Published online Oct 6, 2024. doi: 10.12998/wjcc.v12.i28.6222
Revised: May 20, 2024
Accepted: June 20, 2024
Published online: October 6, 2024
Processing time: 142 Days and 15.6 Hours
Langerhans cell histiocytosis (LCH) is a histiocytic proliferative disease caused by clonal proliferation of Langerhans cells, which is currently defined as an inflammatory myeloid tumor. It is rare in adults, with an incidence of 1–2 per million, and is highly heterogeneous in clinical presentation, with unpredictable disease progression and outcome.
A 52-year-old postmenopausal female patient presented to the gynecology department in July 2023 with bilateral vulvar masses. She was diagnosed with recurrent multisystem LCH. The patient had previously been diagnosed with a single-system and single-focal LCH in October 2021 due to a right maxillofacial mass, which resolved after surgical treatment. A chemotherapy regimen was developed after multidisciplinary consultation. Six cycles of chemotherapy resulted in partial remission, and maintenance chemotherapy is currently being administered.
Recurrent LCH involving the bilateral vulva has been poorly reported. Comprehensive imaging and pathological evaluation is important for diagnosis. The model of joint multidisciplinary specialist diagnosis and treatment is worthy of clinical application.
Core Tip: The pathogenesis of Langerhans cell histiocytosis (LCH) is unclear and it is currently classified as a neoplastic disease. Recurrent LCH involving the female genitalia is rare. Pathological diagnosis is considered the gold standard. For patients with extensive lesions or relapses, we recommend a hematology-based multidisciplinary diagnostic and treatment model. New combination chemotherapy regimens and maintenance chemotherapy may result in better clinical outcomes.
- Citation: Yuan CY, Zhang ZR, Guo MF, Zhang N. Recurrent multisystem Langerhans cell histiocytosis involving the female genitalia: A case report. World J Clin Cases 2024; 12(28): 6222-6229
- URL: https://www.wjgnet.com/2307-8960/full/v12/i28/6222.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i28.6222
Langerhans cell histiocytosis (LCH) is a rare neoplastic disease[1,2]. LCH is frequently observed in children and rare in adults. LCH is more common in men, with an incidence 3.7 times higher than in women[3-6]. The etiology of LCH remains uncertain, with mutations in the BRAF-V600E gene detected in certain patients. The clinical manifestations of LCH in adults are nonspecific, such as bone pain, cutaneous lesions, cough, nausea, headache, weight loss, fever and neuropsychiatric symptoms. Bone pain represents the most prevalent symptom, followed by cutaneous lesions. Cutaneous manifestations of LCH are typically erythema and papular eruptions accompanied by scaling, with the genital region showing less involvement.
Baseline 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) is a valuable imaging tool for aiding diagnosis and determining the extent of disease, while organ-specific imaging techniques have been recommended for further evaluation of the site of disease involvement based on initial imaging. Pathological diagnosis is considered the gold standard. The clinical course of LCH is highly variable, ranging from a self-healing solitary bone lesion to widely disseminated life-threatening disease. In particular, patients with multisystem and multiorgan involvement face a great challenge in terms of treatment[7].
A 52-year-old postmenopausal female patient had bilateral vulvar masses for 5 months. The masses gradually increased in size and were accompanied by pain.
In October 2021, the patient was diagnosed with right maxillofacial LCH with an isolated lesion. The patient underwent successful local excision and postoperative healing. The scope of the surgery included right cervical lymph node dissection, excision of the right oral and maxillofacial mass, and fascial tissue flap plasty. Postoperative pathology revealed (right neck, isthmus) LCH and immunohistochemistry was positive for CD1α, Langerin, S100 and CD21. The patient presented to the hospital in July 2023 following the discovery of bilateral vulvar masses accompanied by pain. The patient was also drinking more water and had polyuria, with a daily urine output of > 5 L.
The patient has had type 2 diabetes mellitus for > 10 years and has stable glycemic control with NovoMix 30 injections (10 U in the morning and 10 U in the evening). There was no previous medical history of vulvar leukoplakia, erosions, ulcers, or pruritus. The patient denied any prior history of vulvar trauma.
The patient did not have a history of smoking and had no other specific personal or family medical history.
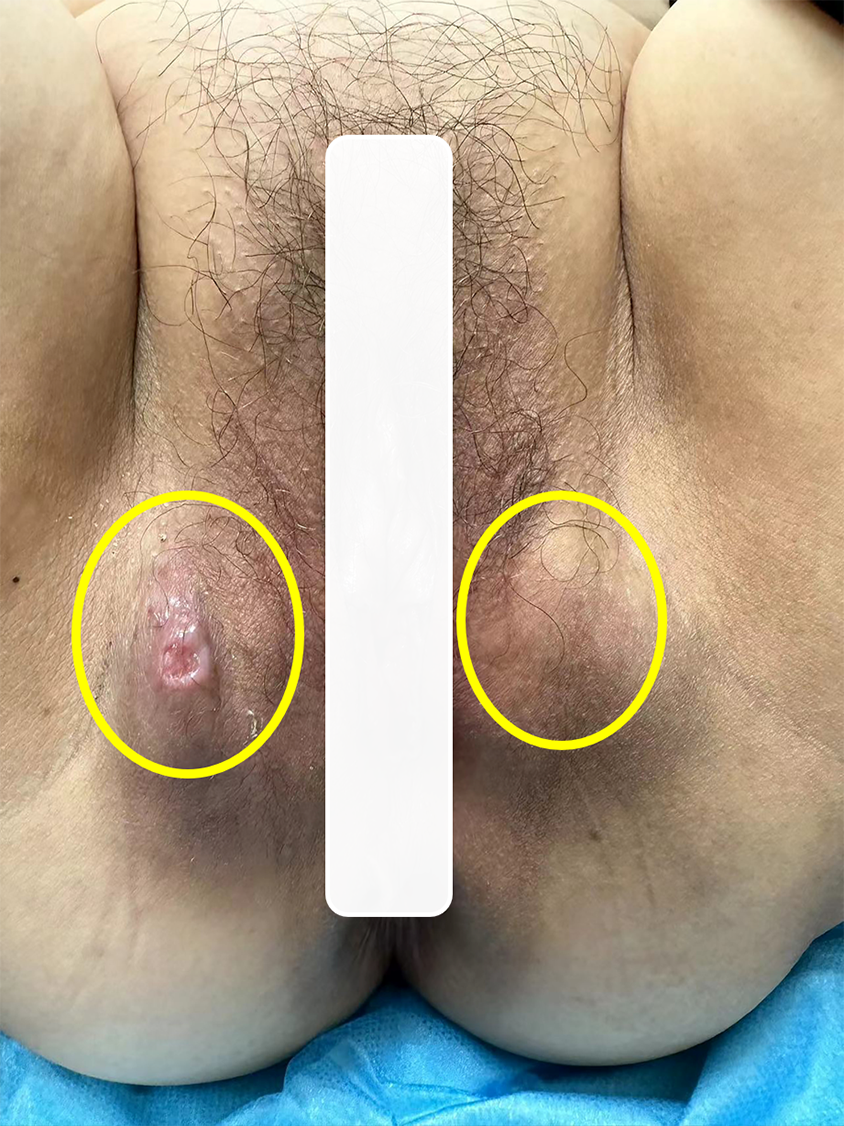
Gynecological examination revealed a 3.0 cm × 3.0 cm × 2.0 cm mass in the right vulvar region, displaying a medium texture and no tenderness. On the skin surface, there was a vesicular area of approximately 0.5 cm × 0.5 cm, accompanied by a small amount of yellowish discharge. No redness or swelling was observed in the surrounding skin. During the examination, a 3.0 cm × 3.0 cm × 3.0 cm mass was identified in the left vulvar region, displaying a medium texture and absence of tenderness. The skin surface appeared intact, with no observed erythema in the surrounding area (Figure 1). The vagina was patent and the mucosa was smooth; the cervix was prototypical, with a smooth surface and no erosions, ulcers, nodules, bloody or purulent discharges; the uterus was normal in size and had no pressure; and no masses were detected in the adnexal regions bilaterally.
Thyroid function test results were as follows: Triiodothyronine 1.1 nmol/L (normal range, 1.3–3.1), free-triiodothyronine 3.06 pmol/L (normal range, 3.1–6.8), thyroxine 64 nmo1/L (normal range, 66–181), and thyroid-stimulating hormone 6.02 uIU/mL (normal range, 0.27–4.2). Fasting blood glucose was 10–13.8 mmol/L; 2-hours postprandial blood glucose was 10.8–21.1 mmol/L; glycosylated hemoglobin was 10.7% (range, 3.6%–6%). Total T-lymphocyte, CD8 lymphocyte, CD4 lymphocyte, natural killer cell and B-lymphocyte counts were below normal. Tests for Epstein–Barr virus, hepatitis B virus, rubella virus, cytomegalovirus, and herpes simplex virus were negative. Routine blood tests, liver and kidney function, and coagulation test results were normal.
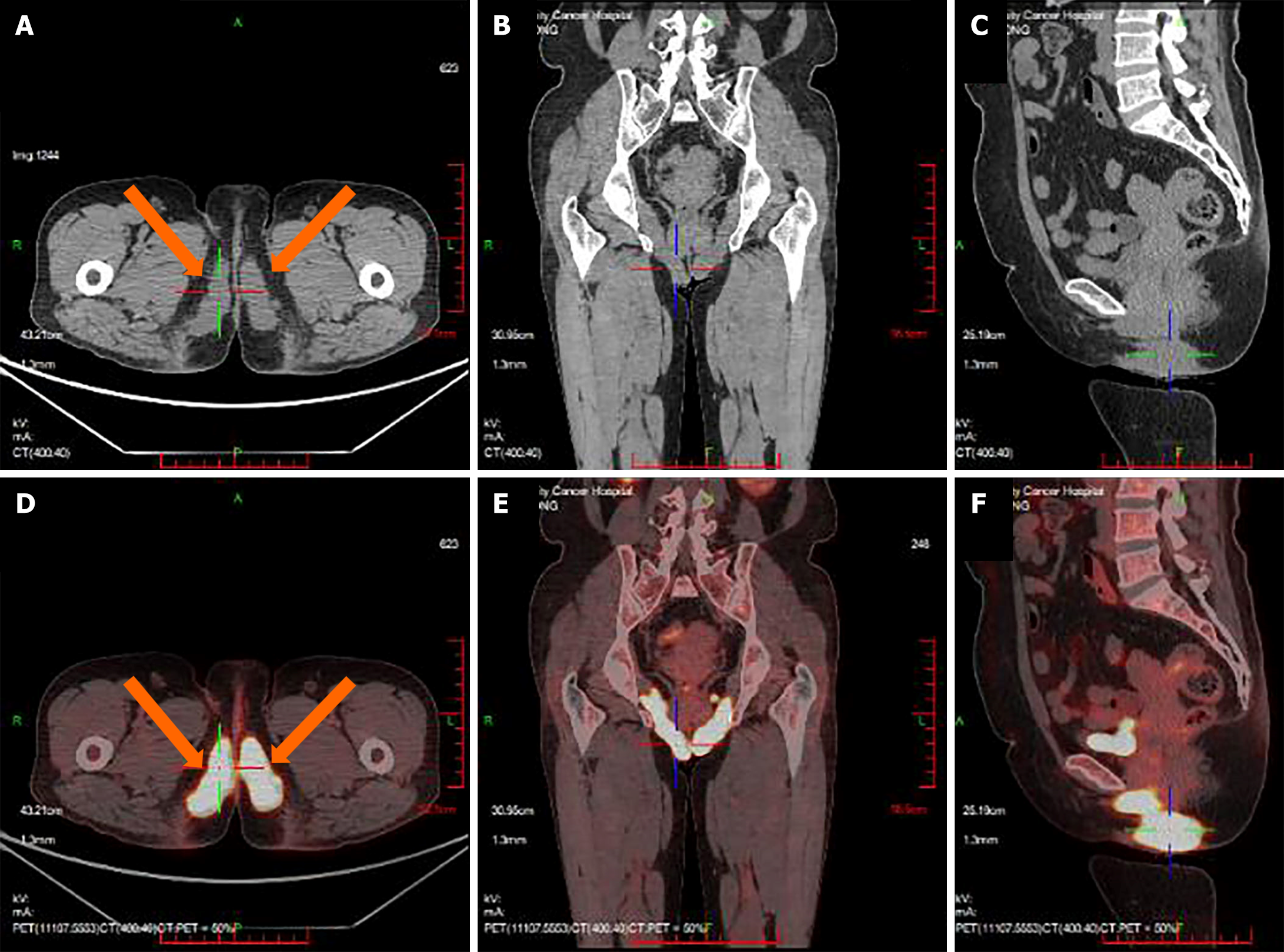
PET/CT imaging (Figure 2) revealed a hypermetabolic soft tissue mass in the left neck area measuring approximately 7.7 cm × 3.4 cm, with a maximum standardized uptake value (SUVmax) of 14.5. Bilateral thickening of the sternocleidomastoid muscles with increased metabolism (SUVmax 12.7) was mainly noted on the left side. In the perineal area, striated, nodular soft-tissue shadows were observed (SUVmax 9.6), showing a symmetrically distributed "figure-of-eight" pattern, with a larger area measuring 7.4 cm × 2.6 cm on the right side. The lesion involved the posterior aspect of the obturator internus muscle on both sides and the bilateral vulva. Uneven density of thyroid parenchyma with increased metabolism was observed, which was more pronounced on the right side (SUVmax 7.4).
Enhanced magnetic resonance imaging (MRI) of the pelvis revealed bilateral involvement of the pelvic floor along the sciatic and subpubic branches, as well as the medial aspect of the obturator internus muscle extending to the perineal region. These regions exhibited a striated T2 slightly hyperintense signal with unclear margins, demonstrating significant enhancement and an increased thickness of approximately 2.5 cm on both sides. Enhanced MRI of the saddle region did not show any abnormalities.
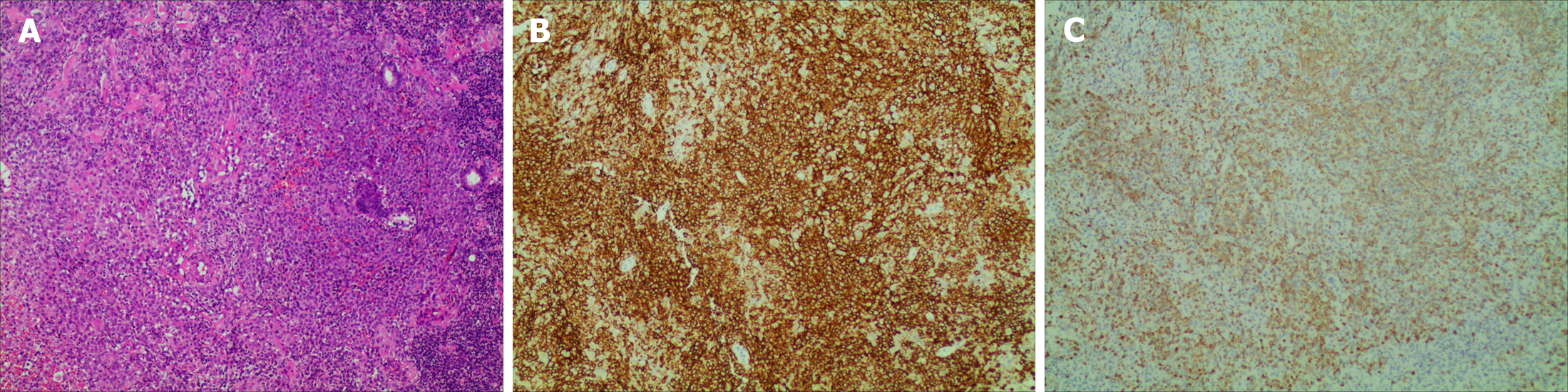
Pathology of the right vulvar mass biopsy indicated clusters of medium-sized cells with dense infiltration of eosinophils. The immunohistochemical profile included: Langerin positive, CD1a positive, CD68 partially positive, CD163 partially positive, CD45 positive, vimentin positive, and Ki-67 (hotspot area approximately 40% positive) (Figure 3). Bone marrow aspiration demonstrated evidence of proliferative activity, while flow cytometry did not reveal any abnormal cell expression.
A multidisciplinary team comprising specialists in hematology, oncology, gynecological oncology, imaging, pathology, and endocrinology reached the following diagnoses: Recurrent multisystem LCH; diabetes insipidus; diabetes mellitus, and hypothyroidism.
Informed consent was obtained from the patient before treatment. The lesions on both sides of the vulva were vastly expansive. If surgical excision were carried out, the lesions would not have been completely removed, and the surgery would have been so traumatic that the vulva may not have healed completely after the operation. If local radiotherapy were used, the large vulvar area of irradiation could have aggravated the damage to the surrounding normal tissues. Chemotherapy was administered from July 17, 2023 after multidisciplinary expert consultation, which consisted of vindesine 3.0 mg/m2 on day 1, cyclophosphamide 750.0 mg/m2 on day 1, dexamethasone 15.0 mg/d on days 1–5, and etoposide 0.1 g/m2 on days 1–4. The patient was treated with oral desmopressin acetate for diabetes insipidus and oral levothyroxine sodium tablets for hypothyroidism.
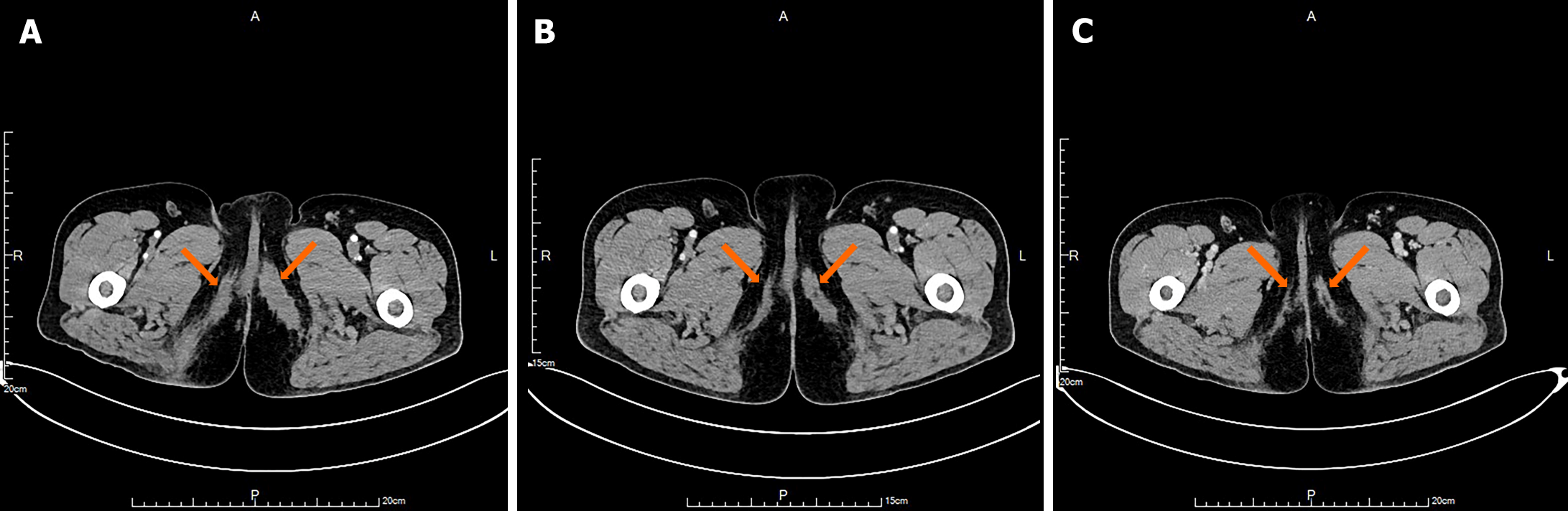
After one cycle of chemotherapy, the patient developed a localized infection at the site of the right vulvar biopsy. The suture at the biopsy site had ruptured, exposing a longitudinal laceration measuring approximately 2.5 cm × 1.0 cm that was filled with yellow, fish-like tissue. The surrounding skin was swollen and mildly erythematous, measuring approximately 5.0 cm × 4.0 cm × 4.0 cm. When pressure was applied to the swollen area, a thin greyish-brown fluid was expelled. We administered anti-infective therapy, and performed local wound debridement and dressing changes. The local infection in the right vulva was successfully resolved, leading to wound healing. Following six cycles of chemotherapy, there was a substantial reduction in the multisystem lesions, and efficacy was evaluated as partial response (Figure 4). Currently, the patient is undergoing maintenance therapy with vindesine 4 mg/d on days 1–5 and prednisone 60 mg/d on days 1–5, and has successfully completed four cycles of treatment. The main adverse reaction of chemotherapy was grade II myelosuppression which was restored after treatment with recombinant human granulocyte-colony stimulating factor.
In 1939, Lane and Smith reported the first case of LCH invading the genital tract of a 6-year-old child[8]. LCH involving the female genital tract is rare, and the available literature comprises almost exclusively case reports. Fewer than 60 cases of LCH invading the vulva have been reported; nearly half of which were isolated cases, and the remainder were multisystemic LCH involving the vulva[9]. The peak onset age for LCH in adults is between 20 and 40 years[10]. The pathogenesis of LCH is unclear and it has previously been classified as an immunological disorder. In recent years, research has found that > 50% of LCH patients have the BARF-V600E gene mutation[2]. BRAF-V600E is a key factor in the mitogen-activated protein kinase signaling cascade of MEK–ERK, and is extensively involved in regulating cell growth, differentiation, senescence and apoptosis[11,12]. The BRAF-V600E mutation can activate the kinase pathway, leading to activation and proliferation of pathological Langerhans cells (LCs), confirming that LCH belongs to the category of neoplastic diseases[1].
In addition to presenting as a single-lesion or single-system multifoci, LCH can also invade multiple systems and organs[13]. Common sites of LCH involvement include bone, skin, pituitary gland, bone marrow, liver, spleen, lungs and lymph nodes[1]. Female genital LCH includes four patterns: (1) Genital LCH alone; (2) Genital LCH with multiorgan involvement; (3) Oral or cutaneous LCH with genital and multiorgan involvement; and (4) Diabetes insipidus with organ involvement[8]. The prognosis of LCH is highly variable, depending on the extent of the disease and which organs are affected. Studies have reported a linear relationship between the number of organs involved and the mortality rate: 25% for two organs, 33% for three or four organs, 72% for five organs and 100% for more than six organs[14].
Our patient presented initially with a right maxillofacial mass and a solitary lesion. Twenty-one months after surgical intervention, the primary manifestation of recurrence was distant bilateral vulvar masses with concomitant diabetes insipidus, which fully reflects the heterogeneity and diversity of the clinical manifestations of LCH. LCH involvement of the vulva more often presents as erythema, papules, ulcers, pruritic rashes, and less often as masses[15,16]. This disease may be confused with other common vulvar lesions such as seborrheic dermatitis, eczema, herpes, genital tuberculosis, sexually transmitted diseases, trauma, sclerosing moss, malignant melanoma, sarcoma, squamous cell carcinoma, and Paget's disease of the vulva, making diagnosis a challenge for clinicians. The incidence of diabetes insipidus in LCH ranges from 15% to 20%, and pituitary dysfunction ranges from 5% to 20%[14].When diabetes insipidus occurs, it is important to examine other hormonal deficiencies in addition to focusing on pituitary lesions. The multisystem lesions of this patient with LCH all presented with a mass. The patient suffered from diabetes insipidus, but no pituitary, thyroid, thymus or adrenal lesions were found. Thyroid function tests confirmed hypothyroidism, and it was hypothesized that the patient's diabetes insipidus was related to endocrine dysfunction.
Histopathology combined with immunohistochemistry was used to confirm the diagnosis. LCs are large, oval shaped and eosinophilic, and are found in the cytoplasm. Typical histopathological features of LCH are an inflammatory infiltrate formed by eosinophils, macrophages, FoxP3+CD4+Treg lymphocytes, and multinucleated giant cells. Positivity for CD1a and/or CD207 (Langerin) is of specific adjunctive diagnostic value. In addition, S100 and CD68 can be positive. CD207-positive dendritic cells have a high affinity for bone, skin, lung and the pituitary gland. Electron microscopy was used to determine the presence of Birkbeck particles[17,18].
In this case, the pathology was consistent with the above presentation. Timely and appropriate assessment of disease status is crucial to effectively address relapsed or refractory LCH. Pathological specimens can be obtained by either excision or puncture, depending on the site and size of the lesion. When adequate tissue specimens are available, it is recommended to opt for complete resection biopsy that includes some normal tissue border, as this helps to reduce the incidence of localized infection within the lesion. Puncture biopsy should avoid areas of ulceration, erythema, and swelling to help reduce the incidence of local infection after biopsy.
LCH occurring in the vulva is rare and treatment options vary widely, with no uniform standard of care available. The prognosis of LCH is closely related to age of onset, number of organs involved, degree of functional impairment, and response to treatment. The basis of treatment is to recommend individualized treatment based on the site of involvement and risk stratification. In multisystem LCH, patients can be classified into a high-risk or low-risk group based on whether high-risk organs are affected. High-risk is defined as involvement of the liver, spleen and bone marrow[19].
Single lesions are largely curable with localized treatment, which includes surgical excision, radiotherapy, and topical medications such as corticosteroids and imiquimod[20]. For multiple foci and systems, single- or multi-agent combination chemotherapy is recommended, while other treatments include targeted therapy, hematopoietic stem cell transplantation and immunotherapy. Studies have shown limited efficacy of treatment regimens including vincristine, prednisone/cyclophosphamide and etoposide[21]. There are case reports of patients treated with cladribine combined with cytarabine for refractory LCH, but high doses of cytarabine can cause severe myelosuppression[22]. Thalidomide had been found to be effective for treatment of genital LCH, but the response was temporary and recurrence rates were high. In addition to teratogenesis, long-term use of thalidomide may lead to substantial neuropathy[8].
In our case, the lesions mainly involved the bilateral vulva and soft tissues of the neck with endocrine disease and did not involve the parenchymal organs. Our patient was treated with six courses of chemotherapy with vindesine, cyclophosphamide, dexamethasone and etoposide (COEP regimen), and the efficacy was evaluated as partial response, followed by continuation of maintenance therapy with vindesine and prednisone (VP regimen). The above chemotherapy regimens have not been reported in relapsed or multisystem LCH, with good efficacy and mild side effects, which can be promoted for clinical practice.
Vulvar LCH is uncommon and the differential diagnosis is broad. When patients present with nonspecific lesions in the vulva, the possibility of LCH should be considered and an accurate pathological diagnosis is necessary. Vulvar LCH may be a local manifestation of systemic disease. Radiological examination of other organs for involvement, especially with the use of PET/CT, can help guide individualized treatment of patients. For patients with extensive lesions or relapse, we recommend a hematology-based multidisciplinary diagnostic and treatment model with individualized application of drugs, targeted therapies, stem cell transplantation or immunotherapy. New combination chemotherapy regimens and maintenance chemotherapy may achieve better clinical outcomes. In cases where second-line therapy is required, BARF-V600E genetic testing or second-generation sequencing is recommended in combination with novel therapies such as targeted therapies to improve patient survival.
The authors thank the patient for her permission to publish this case report.
| 1. | Emile JF, Abla O, Fraitag S, Horne A, Haroche J, Donadieu J, Requena-Caballero L, Jordan MB, Abdel-Wahab O, Allen CE, Charlotte F, Diamond EL, Egeler RM, Fischer A, Herrera JG, Henter JI, Janku F, Merad M, Picarsic J, Rodriguez-Galindo C, Rollins BJ, Tazi A, Vassallo R, Weiss LM; Histiocyte Society. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 965] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 2. | Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, Kuo FC, Ligon AH, Stevenson KE, Kehoe SM, Garraway LA, Hahn WC, Meyerson M, Fleming MD, Rollins BJ. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 854] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 3. | El Demellawy D, Young JL, de Nanassy J, Chernetsova E, Nasr A. Langerhans cell histiocytosis: a comprehensive review. Pathology. 2015;47:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Goyal G, Shah MV, Hook CC, Wolanskyj AP, Call TG, Rech KL, Go RS. Adult disseminated Langerhans cell histiocytosis: incidence, racial disparities and long-term outcomes. Br J Haematol. 2018;182:579-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Su M, Gao YJ, Pan C, Chen J, Tang JY. Outcome of children with Langerhans cell histiocytosis and single-system involvement: A retrospective study at a single center in Shanghai, China. Pediatr Hematol Oncol. 2018;35:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Makras P, Stathi D, Yavropoulou M, Tsoli M, Kaltsas G. The annual incidence of Langerhans cell histiocytosis among adults living in Greece. Pediatr Blood Cancer. 2020;67:e28422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Sato A, Kobayashi M, Yusa N, Ogawa M, Shimizu E, Kawamata T, Yokoyama K, Ota Y, Ichinohe T, Ohno H, Mori Y, Sakaida E, Kondo T, Imoto S, Nannya Y, Mitani K, Tojo A. Clinical and prognostic features of Langerhans cell histiocytosis in adults. Cancer Sci. 2023;114:3687-3697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Santillan A, Montero AJ, Kavanagh JJ, Liu J, Ramirez PT. Vulvar Langerhans cell histiocytosis: a case report and review of the literature. Gynecol Oncol. 2003;91:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Martínez-Castillón D, Sanz-Cardiel A, Gilaberte-Calzada Y, Borderías-Clau L, Vera Álvarez J, Ramón Y Cajal JM. Langerhans cell Histiocytosis of the vulva. Rev Clin Esp (Barc). 2015;215:e5-e7. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Kobayashi M, Ando S, Kawamata T, Makiyama J, Yokoyama K, Imai Y, Tojo A. Clinical features and outcomes of adult Langerhans cell histiocytosis: a single-center experience. Int J Hematol. 2020;112:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Héritier S, Emile JF, Barkaoui MA, Thomas C, Fraitag S, Boudjemaa S, Renaud F, Moreau A, Peuchmaur M, Chassagne-Clément C, Dijoud F, Rigau V, Moshous D, Lambilliotte A, Mazingue F, Kebaili K, Miron J, Jeziorski E, Plat G, Aladjidi N, Ferster A, Pacquement H, Galambrun C, Brugières L, Leverger G, Mansuy L, Paillard C, Deville A, Armari-Alla C, Lutun A, Gillibert-Yvert M, Stephan JL, Cohen-Aubart F, Haroche J, Pellier I, Millot F, Lescoeur B, Gandemer V, Bodemer C, Lacave R, Hélias-Rodzewicz Z, Taly V, Geissmann F, Donadieu J. BRAF Mutation Correlates With High-Risk Langerhans Cell Histiocytosis and Increased Resistance to First-Line Therapy. J Clin Oncol. 2016;34:3023-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 234] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 12. | Stathi D, Yavropoulou MP, Allen CE, Abhyankar H, Scull B, Tsoli M, Andreakos E, Kaltsas G, Makras P. Prevalence of the BRAF (V600E) mutation in Greek adults with Langerhans cell histiocytosis. Pediatr Hematol Oncol. 2022;39:540-548. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Goyal G, Tazi A, Go RS, Rech KL, Picarsic JL, Vassallo R, Young JR, Cox CW, Van Laar J, Hermiston ML, Cao XX, Makras P, Kaltsas G, Haroche J, Collin M, McClain KL, Diamond EL, Girschikofsky M. International expert consensus recommendations for the diagnosis and treatment of Langerhans cell histiocytosis in adults. Blood. 2022;139:2601-2621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 123] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 14. | Gadner H, Grois N, Arico M, Broadbent V, Ceci A, Jakobson A, Komp D, Michaelis J, Nicholson S, Pötschger U, Pritchard J, Ladisch S; Histiocyte Society. A randomized trial of treatment for multisystem Langerhans' cell histiocytosis. J Pediatr. 2001;138:728-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 296] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Zudaire T, Guarch R, Valcayo A, García K, Resano MÁ, Requena D, Rodríguez M. Primary Langerhans Cell Histiocytosis of the Vulva: Case Report and Review of the Literature. Int J Gynecol Pathol. 2017;36:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Sirka CS, Sethy M, Rout AN, Sahu K. Langerhans cell histiocytosis of vulva and perineum. Indian J Dermatol Venereol Leprol. 2021;87:75-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Dietrich JE, Edwards C, Laucirica R, Kaufman RH. Langerhans cell histiocytosis of the vulva: two case reports. J Low Genit Tract Dis. 2004;8:147-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Kuhn I, Stephenson FH, Boyer HW, Greene PJ. Positive-selection vectors utilizing lethality of the EcoRI endonuclease. Gene. 1986;42:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 348] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 19. | Lai CC, Huang WC, Cheng SN. Successful treatment of refractory Langerhans cell histiocytosis by allogeneic peripheral blood stem cell transplantation. Pediatr Transplant. 2008;12:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Minkov M, Grois N, Heitger A, Pötschger U, Westermeier T, Gadner H. Treatment of multisystem Langerhans cell histiocytosis. Results of the DAL-HX 83 and DAL-HX 90 studies. DAL-HX Study Group. Klin Padiatr. 2000;212:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Duan MH, Han X, Li J, Zhang W, Zhu TN, Han B, Zhuang JL, Wang SJ, Cao XX, Cai HC, Chen M, Yang C, Zhou DB. Comparison of vindesine and prednisone and cyclophosphamide, etoposide, vindesine, and prednisone as first-line treatment for adult Langerhans cell histiocytosis: A single-center retrospective study. Leuk Res. 2016;42:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Donadieu J, Bernard F, van Noesel M, Barkaoui M, Bardet O, Mura R, Arico M, Piguet C, Gandemer V, Armari Alla C, Clausen N, Jeziorski E, Lambilliote A, Weitzman S, Henter JI, Van Den Bos C; Salvage Group of the Histiocyte Society. Cladribine and cytarabine in refractory multisystem Langerhans cell histiocytosis: results of an international phase 2 study. Blood. 2015;126:1415-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |