion restriction in both cases with normalization at follow-up in case two | NA | NA | NA | Persistent deficits in both cases |
| Jang et al[27] | 2017 | 50 | M | Carbon monoxide poisoning | None | Myoclonus, dysarthria, decreased level of consciousness | 26 days | T2WI: Bilateral basal ganglia hyperintensities. DTT: Dysconnectivity involving the ascending reticular activating system | NA | Supportive | None | Discharge to rehabilitation facility six weeks from initial presentation. No significant improvement |
| Jayakrishnan et al[28] | 2021 | 68 | F | Myocardial infarction | Hypertension, hyperlipidemia, and myocardial infarction | Drowsiness, behavioral changes, urinary incontinence | 21 days | T2WI: Diffuse hyperintensities involving the cortex. ADC: Diffusion restriction involving the basal ganglia | NA | Supportive | None | Discharge to hospice |
| Jingami et al[29] | 2024 | 47 | M | Opioid intoxication | None | Decreased level of consciousness | 20 days | T2WI: Confluent cerebral white matter hyperintensities. N-isopropyl-(123I)-p-iodoamphetamine | CSF: Elevated myelin basic protein 135.5 pg/mL | Hyperbaric oxygen (2.0 ATA, 60 minutes, 63 total) | None | Improvement in mini-mental status exam from unmeasurable to 15 on day 40 of hospitalization |
| Kim et al [30] | 2002 | 54-71 | Carbon monoxide poisoning | NA | Memory loss, confabulations, and akinetic mutism | 1-4 weeks | T2WI: Confluent white matter hyperintensities in the brain | NA | NA | NA | 4 patients with significant improvement | |
| Law-ye et al[31] | 2018 | 58 | M | Carbon monoxide poisoning | None | Encephalopathy | 14 days | T2WI: Confluent white matter hyperintensities in the brain. ADC: Diffusion restriction in the corresponding area | CSF: Normal | Supportive | None | Significant improvement |
| Lee et al[32] | 2001 | 71 | F | Benzodiazepine overdose | None | Encephalopathy | 14 days | T2WI: Confluent cerebral white matter hyperintensities | CSF: Normal. EEG: Diffuse delta wave pattern | Supportive | None | Significant improvement with discharge to rehabilitation facility on day 47 |
| Lou et al[33] | 2009 | 62 | F | Cardiac arrest after gastrointestinal hemorrhage | NA | Akinetic mutism, rigidity | 14-21 days | T2WI: Confluent cerebral white matter hyperintensities involving the globus pallidi, and basal ganglia | NA | NA | NA | No significant improvement |
| Manjunath et al[34] | 2021 | 76 | M | Acute respiratory distress syndrome | NA | Cognitive decline | Few weeks | T2WI: Confluent cerebral white matter hyperintensities. ADC: Diffusion restriction in corresponding area | NA | Supportive | None | Significant clinical improvement over 3 months. With significant radiographic improvement in 4 months |
| Mazo et al[35] | 2020 | 66 | M | Carbon monoxide poisoning | None | Encephalopathy | 12 days | T2WI: Increased signal within the bilateral globus pallidus | CSF: Normal | Supportive | None | No significant improvement |
| Meyer et al[36] | 2013 | 43 | F | Benzodiazepine overdose | None | Encephalopathy | -- | T2WI: Confluent cerebral white matter hyperintensities. MRS: High peak for choline and creatinine | EEG: Generalized slowing | Supportive | None | Significant improvement in a few months |
| Mittal et al[37] | 2010 | 38 | M | Polysubstance abuse | NA | Encephalopathy, akinetic mutism | 21 days | T2WI: Confluent cerebral white matter hyperintensities | NA | Steroids and antioxidants | None | Significant improvement |
| Molloy et al[38] | 2006 | 40 | F | Opioid overdose | NA | Agitation, echolalia | 17 days | T2WI: Confluent cerebral white matter hyperintensities. ADC: With associated restricted diffusion | CSF: Normal | Supportive | None | Significant improvement over 6 months |
| Newburn et al[39] | 2024 | 19 | M | Benzodiazepine overdose | Developmental delay | Cognitive decline | -- | DSIR: High signal in the white matter of the brain | NA | Supportive | None | Mild improvement |
| Newburn et al[39] | 2024 | 20 | M | Suicide attempt (hanging) | Substance abuse | Cognitive decline | -- | DSIR: High signal in the white matter of the brain | NA | Supportive | None | Mild improvement |
| Nzwalo et al[40] | 2011 | 55 | F | Benzodiazepine overdose | NA | Akinetic mutism | NA | T2WI: Confluent cerebral white matter hyperintensities | CSF: Normal | Supportive | NA | No significant improvement |
| Pfaff et al[41] | 2022 | 81 | M | Unilateral internal carotid artery occlusion | Acute myeloid leukemia, hypertension, hyperlipidemia | Encephalopathy | 13 days | T2WI: Increased signal within the left centrum semiovale | NA | Supportive; mechanical thrombectomy | None | Clinical and radiographic improvement on day 92 of hospitalization |
| Quinn et al[42] | 2014 | 56 | F | Opioid overdose | Schizoaffective disorder, cirrhosis | Catatonia | 21 days | T2WI: Confluent cerebral white matter hyperintensities | EEG: generalized polymorphic theta waves, 2-3 Hz delta waves, and superimposed beta waves | ECT, methylprednisolone | None | No significant improvement |
| Rozen et al[43] | 2012 | 59 | -- | Opioid overdose | NA | Akinetic mutism | 21 days | T2WI: Confluent cerebral white matter hyperintensities including the globus pallidi | NA | IV Magnesium | None | Significant improvement |
| Salazar et al[44] | 2012 | 54 | M | Opioid overdose | NA | Encephalopathy, and rigidity | 21 days | T2WI: Confluent cerebral white matter hyperintensities. ADC: Diffusion restriction involving the globus pallidi | NA | Levodopa for rigidity | None | Significant improvement |
| Singu et al[45] | 2017 | 66 | M | Left main coronary artery occlusion | Hypertension, hyperlipidemia, diabetes mellitus, myocardial infarction | Aphasia, dysexecutive syndrome | 35 days | T2WI: Cerebral white matter hyperintensities involving the L MCA territory. ADC: Corresponding region hypointense. SPECT: 60%-70% decrease in CBF | CSF: Elevated protein | Supportive | None | Moderate improvement |
| Smolinsky et al[46] | 2018 | 16 | F | Traumatic brain injury | None | Encephalopathy | 8 days | DWI/ADC: Restricted diffusion involving right frontoparietal lobes, right temporal lobe, and left parietal lobe, and corpus callosum | None | Amantadine | None | Mild improvement |
| Tahir and Islam[47] | 2021 | 43 | M | ETOH abuse | None | Encephalopathy | 6 days | DWI/ADC: Diffusion restriction involving the bilateral centrum semiovale | CSF: Normal. EEG: Paroxysmal epileptiform activity | Supportive | None | Death |
| Tainta et al[48] | 2018 | 43 | M | Polysubstance abuse | Schizophrenia | Decreased level of consciousness | 21 days | T2WI: Confluent cerebral white matter hyperintensities. DWI/ADC: Diffusion restriction involving the bilateral centrum semiovale | NA | Supportive | None | Significant improvement in 2.5 months |
| Tan and Teo[49] | 2023 | 64 | M | Carbon monoxide poisoning | NA | Psychomotor agitation | 7 days | T2WI: Hyperintensities involving the bilateral globus pallidus | NA | Supportive | None | NA |
| Tormoehlen et al[50] | 2013 | 46 | F | Carbon monoxide poisoning | NA | Pseudobulbar affect | 14 days | T2WI: Confluent cerebral white matter hyperintensities | NA | Supportive | NA | Unknown |
| Wallace et al[51] | 2009 | 28 | M | Polysubstance abuse | ETOH abuse | Encephalopathy | 35 days | T2 BLADE: Hyperintensities involving the bilateral centrum semiovale | EEG: Normal | Supportive | Ventilatory and hemodynamic support | Significant improvement at 12 months |
| Wang and Yang[52] | 2003 | 15 | M | Substance abuse | NA | Seizures, dysphagia, dystonia, and altered mental status | NA | T2WI: Bilateral globus pallidi hyperintensities | NA | Supportive | NA | NA |
| Weinberger et al[53] | 1994 | 34 | -- | Benzodiazepine overdose | NA | Encephalopathy, hyperreflexia, clonus, primitive reflexes, and frontal lobe release sign | 24 days | Increased signal within the supratentorial white matter | NA | NA | NA | Persistent cognitive decline |
| Zamora et al[54] | 2015 | 64 | M | Cardiopulmonary arrest | NA | Psychomotor agitation | 23 days | T2WI: Confluent cerebral white matter hyperintensities. ADC: Increased signal less extensive than T2WI | NA | NA | None | Moderate improvement |
| Zamora et al[54] | 2015 | 32 | M | Opioid abuse | NA | Encephalopathy | 32 days | T2WI: Confluent cerebral white matter hyperintensities. ADC: More extensive than T2WI | NA | NA | None | Significant improvement |
| Zamora et al[54] | 2015 | 63 | F | Polysubstance abuse | NA | Akinetic mutism | 35 days | T2WI: Confluent cerebral white matter hyperintensities.ADC: Matched signal to T2WI | NA | NA | None | Significant improvement |
| Zamora et al[54] | 2015 | 65 | M | Polysubstance abuse | NA | Encephalopathy | 14 days | T2WI: Confluent cerebral white matter hyperintensities. ADC: Increased signal less extensive than T2WI | NA | NA | None | Significant improvement |
| Zamora et al[54] | 2015 | 59 | F | Opioid abuse | NA | Catatonia | 14 days | T2WI: Confluent cerebral white matter hyperintensities. ADC: Matched signal to T2WI | NA | NA | None | Deceased |
| Shprecher et al[55] | 2008 | 39-56 | -- | Polysubstance abuse | NA | Catatonia, memory loss, disorientation, encephalopathy | 31 days-38 weeks | T2WI: Confluent cerebral white matter hyperintensities. ADC: Diffusion restriction in 2 cases. MRS: Decreased NAA | NA | NA | NA | No significant improvement |
The most common causes of hypoxia are benzodiazepine overdose, opioid overdose, polysubstance overdose, and CO poisoning. Other lesser reported causes included cervical spinal cord injury, severe acute respiratory syndrome-coro
The most common neuroimaging findings included: The 44/72 diffuse increase in T2 signal throughout the cerebral white matter, 4 basal ganglia, 1 thalamus, and 5 pallidus globus. The case by Jang and Kwon[27] had a completed diffusion tensor tractography showing dysconnectivity between the ascending reticular activating system, the basal ganglia, and the thalami. Jingami et al[29] completed N-isopropyl-(123I)-p-iodoamphetamine SPECT imaging showing hypoperfusion involving the frontal lobe. The case by Meyer[36] had a complete magnetic resonance spectroscopy (MRS) showing an increase in creatinine and choline signals. The case of Gottfried et al[20] (1994) demonstrated a decrease in N-acetylaspartate (NAA), elevated choline, and lactate. Betts et al[11] reported a case series of 3 patients. MRS demonstrated a decrease in NAA, an elevated choline-to-creatinine ratio, and normal lactate within cerebral white matter. One case had diffuse cerebral atrophy, one follow-up imaging nine months later.
Newburn et al[39] (2024) presented a case series of two patients who showed increased signal intensity at divided subtracted inversion recovery (dSIR). In the case series by Zamora et al[54], all cases had confluent cerebral white matter hyperintensities involving the centrum semiovale and two cases had associated diffusion restriction. Furthermore, histopathological evaluation of case 5 from the Zamora et al[54] case series showed significant loss of myelin, axonal swelling, and reactive astroglia with a sparing of the U fibers. Other cases reported nonspecific lesions involving sub
The 20 cases had a decreased apparent diffusion coefficient signal consistent with cytotoxic edema or ischemia, most frequently involving: The 10 cerebral white matter, 4 globus pallidus, 1 basal ganglia and thalamus. There was at least one case of cerebral microbleeds on susceptibility-weighted imaging. Only a few cases showed contrast enhancement. When an electroencephalogram was performed, it often showed diffuse slowing, polymorphic delta activity involving the frontal region, diffuse delta activity, and paroxysmal epileptiform activity. Cerebrospinal fluid studies when completed were often normal. There were few reported cases of elevated myelin basic protein and elevated protein.
The complications reported included herniation syndromes from elevated intracranial pressure, sympathetic hyper
Hsiao et al[24] (2004) retrospectively reviewed 12 patients with DPHLS after CO intoxication, selected from 89 cases. These patients, averaging 54.4 years, initially showed severe disturbances of consciousness and were treated with high flow oxygen or HBOT. They regained consciousness within a week, but developed delayed encephalopathy 14 days to 45 days later, presenting with cognitive impairment, akinetic mutism, sphincter incontinence, gait ataxia, and various movement disorders. Brain MRI revealed lesions mainly in the subcortical white matter and basal ganglia, especially the globus pallidus. During follow-up, sphincter incontinence resolved first, cognitive function improved significantly over months, but involuntary movements showed minimal improvement, with some patients experiencing persistent symp
Quality and risk of bias assessment was completed using the 8-point questionnaire from the JBI assessment tool for case reports and series. A total of 41 records had a low risk of bias and five had a moderate risk of bias. The complete score breakdown is provided in Table 3[7-19,21-55].
| Ref. | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Overall | Risk |
| Aljarallah and Al-Hussain[7] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Arciniegas et al[8] | Y | Y | Y | Y | Y | N | N | Y | 6 | Moderate |
| Arimany et al[9] | Y | Y | Y | Y | N | N | N | Y | 5 | Moderate |
| Beeskow et al[10] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Betts et al[11] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Brovelli et al[12] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Cardona Quiñones et al[13] | Y | Y | Y | Y | N | N | N | Y | 5 | Moderate |
| Chachkhiani et al[14] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Chen et al[15] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Choi et al[16] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Fong et al[17] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Garzón-Hernández et al[18] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Geraldo et al[19] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Hakamifard et al[21] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Hamlin et al[22] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Hori et al[23] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Hsiao et al[24] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Huarcaya-Victoria[25] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Huisa et al[26] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Jang and Kwon[27] | Y | Y | Y | Y | N | Y | Y | Y | 7 | Low |
| Jingami et al[29] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Kim et al[30] | Y | Y | Y | Y | Y | N | Y | Y | 8 | Low |
| Law-ye et al[31] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Lee et al[32] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Lou et al[33] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Manjunath et al[34] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Mazo et al[35] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Meyer et al[36] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Mittal et al[37] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Molloy et al[38] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Newburn et al[39] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Nzwalo et al[40] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Pfaff et al[41] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Quinn et al[42] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Rozen et al[43] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Salazar et al[44] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Singu et al[45] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Smolinsky et al[46] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Tahir and Islam[47] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Tainta et al[48] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Tan and Teo[49] | Y | Y | Y | Y | Y | N | N | Y | 6 | Moderate |
| Tormoehlen et al[50] | Y | Y | Y | Y | Y | N | N | Y | 6 | Moderate |
| Wallace et al[51] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Wang and Yang[52] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Weinberger et al[53] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Zamora et al[54] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Shprecher et al[55] | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
This systematic review encompassed 72 cases of DPHLS, summarizing key demographic, clinical, neuroimaging, and outcome data. The analysis highlights the significant variability in patient presentations, etiologies, and outcomes, underscoring the complexity of this condition. The presence of symptoms in DPHLS was often correlated with neuro
The pathophysiology of DPHL is not fully understood but is believed to predominantly involve hypoxic-ischemic injury to the brain's white matter. White matter, composed of myelinated axons, is highly susceptible to hypoxic damage due to its high metabolic demand and lower capacity for anaerobic metabolism compared to gray matter[56]. Additionally, widely spread anastomoses within the white matter may also influence neuronal injury in the setting of hypoxia[57]. This review highlighted diffuse increases in the T2 signal in cerebral white matter in a significant proportion of cases, indi
CO poisoning leads to hypoxia by binding to hemoglobin with an affinity 200-250 times greater than oxygen, forming carboxyhemoglobin (COHb), which alters oxygen transport and release to tissues[58]. This hypoxic state particularly affects tissues of high metabolic demand, such as the brain. The resulting hypoxic injury initiates a cascade of cellular events, including oxidative stress, excitotoxicity, and inflammatory responses, which contribute to neuronal damage and white matter demyelination[59]. In DPHLS, the delayed onset of symptoms after an acute hypoxic episode, such as that induced by CO poisoning, suggests secondary injury mechanisms. These include the activation of microglia and astro
Initial hypoxic insult can trigger a cascade of cellular and molecular events, including inflammation, oxidative stress, and excitotoxicity, which evolve over time and lead to progressive damage to white matter. Neuroimaging overlaps between delayed DPHLS and metachromatic leukodystrophy (MLD) reveal significant similarities and differences that offer insights into their pathophysiology and diagnostic challenges. Both conditions commonly present with diffuse white matter hyperintensities on T2-FLAIR MRI sequences, reflecting extensive demyelination[64,65]. Despite these similarities, the pathophysiological mechanisms underlying DPHLS and MLD differ significantly. DPHLS results from hypoxic episodes leading to delayed demyelination and axonal damage, characterized by oxidative stress, excitotoxicity, and inflammation[64]. On the contrary, MLD is a genetic disorder caused by mutations in the ARSA gene, leading to deficient arylsulfatase A enzyme activity and subsequent accumulation of sulfatides, resulting in progressive demyelination[65]. The progression of white matter changes in DPHLS is typically subacute, with symptoms appearing weeks to months after the hypoxic event, while MLD has a more insidious onset, with gradual progression over months to years[66]. Advanced imaging techniques, such as MRS and diffusion tensor imaging (DTI), have revealed further similarities and distinctions. Both conditions show reduced levels of NAA, indicating neuronal loss, and elevated levels of choline, reflecting increased membrane turnover and gliosis[67]. However, MLD can show additional unique metabolic markers due to the specific biochemical abnormalities associated with sulfatide accumulation[68]. DTI studies reveal altered white matter integrity under both conditions, with decreased fractional anisotropy and increased mean diffusivity, although the pattern of white matter tract involvement can differ[66]. Histopathological evaluations revealed significant loss of myelin, axonal swelling, and reactive astroglia, indicating an inflammatory response to hypoxic injury[67]. These findings point to a substantial role for inflammation and immune activation in the progression of DPHL, contributing to the primary and secondary phases of white matter injury.
The cases included in this review exhibit significant heterogeneity in terms of patient demographics, causes of hypoxia, symptomatology, and treatment approaches. This variability makes it difficult to draw definitive conclusions and limits the generalizability of the findings. Most of the included studies are retrospective case reports and series, which can introduce selection bias and limit the ability to establish causality. The retrospective design also relies on the accuracy and completeness of medical records. The absence of standardized diagnostic criteria for DPHLS results in variability in diagnosis and reporting, which can lead to inconsistencies in case identification and classification. Incomplete or inconsistent reporting of clinical results, neuroimaging findings, and therapeutic interventions in included studies limits the ability to perform a comprehensive and uniform analysis. Many studies lack long-term follow-up data, which is crucial for understanding the full path of DPHLS, including the persistence of symptoms, long-term outcomes, and the potential for recovery. Conducting prospective cohort studies with standardized diagnostic criteria and protocols will provide more robust data on the incidence, risk factors, and natural history of DPHLS. These studies can also help establish causality and improve understanding of disease progression. Developing and adopting standardized diagnostic criteria for DPHLS will improve the consistency and reliability of diagnosis and reporting across studies, facilitating more accurate comparisons and meta-analyses. Identifying and validating biomarkers for DPHLS, such as specific neuroche
The findings of Newburn et al[39] suggest that DPHL may be underdiagnosed due to the reliance on conventional MRI and stringent diagnostic criteria. Future research should aim to broaden the diagnostic criteria for DPHL to include less severe cases and recognize the spectrum of clinical presentations. The use of dSIR sequences, as highlighted in the study, may provide greater clinical utility than conventional MRI techniques. Future studies should explore the routine use of advanced MRI sequences like dSIR to improve the detection and characterization of white matter changes in DPHL[39].
Delayed post-hypoxic leukoencephalopathy syndrome (DPHLS), or Grinker’s myelinopathy, is an under-recognized neurological condition that manifests after a latent period after a hypoxic event. It is characterized by delayed onset of neurological and cognitive deficits, which typically present day to weeks after the injury. This systematic review high
| 1. | Bougouin W, Lamhaut L, Marijon E, Jost D, Dumas F, Deye N, Beganton F, Empana JP, Chazelle E, Cariou A, Jouven X; SDEC Co-Investigators. Characteristics and prognosis of sudden cardiac death in Greater Paris: population-based approach from the Paris Sudden Death Expertise Center (Paris-SDEC). Intensive Care Med. 2014;40:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 2. | Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. N Engl J Med. 2001;345:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 233] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2250] [Cited by in RCA: 2153] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 4. | Shprecher D, Mehta L. The syndrome of delayed post-hypoxic leukoencephalopathy. NeuroRehabilitation. 2010;26:65-72. [PubMed] |
| 5. | Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol. 1983;40:433-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 340] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47199] [Article Influence: 2949.9] [Reference Citation Analysis (0)] |
| 7. | Aljarallah S, Al-Hussain F. Acute fatal posthypoxic leukoencephalopathy following benzodiazepine overdose: a case report and review of the literature. BMC Neurol. 2015;15:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Arciniegas DB, Frey KL, Anderson CA, Brousseau KM, Harris SN. Amantadine for neurobehavioural deficits following delayed post-hypoxic encephalopathy. Brain Inj. 2004;18:1309-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Arimany MS, Garriga M, Parellada E. Delayed post-hypoxic leukoencephalopathy: Case report. Eur psychiatr. 2017;41:s839-s839. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 10. | Beeskow AB, Oberstadt M, Saur D, Hoffmann KT, Lobsien D. Delayed Post-hypoxic Leukoencephalopathy (DPHL)-An Uncommon Variant of Hypoxic Brain Damage in Adults. Front Neurol. 2018;9:708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Betts AM, Ritter JL, Kubal WS. Reversible delayed posthypoxic leukoencephalopathy after drug overdose: MRI findings in a collection of patients. Emerg Radiol. 2012;19:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 12. | Brovelli F, Saraceno L, Di Pietro A, Erminio C, Agostoni EC. Delayed post-hypoxic leukoencephalopathy following opioid intoxication. Neurol Sci. 2023;44:761-763. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Cardona Quiñones RA, Salem Hernández SA, Sekimitsu S, Antongiorgi Torres J, Yerstein O, Safar LT. A neuropsychiatric case of delayed post-hypoxic leukoencephalopathy from opioid intoxication resulting in Anton-Babinski syndrome and quadriplegia. Neurocase. 2023;29:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 14. | Chachkhiani D, Chimakurthy AK, Verdecie O, Goyne CT, Mader EC Jr. Delayed Toxic-Hypoxic Leukoencephalopathy As Sequela of Opioid Overdose and Cerebral Hypoxia-Ischemia. Cureus. 2021;13:e20271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Chen Y, Liu Q, Wang J, Li H, Zhang Y, Sun L, Liu J. Delayed Post-Hypoxic Leukoencephalopathy Following Nitrite Poisoning: A Case Report and Review of the Literature. Front Neurol. 2022;13:836844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Choi S, Lee SH, Kim ES, Eoh W. Delayed post-hypoxic leucoencephalopathy presenting akinetic mutism after traumatic cervical cord injury: a case report. Br J Neurosurg. 2013;27:529-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Fong M, Rigby N, Pun P, Mitchell R, Schweitzer D, Swayne A. 098 A case of delayed post-hypoxic leukoencephalopathy complicating drug overdose. J Neurol Neurosurg Psychiatry. 2019;90:A32.1-A32. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Garzón-hernández JP, Pabón-moreno A, Andrade-rondón SA, Álvarez-pabón Y, Silva-sieger FA. Covid-19–associated diffuse posthypoxic leukoencephalopathy and microhemorrhages – A case report. Neuroimmunology Reports. 2022;2:100150. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Geraldo AF, Silva C, Neutel D, Neto LL, Albuquerque L. Delayed leukoencephalopathy after acute carbon monoxide intoxication. J Radiol Case Rep. 2014;8:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Gottfried JA, Mayer SA, Shungu DC, Chang Y, Duyn JH. Delayed posthypoxic demyelination. Association with arylsulfatase A deficiency and lactic acidosis on proton MR spectroscopy. Neurology. 1997;49:1400-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Hakamifard A, Hajiahmadi S, Khorvash F, Azish S. A case study of methadone-induced delayed post-hypoxic leukoencephalopathy with improvement by antioxidant therapy. North Clin Istanb. 2021;8:106-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Hamlin DW, Hussain N, Pathare A. Storms and silence: a case report of catatonia and paroxysmal sympathetic hyperactivity following cerebral hypoxia. BMC Psychiatry. 2020;20:473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Hori A, Hirose G, Kataoka S, Tsukada K, Furui K, Tonami H. Delayed postanoxic encephalopathy after strangulation. Serial neuroradiological and neurochemical studies. Arch Neurol. 1991;48:871-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Hsiao CL, Kuo HC, Huang CC. Delayed encephalopathy after carbon monoxide intoxication--long-term prognosis and correlation of clinical manifestations and neuroimages. Acta Neurol Taiwan. 2004;13:64-70. [PubMed] |
| 25. | Huarcaya-Victoria J, Podestá-Ampuero A, Ledesma-Gastañadui M, Reinoso-Santa Cruz C. Treatment of Delayed Post-Hypoxic Leukoencephalopathy as a complication of carbon monoxide poisoning with risperidone and hyperbaric oxygen therapy. Actas Esp Psiquiatr. 2018;46:68-74. [PubMed] |
| 26. | Huisa BN, Gasparovic C, Taheri S, Prestopnik JL, Rosenberg GA. Imaging of subacute blood-brain barrier disruption after methadone overdose. J Neuroimaging. 2013;23:441-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Jang SH, Kwon HG. Injury of ascending reticular activating system associated with delayed post-hypoxic leukoencephalopathy: a case report. BMC Neurol. 2017;17:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Jayakrishnan R, Rashid N, Cheenath RT, Verghese J. Grinker's myelinopathy: The rarely reported consequence of hypoxic brain injury. Radiol Case Rep. 2021;16:3311-3314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Jingami N, Cho K, Nitta T, Takatani M, Kobayashi K, Takenaka R, Sugita N, Ohtsuru S. Case report: Consecutive hyperbaric oxygen therapy for delayed post-hypoxic leukoencephalopathy resulting from CHANTER syndrome caused by opioid intoxication. Front Med (Lausanne). 2024;11:1364038. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Kim HY, Kim BJ, Moon SY, Kwon JC, Shon YM, Na DG, Lee KH, Na DL. Serial diffusion-weighted MR Imaging in delayed postanoxic encephalopathy. A case study. J Neuroradiol. 2002;29:211-215. [PubMed] |
| 31. | Law-Ye B, Dodet P, Hermann B, Trunet S, Dormont D, Pyatigorskaya N, Leclercq D. Progressive white-matter demyelination in delayed CO poisoning encephalopathy. J Neuroradiol. 2018;45:59-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Lee HB, Lyketsos CG. Delayed post-hypoxic leukoencephalopathy. Psychosomatics. 2001;42:530-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Lou M, Jing CH, Selim MH, Caplan LR, Ding MP. Delayed substantia nigra damage and leukoencephalopathy after hypoxic-ischemic injury. J Neurol Sci. 2009;277:147-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Manjunath V, Nadaf S, Chakor RT. Delayed Post-hypoxic Leukoencephalopathy with Neuroradiological Recovery. Indian J Crit Care Med. 2021;25:1326-1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Mazo J, Mukhtar E, Mazo Y, Nagaraj A, Mantello MT. Delayed brain injury post carbon monoxide poisoning. Radiol Case Rep. 2020;15:1845-1848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Meyer MA. Delayed post-hypoxic leukoencephalopathy: case report with a review of disease pathophysiology. Neurol Int. 2013;5:e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Mittal M, Wang Y, Reeves A, Newell K. Methadone-induced delayed posthypoxic encephalopathy: clinical, radiological, and pathological findings. Case Rep Med. 2010;2010:716494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Molloy S, Soh C, Williams TL. Reversible delayed posthypoxic leukoencephalopathy. AJNR Am J Neuroradiol. 2006;27:1763-1765. [PubMed] |
| 39. | Newburn G, Condron P, Kwon EE, McGeown JP, Melzer TR, Bydder M, Griffin M, Scadeng M, Potter L, Holdsworth SJ, Cornfeld DM, Bydder GM. Diagnosis of Delayed Post-Hypoxic Leukoencephalopathy (Grinker's Myelinopathy) with MRI Using Divided Subtracted Inversion Recovery (dSIR) Sequences: Time for Reappraisal of the Syndrome? Diagnostics (Basel). 2024;14:418. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 40. | Nzwalo H, Sá F, Cordeiro I, Ferreira F, Basílio C. Delayed hypoxic-ischemic leukoencephalopathy. BMJ Case Rep. 2011;2011:bcr0620114344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Pfaff JAR, Machegger L, Trinka E, Mutzenbach JS. Unilateral delayed post-hypoxic leukoencephalopathy: a case report. J Med Case Rep. 2022;16:480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 42. | Quinn DK, Abbott CC. Catatonia after cerebral hypoxia: do the usual treatments apply? Psychosomatics. 2014;55:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Rozen TD. Rapid resolution of akinetic mutism in delayed post-hypoxic leukoencephalopathy with intravenous magnesium sulfate. NeuroRehabilitation. 2012;30:329-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Salazar R, Dubow J. Delayed posthypoxic leukoencephalopathy following a morphine overdose. J Clin Neurosci. 2012;19:1060-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Singu T, Inatomi Y, Yonehara T, Ando Y. Delayed leukoencephalopathy after recanalized cardioembolic stroke: Two case reports. J Neurol Sci. 2017;379:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 46. | Smolinsky K, Sediva I. A Case of Delayed-Onset Posthypoxic Leukoencephalopathy in a Pediatric Patient. Child Neurol Open. 2018;5:2329048X18792441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Tahir I, Islam MU. Delayed post-hypoxic leukoencephalopathy following alcohol consumption and cardiopulmonary arrest. Radiol Case Rep. 2021;16:3039-3043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 48. | Tainta M, de la Riva P, Urtasun MÁ, Martí-Massó JF. Reversible delayed post-hypoxic leukoencephalopathy. Neurologia (Engl Ed). 2018;33:59-61. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 49. | Tan LYC, Teo WS. Too Early to Celebrate? A Case of Delayed Posthypoxic Leukoencephalopathy. Am J Phys Med Rehabil. 2024;103:e91-e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 50. | Tormoehlen LM. Toxic leukoencephalopathies. Psychiatr Clin North Am. 2013;36:277-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Wallace IR, Dynan C, Esmonde T. One confused patient, many confused physicians: a case of delayed post-hypoxic leucoencephalopathy. QJM. 2010;103:193-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Wang XP, Yang RM. Movement disorders possibly induced by traditional chinese herbs. Eur Neurol. 2003;50:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Weinberger LM, Schmidley JW, Schafer IA, Raghavan S. Delayed postanoxic demyelination and arylsulfatase-A pseudodeficiency. Neurology. 1994;44:152-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Zamora CA, Nauen D, Hynecek R, Ilica AT, Izbudak I, Sair HI, Gujar SK, Pillai JJ. Delayed posthypoxic leukoencephalopathy: a case series and review of the literature. Brain Behav. 2015;5:e00364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 55. | Shprecher DR, Flanigan KM, Smith AG, Smith SM, Schenkenberg T, Steffens J. Clinical and diagnostic features of delayed hypoxic leukoencephalopathy. J Neuropsychiatry Clin Neurosci. 2008;20:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Armstrong RC, Mierzwa AJ, Marion CM, Sullivan GM. White matter involvement after TBI: Clues to axon and myelin repair capacity. Exp Neurol. 2016;275 Pt 3:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 57. | Ravens JR, Toole JF, Hasegawa T. Anastomoses in the vascular bed of the human cerebrum. J Neuropathol Exp Neurol. 1968;27:123-124. [PubMed] |
| 58. | Raub JA, Mathieu-Nolf M, Hampson NB, Thom SR. Carbon monoxide poisoning--a public health perspective. Toxicology. 2000;145:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 363] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 59. | Piantadosi CA. Carbon monoxide poisoning. N Engl J Med. 2002;347:1054-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Prockop LD, Chichkova RI. Carbon monoxide intoxication: an updated review. J Neurol Sci. 2007;262:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 367] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 61. | Rose JJ, Wang L, Xu Q, McTiernan CF, Shiva S, Tejero J, Gladwin MT. Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy. Am J Respir Crit Care Med. 2017;195:596-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 434] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 62. | Hampson NB, Weaver LK. Carbon monoxide poisoning: a new incidence for an old disease. Undersea Hyperb Med. 2007;34:163-168. [PubMed] |
| 63. | Thom SR. Hyperbaric-oxygen therapy for acute carbon monoxide poisoning. N Engl J Med. 2002;347:1105-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Chang KH, Han MH, Kim HS, Wie BA, Han MC. Delayed encephalopathy after acute carbon monoxide intoxication: MR imaging features and distribution of cerebral white matter lesions. Radiology. 1992;184:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 109] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Kehrer C, Blumenstock G, Raabe C, Krägeloh-Mann I. Development and reliability of a classification system for gross motor function in children with metachromatic leucodystrophy. Dev Med Child Neurol. 2011;53:156-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 66. | Amedick LB, Martin P, Beschle J, Strölin M, Wilke M, Wolf N, Pouwels P, Hagberg G, Klose U, Naegele T, Kraegeloh-Mann I, Groeschel S. Clinical Significance of Diffusion Tensor Imaging in Metachromatic Leukodystrophy. Neuropediatrics. 2023;54:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 67. | Feldmann J, Martin P, Bender B, Laugwitz L, Zizmare L, Trautwein C, Krägeloh-Mann I, Klose U, Groeschel S. MR-spectroscopy in metachromatic leukodystrophy: A model free approach and clinical correlation. Neuroimage Clin. 2023;37:103296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 68. | Sener RN. Metachromatic leukodystrophy: diffusion MR imaging findings. AJNR Am J Neuroradiol. 2002;23:1424-1426. [PubMed] |