Published online Oct 6, 2024. doi: 10.12998/wjcc.v12.i28.6137
Revised: June 8, 2024
Accepted: July 3, 2024
Published online: October 6, 2024
Processing time: 158 Days and 1.6 Hours
Acute ischemic stroke is one of the leading causes of morbidity and mortality worldwide. Restoration of cerebral blood flow to affected ischemic areas has been the cornerstone of therapy for patients for eligible patients as early diagnosis and treatment have shown improved outcomes. However, there has been a paradigm shift in the management approach over the last decade, and with the emphasis currently directed toward including newer modalities such as neuroprotection, stem cell treatment, magnetic stimulation, anti-apoptotic drugs, delayed recanali
Core Tip: Acute ischemic stroke is becoming a more prevalent health concern as life expectancies increase. The fact that thrombolysis and thrombectomy within a very limited timeframe are the only definite therapies for this debilitating disease severely limits options. Extensive research is still ongoing and has shown promise in salvaging neurological function as well as extending the time window in which such therapies can be offered to stroke patients.
- Citation: Nag DS, Swain A, Sahu S, Sen B, Vatsala, Parween S. Stroke: Evolution of newer treatment modalities for acute ischemic stroke. World J Clin Cases 2024; 12(28): 6137-6147
- URL: https://www.wjgnet.com/2307-8960/full/v12/i28/6137.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i28.6137
Stroke can be defined as the rapid development of disturbances in the neurological function of brain due to hypoperfusion of cerebral tissue. The majority (85%) of acute stroke episodes are acute ischemic stroke (AIS) while around 15% of such episodes have a hemorrhagic cause[1]. Another easy way to classify etiology is the “rule of quarters’’ i.e., 25% cardiac emboli, 25% thromboembolic, 25% lacunar, and 25% due to other causes[2]. While there is still a lot of ongoing research into modalities that could improve outcomes in stroke, an important dictum that is still relevant is “Time is Brain, save the Penambra”[3]. Among the fewer established modalities of stroke management, intravascular thrombolysis (IVT) with tissue plasminogen activator (tPA/alteplase) is the only treatment approved by Food and Drug Administration (FDA) presently[4-7]. More recently, mechanical endovascular thrombectomy (EVT) of large vessel occlusion (LVO) has emerged as an effective emergency time-dependent treatment in selected population[8]. Neuroprotective strategies aimed at mitigating the secondary damage cascade following ischemic insult have taken a prominence. From neuroinflammation modulation to neuroregeneration promotion, novel pharmacology, and neurorehabilitation techniques are promising. Nonpharmacological approaches such as telestroke facility, artificial intelligence (AI) gene
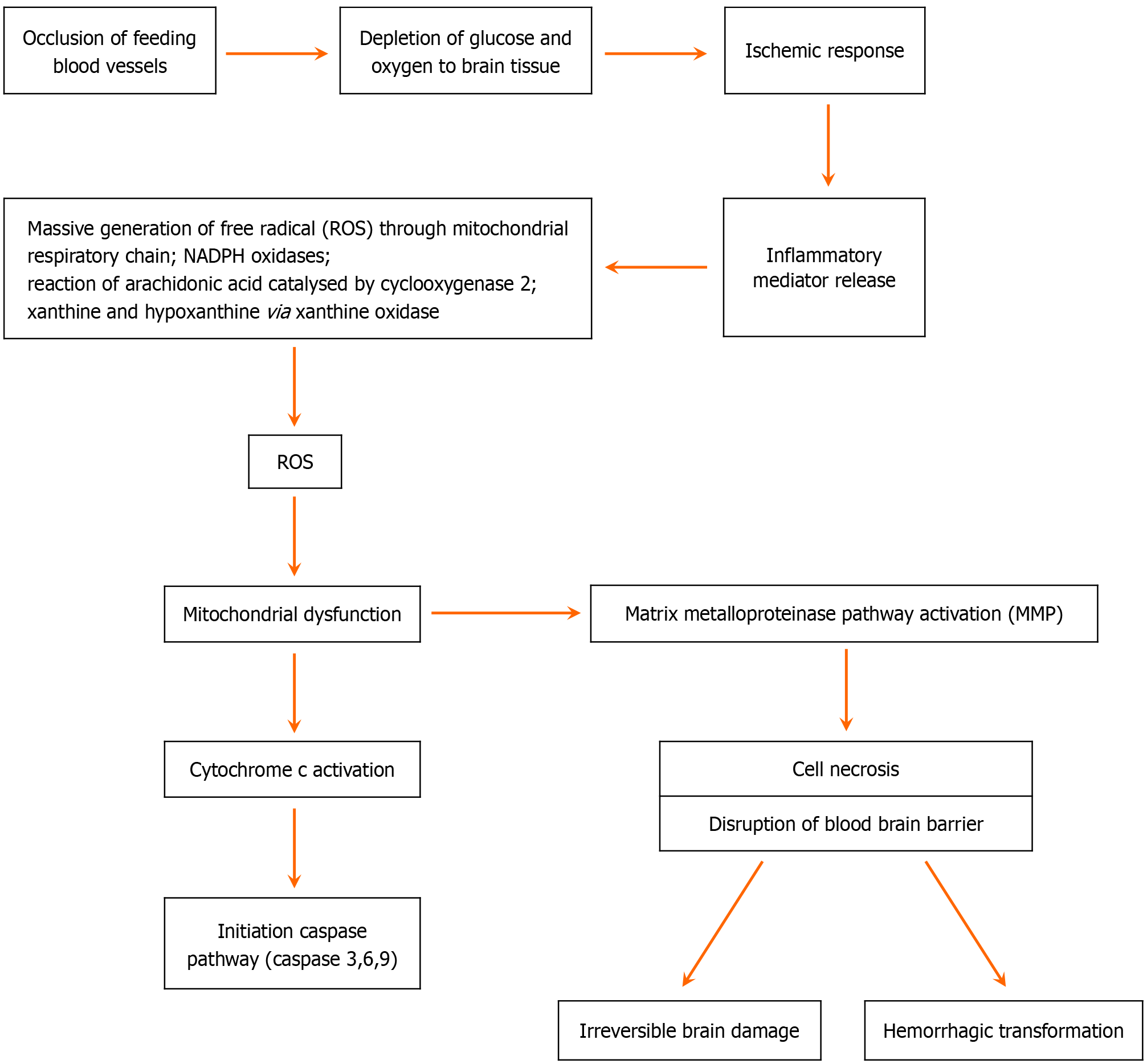
The primary pathophysiology for AIS is an acute onset reduction in the blood supply to the brain tissue and its consequent dysfunction. Advanced studies have uncovered that the molecular pathogenesis is much more complex. These molecular events are responsible for the acute and subacute complication as well as pathogenic sequel. With the onset of hypoperfusion, intrinsic and extrinsic inflammatory pathways get activated. Free radical mediated oxidative stress leads to mitochondrial damage which in turn results in apoptotic factor (cytochrome c)-mediated caspase (caspase-3, caspase-6, caspase-9) pathway activation, and cell death. Finally, the disruption of blood brain barrier leads to cerebral edema and hemorrhagic transformation (HT) (Figure 1)[9-10].
The medical management of acute stroke encompasses negotiating numerous challenges beginning from the prehospital area. Rapid transfer, early assessment, early brain imaging, and early intervention have been shown to help save the brain tissue and reduce the size of penumbra. The standard emergency medical services include performing routine airway, breathing, and circulation assessments; administering supplemental oxygen as needed; checking blood/capillary glucose and treating hypoglycemia. Concurrently, it is also important to perform a validated stroke severity scale examination such as Field Assessment Stroke Triage-Emergency Destination[11], Rapid Artery Occlusion Evaluation[12], Los Angeles Motor Scale[13], Cincinnati Prehospital Stroke Scale[14]. Ultimately, if the patient is manifesting physical signs of a stroke in the erectile dysfunction and satisfies the American Heart Association/American Stroke Association criteria for patient selection, then thrombolytics should be offered and administered[15-17]. The recent utilization of “tele stroke” networks (hub-and-spoke model) has helped in ameliorating the shortage of neurologists in rural areas and has demonstrated high rates of safe administration of thrombolytics while decreasing the time to initiate thrombolytics[18,19]. A recurring concern with stroke therapy is that while a lot of modalities have shown promise and have been or are being investigated for their efficaciousness, only time-dependent thrombolysis and thrombectomy have been found to improve outcome[4-7]. While antiplatelet therapy (aspirin) has been shown to be effective in patients with AIS, its use is controversial and fraught with ethical dilemmas in patients with hemorrhagic stroke. A recent editorial eloquently describes these concerns while elucidating on recent evidence showing benefit of aspirin in small volume intracranial hemorrhages[20].
Most of the treatment strategies for management of AIS are directed toward reopening the thrombosed vessels within a fixed time of the insult. Beyond a critical time, instead of preserving brain tissues, restoration of oxygen amplifies the destruction of an already deranged neurovascular and brain parenchymal environment. This topic has been a cause of scientific intrigue even before the evolution of thrombolytic therapies. Prolonged hypoperfusion and subsequent reperfusion cause free radical injury and disruption of blood brain barrier which leads to HT. In the Safe Implementation of Thrombolysis in Stroke-Monitoring Study: An observational study, HT was seen in up to 7.3% of ischemic stroke patients undergoing thrombolysis[21]. A much better insight into the epidemiology of thrombectomy-based reperfusion injury can be sourced from the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES) meta-analysis by Goyal et al[22], where HT was observed in 4.4% cases. In the recently concluded extended window endovascular trials, DEFUSE-3 and dawn, HT was reported in 7% and 6% cases respectively[23,24].
Targeting the inflammatory pathways and the relevant molecular cascade for managing reperfusion injury in pre-thrombectomy and post-thrombectomy period is coming into vogue in the treatment of stroke. It has been unequivocally demonstrated that the inactivation of a single molecule of the cascade can attenuate the inflammatory chain as well as the oxidative stress, thus halting its catastrophic sequel[3].
Reactive oxygen species (ROS) which are primarily blamed for cellular insult during ischemia and post-reperfusion consequences have attracted the attention of present-day researchers. More and more studies are aimed at understanding the molecular basis of preventing ROS formation and scavenging free radicals in an attempt to improve outcomes.
Hydrogen gas therapy: Hydrogen gas inhalation to mop up the ROS into free water has been researched in animal models, although this approach has not yet been explored in human participants[25]. A clinical trial has been tried at a small-scale involving infusion of superoxide dismutase with promising results, although large-scale trials are still awaited[26].
Nicotinamide adenine dinucleotide phosphate oxidase inhibitor: A Rho-kinase inhibitor aimed at inhibiting the nicotinamide adenine dinucleotide oxidase and further limiting ROS production has shown promise in rat models of ischemia/reperfusion injury, which is similar to apocynin[27]. Low-dose apocynin via oral route reaches the CNS parenchyma in a sufficient concentration to inhibit microglial oxidative stress. Although their usefulness has been explored in asthmatic patients, further research and human clinical trials are still warranted in human populations.
A new promising free radical scavenger: Free radicals activate the matrix metalloproteinase (MMP) pathways, which leads to collagen and laminin degradation including the breakdown of the blood brain barrier[10]. Sumii et al[28] found that MMPs participate in the tPA-associated hemorrhage progress. Additionally, tPA can upregulate MMPs (especially MMP-9) by the lipoprotein receptor signaling pathway and can reduce hemorrhagic volumes by using MMP inhibitors[28]. Edaravone is a low molecular weight lipophilic hydrophobic free radical scavenger which readily crosses the blood brain barrier. It prevents formation of hydroxyl, peroxyl, and super oxide anion radicals and retards lipid peroxidation by inhibiting MMPs in neuronal cell membrane[29-32]. Recent research has shown that edaravone can suppress delayed neuronal death, counteract microglia-induced neurotoxicity, and reduce the long-term inflammation. It has been suggested that it may prevent the development of edema following a stroke by inhibiting astrocyte production of expression of vascular endothelial growth factor[33]. Side effects include deranged liver function, renal toxicity, thrombocytopenia, lymphoma, and pneumonia, although the incidence is very low. Larger scale human trials will help study the toxicity profile of this drug as well as establish its effects on outcome.
Sphingosine 1-phosphate receptor modulator: Fingolimod is an immunosuppressant approved by FDA for multiple sclerosis[34]. It downregulates the Sphingosine 1-phosphate receptor in T lymphocyte which prevents egress of lymphocyte into inflamed tissue. Synergistically, it also acts on glycoprotein receptors located on platelets and prevents platelet aggregation and further thrombus formation[34]. Combination use of fingolimod along with thrombolytic agents has resulted in promising outcomes[34]. When used along with tPA, fingolimod demonstrated a reduced microvascular permeability and attenuated the risk of hemorrhagic complications in ischemic stroke patients. Clinical trials have been done in assessing the efficacy of a combination of fingolimod with reperfusion therapies in attenuating reperfusion injury in patients with LVO treated within 6 hours of stroke onset[35]. Given that tPA exacerbates blood brain barrier breakdown after brain ischemia, it is plausible that fingolimod could prevent damaging effects of tPA on the blood brain barrier. Further studies are warranted in elucidating the complex effects which fingolimod seems to have on the brain vasculature following ischemic stroke[36].
Oxidative damage to mitochondria causes cytochrome c-mediated caspase activation leading to apoptotic cell death after hypoperfusion and reperfusion period. Impact of caspase activity is not only on neurodegeneration through inflammatory activity, but it has also been shown to diminish glial cell activity. Death receptor blockage, genetic manipulation, and catalytic inhibition are the three major strategies to prevent caspase chain in stroke[37]. VX-740 (pralnacasan) and VX-765 (belnacasan) are two analog peptidomimetic inhibitors of caspase 1[38]. Animal trials have demonstrated a hindrance of drug delivery to specific ischemic area due to blood brain barrier. Further human studies at a larger scale will hopefully elucidate the exact mechanisms.
Metformin combination treatment for 2 weeks has shown improved neurological outcomes [Mini-Mental State Examination, National Institutes of Health Stroke Scale (NIHSS), and activities of daily living scores] and reduced oxidative stress (increased glutathione peroxidase and superoxide dismutase) in patients with stroke[39]. Uric acid in combination with intravenous thrombolysis and mechanical thrombectomy improved modified Rankin Scale (mRS) 0–2 at 90 days and reduced mortality[40]. These two neuroprotective treatments have demonstrated good results in human studies[39,40].
Activated protein C (APC), a protease with anticoagulant and cytoprotective activities, protects neurons and cere
Studies with intravenous glyburide have shown improvement in midline shift, MMP9 levels, NIHSS, and fewer deaths attributed to edema in cases of ischemic stroke[42].
Artificial intelligence: Artificial intelligence (AI) seems to be the most evolving and promising technology which can change the stroke treatment scenario. AI is a generic term which refers to the simulation of thought processes by computer systems, enabling them to perform tasks which typically require human intelligence, such as understanding natural language, recognizing patterns, solving problems, and making decisions[43]. With established treatment protocols, the main hindrance in the rush to save the brain has been due to delayed prehospital assessment, imaging, and in selecting the patient for treatment (IVT/EVT). AI has hastened the prehospital assessment of stroke patients by facilitating early emergent imaging of such patients in the ambulance leading to early diagnosis and prompt selection for further treatment and by promptly alerting the stroke center as well. While traditionally radiologists summarize an image with a few key descriptors (e.g., hemorrhage volume), machine learning (ML) algorithms employed by AI attempt to do the same with a matrix of voxels in fraction of time[44]. A matter of concern with ML algorithms is the lack of clarity on decision-making process. This is particularly true of the most popular deep neural network approaches currently in use and is termed the “black box problem”[45].
Repetitive transcranial magnetic stimulation: Repetitive transcranial magnetic stimulation can improve the functional recovery after stroke by modulating cortical excitability and recovering the balance between the brain hemispheres, something which is disrupted by stroke and the leading cause of motor impairment. It can be considered as a feasible nonpharmacological tool in assisting the neurorehabilitation of different motor and nonmotor clinical manifestations of stroke[46].
Stem cell therapy: Stem cell therapy for stroke offers promise because of its potential in providing neurorestorative benefits. Stem cell-based therapies aim to promote neurogenesis and replacement of lost neurons or protect surviving neurons in order to improve neurological recovery depending on the route of administration and source of stem cell retrieval (umbilical cord, neural, bone marrow, mesenchymal)[47]. As results of the phase three clinical trial, Pisces III, have demonstrated, intracerebral neural stem cell transplants can be explored as an avenue for neuroregeneration through direct replacement of lost neurons, and when compared to other stem cell therapies, may provide the longest window for therapeutic intervention[48].
Intracerebral implantation by stereotactic injection to the putamen ipsilateral to the cerebral infarct of allogeneic human neural stem cell line CTX0E03 in multicenter study which has shown significant improvement in upper limb function occurred at 3 months, 6 months, and 12 months, but not in those with absent upper limb movement at baseline[49].
Nano technology for targeted drug delivery: Even after successful recanalization, ischemia-reperfusion injury represents an additional challenge. The use of tPA may further disrupt the blood brain barrier integrity and is neurotoxic, aggravating reperfusion injury. Nanoparticle-based approaches have the potential to circumvent the aforementioned issues and develop a thrombolytic agent which can be administered safely beyond the time window for tPA treatment. Nanoparticle-containing imaging particles, thrombolytic agents, antioxidants, neuroprotective drugs, and target ligands have all shown improved outcomes in experimental animals. Nanoparticle-containing magnetic particles with thrombolytic agents have been studied for loosening of clot by sono-stimulation and magnetic stimulation[50]. It must also be recognized that most preclinical studies are carried out in otherwise healthy animals, and patients suffering from stroke may in turn have reduced cerebrovascular reserve or reduced benefits from protective agents due to underlying pathologies or aging[51]. Research in this area is ongoing, and while there is significant promise, we are far from clinical applications. Nonetheless, nanotechnology represents a frontier in stroke management which could revolutionize treatment and improve outcomes for patients.
The role of neuroprotection: Neuroprotective agents can act as an adjunct to iv thrombolysis in reducing the rapidly progressing insult of the ischemic penumbra, which improves the outcome of stroke[52]. Neuroprotection research has found that testing works in animal models, but the outcomes in clinical trials have been disappointing. Patients have showed promising improvement in the functional outcome of patients with AIS involving middle cerebral artery (MCA) territory at 90 days receiving citicoline, edaravone, and cerebrolysin. Other therapies such as transcranial direct current stimulation after thrombectomy (TESSERACT-BA), low-frequency pulsed electromagnetic fields (ELF-MF), Rheo-erythrocrine dysfunction as a biomarker for reduced-intensity conditioning treatment, regional hypothermia in combination with endovascular thrombectomy, stem cell-based treatment modalities, glibenclamide combined with recombinant tPA are being explored.
Nerinetide: Nerinetide is a neuroprotective eicosapeptide which inhibits the interaction of N-methyl-D-aspartate receptor/post-synaptic density protein 95, preventing the neurotoxic signaling of neuronal nitric oxide synthase in AIS[53]. It has proven to be efficacious in animal models, mainly in primates, to protect nerve tissue and function against schemia/reperfusion injury. A randomized, double-blind, placebo-controlled phase 2 trial in patients with iatrogenic stroke after endovascular aneurysm repair showed that this drug is safe and efficacious in reducing the infarct volume in the brain[53].
The efficacy and safety of nerinetide for the treatment of acute ischemic stroke study was undertaken according to the positive results of various preclinical and clinical trials[54]. While the overall result of this phase III trial was disappointing and failed to develop a new effective drug for AIS, it still brings hope that developing a neuroprotective drug for AIS is possible. The trial also found that there was a treatment effect of nerinetide in patients without the usual care of thrombolytic therapy with alteplase[54]. The result of the trial encourages further development of other neuroprotective drugs.
HERMES Trials study is the time sensitivity of the treatment. The benefits of mechanical thrombectomy diminish beyond 6 hours[55]. Patients with poor collaterals, described as “fast progressors,” are found to be highly dependent on the timely administration of reperfusion therapy. In contrast, those with good collaterals, labeled as “slow progressors,” show less sensitivity to the time factor and may still benefit from embolectomy even beyond the conventional time windows[55]. The role of collaterals in determining the progression of irreversibly damaged brain tissue (ischemic core) at the expense of the penumbra, but still viable brain which could undergo infarction if vessel occlusion persists. Patients with good collaterals may present with substantial mismatch between the infarct volume and the total volume of critically hypoperfused brain, indicating a potential for benefiting from reperfusion therapy beyond 6 hours[55].
Novel therapeutic applications are made possible by recent interventional techniques. Combining balloon techniques with mechanical thrombectomy or direct aspiration can enhance the local effectiveness of intra-arterial therapies[56]. This approach may temporarily improve bioavailability, delaying washout and systemic dilution, particularly beneficial for therapies targeting the blood brain barrier and endothelium. This could enhance the efficacy of therapeutics limited by systemic dosing or reduced effectiveness during normal arterial perfusion[56]. Intra-arterial administration of thera
In cases of MCA occlusions, studies indicate that recanalization occurring beyond 24 hours up to 60 days since the stroke onset has shown improved outcomes[57,58]. For basilar artery occlusions, reperfusion at 36 hours, 50 hours, and beyond 2 days led to fully restored neurologic function, complete functional recovery, and 77% achieving a mRS of 0–3, respectively[59]. In internal carotid artery occlusions, recanalization from 1 month up to 27 months resulted in favorable outcomes, including some patients achieving full recovery[60,61]. Basic science research, particularly in MCAO rat models, demonstrates that delayed recanalization administered at 3 days, 7 days, and 14 days following stroke onset can improve neurological function and even reduce infarct volume when compared to permanent MCAO groups[62-65].
With the advancement of medical science, as millions of people are heading toward the age where nearly three quarters of all strokes occur (> 65 years old), the world seems to be careening toward a major stroke crisis. Currently, there is no gold standard therapy for this impending public health disaster. Table 1 summarizes the existing and novel treatment modalities in various stages of evolution of AIS (Table 1)[34,35,37-43,48-50,66-71]. To date, an absolute treatment is an unmet need. Prehospital interventions showing promise include lifestyle modification and stroke protocol. This also includes imaging and patient selection for specific treatments progressing to in-hospital management, which includes thrombolysis. The main concern is time for neuroprotection. The search is still on in finding the magic bullet for stroke. Innovative treatments hold the key in unlocking brighter prospects for stroke patients, which ushers in an era of hope, resilience, and renewed possibilities in the journey toward recovery.
| Ref. | Basis | Advantage | Disadvantage |
| Established treatment modalities[66] | |||
| Alteplase, reteplasea, and tenecteplase | Thrombolytic (fibrin)-convert plasminogen to plasmin directly and within 4.5 hours | FDA approved treatment modality. Good outcome when treated within specified time window. Incomplete recanalization | Disrupts blood brain barrier. Chances of hemorrhagic transformation. Reperfusion injury and brain swelling |
| Streptokinase and staphylokinase | Thrombolytic (non-fibrin activators) | Same as above | Same as above |
| Ancrod | Defibrinogenating agent derived from snake venom | Favourable functional recovery. Lower prevalence of intracerebral hemorrhage | Potential risk of bleeding |
| Endovascular therapy | Overall outcome of acute ischemic stroke patients with proximal middle cerebral artery or internal carotid artery occlusion was improved when endovascular thrombectomy was performed within either 6 hours, 8 hours, or 12 hours of symptom onset | FDA approved invasive treatment modality. Promising result. Less chances of disrupting blood brain barrier. Less chances of haemorrhagic transformation | Applicable in anatomically more proximal large vessel occlusion only not for small vessels. Needs expertise. Done in selective centres only. Reperfusion injury. Downstream embolization |
| Nimodipine[67] | Second-generation 1,4-dihydropyridine calcium channel blocker. Neuroprotective effect by preventing calcium overload in ischemic neurons | Useful in aneurysmal SAH. Can also be administered through nasogastric tube | Risk of hypotension. Drug interactions need consideration |
| Early drain (outcome after early lumbar cerebrospinal fluid-drainage in aneurysmal SAH)[68] | Lumber drainage vs external ventricular drain. Erythrocyte sedimentation by weight and potentially easier removal by lipid droplets | Associated with benefits in relation to rates of vasospasm, secondary cerebral infarctions, and mortality, without an increased risk of adverse events | Needs further trials. Limited to aneurysmal SAH cases only |
| Antiseizure medications | For 7 days in patients with seizures but without prior epilepsy | Prevents post stroke epilepsy | Needs further research |
| Newer modalities | |||
| Hydrogen gas therapy[69] | Mop up the reactive oxygen species into free water. Buffer the effects of oxidative stress | Basic study on neutralizing free oxygen radical and reducing oxidative stress | Researched in animal model but yet to be exploded in human |
| Nicotinamide adenine dinucleotide phosphate oxidase inhibitor[70] | Rho-kinase inhibitor inhibits the nicotinamide adenine dinucleotide oxidase and limits reactive oxygen species production | Reduces oxidative stress thus delayed neuronal death | Researched in rat models of ischemia/reperfusion injury. Studied in asthmatic pt not in stroke pt |
| Edaravone (matrix metalloproteinase inhibitor) | Scavenges free radical by preventing matrix metalloproteinase in neuronal cell membrane | Prevent blood brain barrier leakage. Reduces brain edema. Attenuate microglia induced neuronal death | Further research is needed. Side effects are lymphoma, deranged lung function test, renal toxicity, thrombocytopenia, pneumonia |
| Fingolipod[34,35,71] | S1P1 receptor modulator. It downregulates the S1P1 receptor in T lymphocyte which prevents egress of lymphocyte to inflamed tissue. Acts synergistically on glycoprotein receptor on platelet and prevent platelet aggregation and further thrombus formation | More effective after receiving tPA treatment by reducing haemorrhagic transformation (fingolimod plus alteplase < 6 hours from last known well) in human study. Also attenuates blood brain barrier leakage and reduces infarct volume, reduced blood lymphocyte counts, and improved mRS at day 90 | Ineffective in thrombocytopenic pt and patients on antiplatelet therapy. More human study needed. Small trials |
| Caspase Inhibitors[37,38] | Cytochrome c mediated caspase activation which leads to apoptotic cell death after hypoperfusion and reperfusion period VX-740 (pralnacasan) and VX-765 (belnacasan) are two analogue peptidomimetic inhibitors of caspase 1 | Protect blood brain barrier integrity. Post-experimental mice are healthy and fertile | Targeted drug delivery is a major hindrance for blood brain barrier. Liver toxicity in long term use. Very few clinical trials in animal |
| Uric Acid[40] | Uric acid in combination with tPA has good stroke outcome by its free radical scavenging property | Uric acid in combination to intravenous thrombolysis and mechanical thrombectomy reduced mortality (8%–4%), good result in human study | More trials are needed |
| Metformin therapy[39] | Metformin acts by adenosine monophosphate activated protein kinase activation in brain tissue and also by reducing oxidative stress | Post-stroke chronic treatment enhances long-term functional recovery and brain repair within clinically relevant therapeutic windows | Very narrow therapeutic window in stroke pt. Time of administration is also an issue. Needs more studies |
| 3K3A[41] | APC, a protease with anticoagulant and cytoprotective activities. Protects neurons and cerebrovascular endothelium from ischemic injury. Newer recombinant 3K3A-APC has been studied with less anticoagulant effect and more neuroprotective activity | Though rAPC bleeding is an expected pharmacological side effect, there was no other systemic side effect noted in experimental animals and that is a strong support for proposal for human study | Recombinant APC, drotrecogin alfa (activated) was approved by the FDA for the treatment of sepsis but has dose limited chance of bleeding. Still in preclinical trial for stroke (used in severe sepsis which is FDA approved) |
| Iv glyburide[42] | Acts by blocking sulfonylurea receptor 1-transient receptor potential melastatin 4 chnnel | Prevent midline shift by reducing penumbral edema | Results of human studies are promising |
| AI[43] | AI has changed prehospital assessment by imaging in ambulance, diagnosing, selecting patient for further treatment | Massive impact in golden hours in form of prompt imaging, diagnosis and treatment. Cost effective. Time saving. Can be started treatment during prehospital transit | Necessity for high quality data, “black box problem” i.e. machine needs continuous upgradation with newer data for reasoning and accuracy |
| Stem cell therapy[48,49] | Neurorestorative benefits. Three types are in study BMSC, NSC and umbilical cord blood stem cell | NSC has robust safety margin but pending further trial. MSC and BMSC has also immunomodulatory property to attenuate poststroke inflammation along with neuro regenerative property | BMSC treatment of stroke has proven safe, but otherwise ineffective in clinical trials. All are in preclinical trial stage |
| Nano technology for targeted drug deliver[50] | Nano particle containing magnetic particle with thrombolytic agent has been studied for loosening of clot by sono stimulation and magnetic stimulation | More powerful effect. Longer half life. Increased clot penetration. Making the thrombolytic agent safe. Precise delivery confirms more detail delineation of image | Studies have been conducted in otherwise healthy animal but patients suffering from stroke may have reduced cerebral reserve |
| 1. | Musuka TD, Wilton SB, Traboulsi M, Hill MD. Diagnosis and management of acute ischemic stroke: speed is critical. CMAJ. 2015;187:887-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 2. | Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58:688-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 463] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 3. | Widimsky P, Snyder K, Sulzenko J, Hopkins LN, Stetkarova I. Acute ischaemic stroke: recent advances in reperfusion treatment. Eur Heart J. 2023;44:1205-1215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 4. | Lapchak PA. Development of thrombolytic therapy for stroke: a perspective. Expert Opin Investig Drugs. 2002;11:1623-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Schellinger PD, Fiebach JB, Mohr A, Ringleb PA, Jansen O, Hacke W. Thrombolytic therapy for ischemic stroke--a review. Part II--Intra-arterial thrombolysis, vertebrobasilar stroke, phase IV trials, and stroke imaging. Crit Care Med. 2001;29:1819-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Schellinger PD, Fiebach JB, Mohr A, Ringleb PA, Jansen O, Hacke W. Thrombolytic therapy for ischemic stroke--a review. Part I--Intravenous thrombolysis. Crit Care Med. 2001;29:1812-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Verstraete M. Newer thrombolytic agents. Ann Acad Med Singap. 1999;28:424-433. [PubMed] |
| 8. | Hassan AE, Chaudhry SA, Grigoryan M, Tekle WG, Qureshi AI. National trends in utilization and outcomes of endovascular treatment of acute ischemic stroke patients in the mechanical thrombectomy era. Stroke. 2012;43:3012-3017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Margaill I, Plotkine M, Lerouet D. Antioxidant strategies in the treatment of stroke. Free Radic Biol Med. 2005;39:429-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 10. | Miller AA, Drummond GR, De Silva TM, Mast AE, Hickey H, Williams JP, Broughton BR, Sobey CG. NADPH oxidase activity is higher in cerebral versus systemic arteries of four animal species: role of Nox2. Am J Physiol Heart Circ Physiol. 2009;296:H220-H225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Lima FO, Silva GS, Furie KL, Frankel MR, Lev MH, Camargo ÉC, Haussen DC, Singhal AB, Koroshetz WJ, Smith WS, Nogueira RG. Field Assessment Stroke Triage for Emergency Destination: A Simple and Accurate Prehospital Scale to Detect Large Vessel Occlusion Strokes. Stroke. 2016;47:1997-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 12. | Pérez de la Ossa N, Carrera D, Gorchs M, Querol M, Millán M, Gomis M, Dorado L, López-Cancio E, Hernández-Pérez M, Chicharro V, Escalada X, Jiménez X, Dávalos A. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 332] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 13. | Kim JT, Chung PW, Starkman S, Sanossian N, Stratton SJ, Eckstein M, Pratt FD, Conwit R, Liebeskind DS, Sharma L, Restrepo L, Tenser MK, Valdes-Sueiras M, Gornbein J, Hamilton S, Saver JL; FAST-MAG Trial (Field Administration of Stroke Therapy–Magnesium) Nurse-Coordinators and Investigators. Field Validation of the Los Angeles Motor Scale as a Tool for Paramedic Assessment of Stroke Severity. Stroke. 2017;48:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Kothari RU, Pancioli A, Liu T, Brott T, Broderick J. Cincinnati Prehospital Stroke Scale: reproducibility and validity. Ann Emerg Med. 1999;33:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 411] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146-e603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6484] [Cited by in RCA: 6378] [Article Influence: 797.3] [Reference Citation Analysis (0)] |
| 16. | Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344-e418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 4089] [Article Influence: 681.5] [Reference Citation Analysis (0)] |
| 17. | Demaerschalk BM, Kleindorfer DO, Adeoye OM, Demchuk AM, Fugate JE, Grotta JC, Khalessi AA, Levy EI, Palesch YY, Prabhakaran S, Saposnik G, Saver JL, Smith EE; American Heart Association Stroke Council and Council on Epidemiology and Prevention. Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016;47:581-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 481] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 18. | Demaerschalk BM, Berg J, Chong BW, Gross H, Nystrom K, Adeoye O, Schwamm L, Wechsler L, Whitchurch S. American Telemedicine Association: Telestroke Guidelines. Telemed J E Health. 2017;23:376-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Schwamm LH, Chumbler N, Brown E, Fonarow GC, Berube D, Nystrom K, Suter R, Zavala M, Polsky D, Radhakrishnan K, Lacktman N, Horton K, Malcarney MB, Halamka J, Tiner AC; American Heart Association Advocacy Coordinating Committee. Recommendations for the Implementation of Telehealth in Cardiovascular and Stroke Care: A Policy Statement From the American Heart Association. Circulation. 2017;135:e24-e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 20. | Buddhavarapu V, Kashyap R, Surani S. Early antiplatelet therapy used for acute ischemic stroke and intracranial hemorrhage. World J Clin Cases. 2024;12:677-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (3)] |
| 21. | Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, Lees KR, Roine RO, Soinne L, Toni D, Vanhooren G; SITS-MOST investigators. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1562] [Cited by in RCA: 1661] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 22. | Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millán M, Davis SM, Roy D, Thornton J, Román LS, Ribó M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4230] [Cited by in RCA: 5370] [Article Influence: 596.7] [Reference Citation Analysis (0)] |
| 23. | Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG; DAWN Trial Investigators. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3099] [Cited by in RCA: 3782] [Article Influence: 540.3] [Reference Citation Analysis (0)] |
| 24. | Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG; DEFUSE 3 Investigators. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2796] [Cited by in RCA: 3398] [Article Influence: 485.4] [Reference Citation Analysis (0)] |
| 25. | Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1678] [Article Influence: 93.2] [Reference Citation Analysis (1)] |
| 26. | Marzi I, Bühren V, Schüttler A, Trentz O. Value of superoxide dismutase for prevention of multiple organ failure after multiple trauma. J Trauma. 1993;35:110-9; discussion 119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006;1090:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 302] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 29. | Higashi Y. Edaravone for the treatment of acute cerebral infarction: role of endothelium-derived nitric oxide and oxidative stress. Expert Opin Pharmacother. 2009;10:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Kono H, Woods CG, Maki A, Connor HD, Mason RP, Rusyn I, Fujii H. Electron spin resonance and spin trapping technique provide direct evidence that edaravone prevents acute ischemia-reperfusion injury of the liver by limiting free radical-mediated tissue damage. Free Radic Res. 2006;40:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Banno M, Mizuno T, Kato H, Zhang G, Kawanokuchi J, Wang J, Kuno R, Jin S, Takeuchi H, Suzumura A. The radical scavenger edaravone prevents oxidative neurotoxicity induced by peroxynitrite and activated microglia. Neuropharmacology. 2005;48:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Shichinohe H, Kuroda S, Yasuda H, Ishikawa T, Iwai M, Horiuchi M, Iwasaki Y. Neuroprotective effects of the free radical scavenger Edaravone (MCI-186) in mice permanent focal brain ischemia. Brain Res. 2004;1029:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Ishikawa A, Yoshida H, Metoki N, Toki T, Imaizumi T, Matsumiya T, Yamashita K, Taima K, Satoh K. Edaravone inhibits the expression of vascular endothelial growth factor in human astrocytes exposed to hypoxia. Neurosci Res. 2007;59:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, Dong Y, Xu X, Liu Q, Huang D, Shi FD. Combination of the Immune Modulator Fingolimod With Alteplase in Acute Ischemic Stroke: A Pilot Trial. Circulation. 2015;132:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 35. | Zhang S, Zhou Y, Zhang R, Zhang M, Campbell B, Lin L, Shi FD, Lou M. Rationale and design of combination of an immune modulator Fingolimod with Alteplase bridging with Mechanical Thrombectomy in Acute Ischemic Stroke (FAMTAIS) trial. Int J Stroke. 2017;12:906-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Salas-Perdomo A, Miró-Mur F, Gallizioli M, Brait VH, Justicia C, Meissner A, Urra X, Chamorro A, Planas AM. Role of the S1P pathway and inhibition by fingolimod in preventing hemorrhagic transformation after stroke. Sci Rep. 2019;9:8309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Akpan N, Troy CM. Caspase inhibitors: prospective therapies for stroke. Neuroscientist. 2013;19:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Ye X, Song G, Huang S, Liang Q, Fang Y, Lian L, Zhu S. Caspase-1: A Promising Target for Preserving Blood-Brain Barrier Integrity in Acute Stroke. Front Mol Neurosci. 2022;15:856372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Zhao M, Li XW, Chen Z, Hao F, Tao SX, Yu HY, Cheng R, Liu H. Neuro-Protective Role of Metformin in Patients with Acute Stroke and Type 2 Diabetes Mellitus via AMPK/Mammalian Target of Rapamycin (mTOR) Signaling Pathway and Oxidative Stress. Med Sci Monit. 2019;25:2186-2194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 40. | Chamorro Á, Amaro S, Castellanos M, Gomis M, Urra X, Blasco J, Arenillas JF, Román LS, Muñoz R, Macho J, Cánovas D, Marti-Fabregas J, Leira EC, Planas AM; URICO-ICTUS Investigators. Uric acid therapy improves the outcomes of stroke patients treated with intravenous tissue plasminogen activator and mechanical thrombectomy. Int J Stroke. 2017;12:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Williams PD, Zlokovic BV, Griffin JH, Pryor KE, Davis TP. Preclinical safety and pharmacokinetic profile of 3K3A-APC, a novel, modified activated protein C for ischemic stroke. Curr Pharm Des. 2012;18:4215-4222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Sheth KN, Simard JM, Elm J, Kronenberg G, Kunte H, Kimberly WT. Human Data Supporting Glyburide in Ischemic Stroke. Acta Neurochir Suppl. 2016;121:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Dresser LP, Kohn MA. Artificial Intelligence and the Evaluation and Treatment of Stroke. Dela J Public Health. 2023;9:82-84. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 44. | Soun JE, Chow DS, Nagamine M, Takhtawala RS, Filippi CG, Yu W, Chang PD. Artificial Intelligence and Acute Stroke Imaging. AJNR Am J Neuroradiol. 2021;42:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 45. | O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 1044] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 46. | Fisicaro F, Lanza G, Grasso AA, Pennisi G, Bella R, Paulus W, Pennisi M. Repetitive transcranial magnetic stimulation in stroke rehabilitation: review of the current evidence and pitfalls. Ther Adv Neurol Disord. 2019;12:1756286419878317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 47. | Chrostek MR, Fellows EG, Crane AT, Grande AW, Low WC. Efficacy of stem cell-based therapies for stroke. Brain Res. 2019;1722:146362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 48. | Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, Muir KW. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388:787-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 307] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 49. | Muir KW, Bulters D, Willmot M, Sprigg N, Dixit A, Ward N, Tyrrell P, Majid A, Dunn L, Bath P, Howell J, Stroemer P, Pollock K, Sinden J. Intracerebral implantation of human neural stem cells and motor recovery after stroke: multicentre prospective single-arm study (PISCES-2). J Neurol Neurosurg Psychiatry. 2020;91:396-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 50. | Hagisawa K, Nishioka T, Suzuki R, Maruyama K, Takase B, Ishihara M, Kurita A, Yoshimoto N, Nishida Y, Iida K, Luo H, Siegel RJ. Thrombus-targeted perfluorocarbon-containing liposomal bubbles for enhancement of ultrasonic thrombolysis: in vitro and in vivo study. J Thromb Haemost. 2013;11:1565-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Toljan K, Ashok A, Labhasetwar V, Hussain MS. Nanotechnology in Stroke: New Trails with Smaller Scales. Biomedicines. 2023;11:780. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 52. | Ghozy S, Reda A, Varney J, Elhawary AS, Shah J, Murry K, Sobeeh MG, Nayak SS, Azzam AY, Brinjikji W, Kadirvel R, Kallmes DF. Neuroprotection in Acute Ischemic Stroke: A Battle Against the Biology of Nature. Front Neurol. 2022;13:870141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 53. | Hill MD, Martin RH, Mikulis D, Wong JH, Silver FL, Terbrugge KG, Milot G, Clark WM, Macdonald RL, Kelly ME, Boulton M, Fleetwood I, McDougall C, Gunnarsson T, Chow M, Lum C, Dodd R, Poublanc J, Krings T, Demchuk AM, Goyal M, Anderson R, Bishop J, Garman D, Tymianski M; ENACT trial investigators. Safety and efficacy of NA-1 in patients with iatrogenic stroke after endovascular aneurysm repair (ENACT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2012;11:942-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 312] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 54. | Zhou XF. ESCAPE-NA1 Trial Brings Hope of Neuroprotective Drugs for Acute Ischemic Stroke: Highlights of the Phase 3 Clinical Trial on Nerinetide. Neurosci Bull. 2021;37:579-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Jovin TG, Saver JL, Ribo M, Pereira V, Furlan A, Bonafe A, Baxter B, Gupta R, Lopes D, Jansen O, Smith W, Gress D, Hetts S, Lewis RJ, Shields R, Berry SM, Graves TL, Malisch T, Rai A, Sheth KN, Liebeskind DS, Nogueira RG. Diffusion-weighted imaging or computerized tomography perfusion assessment with clinical mismatch in the triage of wake up and late presenting strokes undergoing neurointervention with Trevo (DAWN) trial methods. Int J Stroke. 2017;12:641-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 56. | Matei N, Camara J, Zhang JH. The Next Step in the Treatment of Stroke. Front Neurol. 2020;11:582605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 57. | Zheng M, Song Y, Zhang J, Zhao W, Sun L, Yin H, Zhang J, Wang W, Han J. Endovascular Recanalization of Non-acute Symptomatic Middle Cerebral Artery Total Occlusion and Its Short-Term Outcomes. Front Neurol. 2019;10:484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Ma N, Mo DP, Gao F, Miao ZR. Endovascular recanalization for chronic symptomatic middle cerebral artery total occlusion. J Neurointerv Surg. 2013;5:e15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Cognard C, Weill A, Lindgren S, Piotin M, Castaings L, Moret J. Basilar artery occlusion in a child: "clot angioplasty" followed by thrombolysis. Childs Nerv Syst. 2000;16:496-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Grigoriadis S, Gomori JM, Grigoriadis N, Cohen JE. Clinically successful late recanalization of basilar artery occlusion in childhood: what are the odds? Case report and review of the literature. J Neurol Sci. 2007;260:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Zhao W, Zhang J, Song Y, Sun L, Zheng M, Yin H, Zhang J, Wang W, Han J. Endovascular Recanalization for Symptomatic Subacute to Chronic Atherosclerotic Basilar Artery Occlusion. Front Neurol. 2019;10:1290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Shojima M, Nemoto S, Morita A, Miyata T, Namba K, Tanaka Y, Watanabe E. Protected endovascular revascularization of subacute and chronic total occlusion of the internal carotid artery. AJNR Am J Neuroradiol. 2010;31:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 63. | Namba K, Shojima M, Nemoto S. Wire-probing technique to revascularize subacute or chronic internal carotid artery occlusion. Interv Neuroradiol. 2012;18:288-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Rostambeigi N, Khatri R, Hassan AE, Qureshi AI. Duplex ultrasound assisted endovascular revascularization of chronic internal carotid artery occlusion: technical note. J Vasc Interv Neurol. 2013;6:42-46. [PubMed] |
| 65. | Fan YL, Wan JQ, Zhou ZW, Chen L, Wang Y, Yao Q, Jiang JY. Neurocognitive improvement after carotid artery stenting in patients with chronic internal carotid artery occlusion: a prospective, controlled, single-center study. Vasc Endovascular Surg. 2014;48:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 66. | Kuriakose D, Xiao Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int J Mol Sci. 2020;21:7609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 445] [Cited by in RCA: 604] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 67. | Das JM, Zito PM. Nimodipine. [Updated 2024 May 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534870/. |
| 68. | Jha RM, Sheth KN. 2023 Neurocritical Care Updates in Cerebrovascular Disease. Stroke. 2023;54:2671-2675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 69. | Wood KC, Gladwin MT. The hydrogen highway to reperfusion therapy. Nat Med. 2007;13:673-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 70. | Wang Q, Sun AY, Simonyi A, Kalogeris TJ, Miller DK, Sun GY, Korthuis RJ. Ethanol preconditioning protects against ischemia/reperfusion-induced brain damage: role of NADPH oxidase-derived ROS. Free Radic Biol Med. 2007;43:1048-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 71. | Diaz Diaz AC, Malone K, Shearer JA, Moore AC, Waeber C. Preclinical Evaluation of Fingolimod in Rodent Models of Stroke With Age or Atherosclerosis as Comorbidities. Front Pharmacol. 2022;13:920449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |