Published online Sep 26, 2024. doi: 10.12998/wjcc.v12.i27.6111
Revised: June 24, 2024
Accepted: July 18, 2024
Published online: September 26, 2024
Processing time: 193 Days and 19.6 Hours
HDR syndrome is a rare genetic disease caused by variants in the GATA3 gene and is phenotypically defined by the triad of hypoparathyroidism (H), deafness (D), and renal disease (R). Renal disorders of HDR are mainly developmental ab
A 9-month-old boy was hospitalized with a complaint of diarrhea. Proteinuria was detected in the patient by routine testing for 3 days. No edema, oliguria, fever or abnormal urine color were observed. Routine urinary tests at a local hospital revealed proteinuria (protein 3 +) and microscopic hematuria (red blood cells 5-10/HP). The patient was born by cesarean delivery due to placental abruption at 35 weeks + 4 days of gestation. Intrauterine growth retardation was detected be
We report an infant with HDR syndrome who presented with early-onset nephrotic syndrome in China. We suggest that variants in the GATA3 gene might be associated with infant-onset nephrotic syndrome.
Core Tip: HDR syndrome is a rare genetic disease caused by variants in the GATA3 gene and is phenotypically defined by the triad of hypoparathyroidism (H), deafness (D), and renal disease (R). Patients with HDR syndrome may exhibit the full phenotypic triad or only a subset. Renal disorders of HDR are mainly developmental abnormalities, although renal functional abnormalities can also be observed. Nephrotic syndrome or nephrotic-level proteinuria is rare in HDR syndrome. Here, we report a Chinese infant with HDR syndrome who present with early-onset nephrotic syndrome.
- Citation: Ma LJ, Yang W, Zhang HW. HDR syndrome presented with nephrotic syndrome in a Chinese boy: A case report. World J Clin Cases 2024; 12(27): 6111-6116
- URL: https://www.wjgnet.com/2307-8960/full/v12/i27/6111.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i27.6111
HDR syndrome (OMIM 146255) is a rare autosomal dominant genetic disease caused by variants in the GATA3 gene located on chromosome 10p14. GATA-3 plays an essential role in the embryonic development of the parathyroids, inner ear and kidneys. HDR syndrome is phenotypically defined by the triad of hypoparathyroidism (H), deafness (D), and renal disease (R)[1,2]. HDR syndrome has been reported previously in China[3,4] and other Asian countries[2,5,6]. Here, we report a Chinese boy with HDR syndrome who presented with early-onset nephrotic syndrome.
A 9-month-old boy was hospitalized with a complaint of diarrhea.
Proteinuria was detected in the patient by routine testing for 3 days. The parents denied any occurrence of edema, oliguria, fever or abnormal urine color. Routine urinary tests at a local hospital revealed protein 3 + and red blood cells 5-10/HP.
The boy was born to nonconsanguineous healthy parents. He was born by cesarean delivery due to placental abruption at 35 weeks + 4 days of gestation. Intrauterine growth retardation was detected beginning at 6 months of gestation. His birth weight was 1.47 kg (< P3th), length was 39 cm (< P3th), and head circumference was 28 cm (< P3th). His motor developmental milestones were obviously delayed. He was able to raise his head at 4 months. He was unable to turn over, crawl or sit at 9 months.
There was no abnormal past medical or family history, and no family history of maternal renal disease.
At admission, the patient’s weight was 7.1 kg (< P3th), height was 66 cm (< P3th), and head circumference was 36 cm (< P3th). He was able to make sounds, look and hear with audiovisual stimulation. No obviously abnormal dysmorphic features were revealed in physical examination. No positive signs were found on examination of his lungs, heart and abdomen. No other neurodevelopmental or ophthalmologic deficits were observed.
The results of routine laboratory tests, including routine blood test, electrolytes, liver and kidney function, thyroid hormone, growth hormone and insulin-like growth factor 1, were all normal. All autoantibodies were negative. The levels of C3 and C4 were normal while the levels of immunoglobulin G (IgG) and IgA were decreased. T/B lymphocyte subsets showed some abnormalities. The total parathyroid hormone level was normal. Routine urinary tests displayed protein 3 + and red blood cells 5-10/HP. The urinary microalbumin concentration ranged from 4384-5981 mg/L, and the spot urinary protein-to-creatinine ratio ranged from 2.96-5.87 (Table 1). The results of blood and urine metabolism analyses were all normal. According to the Gesell development schedules, the estimated total DQ was 72, the adaptive behavior DQ was 78, the large motor behavior DQ was 65, the fine motor behavior DQ was 66, the language behavior DQ was 75, and the personal social behavior DQ was 76. Pure tone audiometry indicated sensorineural deafness. His karyotype was 46, XY.
| Items | Results | Normal range |
| Total serum protein | 39.40 g/L | 55-75 |
| Serum albumin | 24.40 g/L | 39-54 |
| Total cholesterol | 5.89 mmol/L | < 6.22 |
| Triglyceride | 2.08 mmol/L | < 1.7 |
| Serum creatinine | 18.40 μmol/L | 13-33 |
| Blood urea nitrogen | 3.54 mmol/L | 1.1-5.9 |
| Serum calcium | 2.32 mmol/L | 2.1-2.8 |
| IgG | 1.07 g/L | 7-16 |
| IgA | 0.28 g/L | 0.7-5 |
| IgM | 0.60 g/L | 0.4-2.8 |
| C3 | 1.12 g/L | 0.78-2.1 |
| C4 | 0.22 g/L | 0.17-0.48 |
| Total parathyroid hormone | 22.22 ng/L | 12-88 |
| 25-OH vitamin D | 62.15 μg/L | 30-100 |
| Total T cell | 62.44% | 56-71 |
| CD4+ T cell | 44.23% | 25-39 |
| CD8+ T cell | 16.71% | 20-34 |
| CD4/CD8 | 2.65 | 0.76-1.61 |
| B cell | 31.15% | 13-25 |
| NK cell | 6.44% | 8-20 |
| Urinary microalbumin | 4384-5981 mg/L | 0-15 |
| Urine protein/creatinine | 2.96-5.87 | < 0.2 |
| Urine red blood cell | 5-10/HP | < 3 |
Ultrasonography revealed that the renal body was normal (6.2-6.6 cm in length and 2.4-3.1 cm in width), and the border of the cortex and medulla was clear. Echocardiography revealed an atrial septal defect (5.0 mm). Electroencephalography and cranial magnetic resonance imaging results were normal.
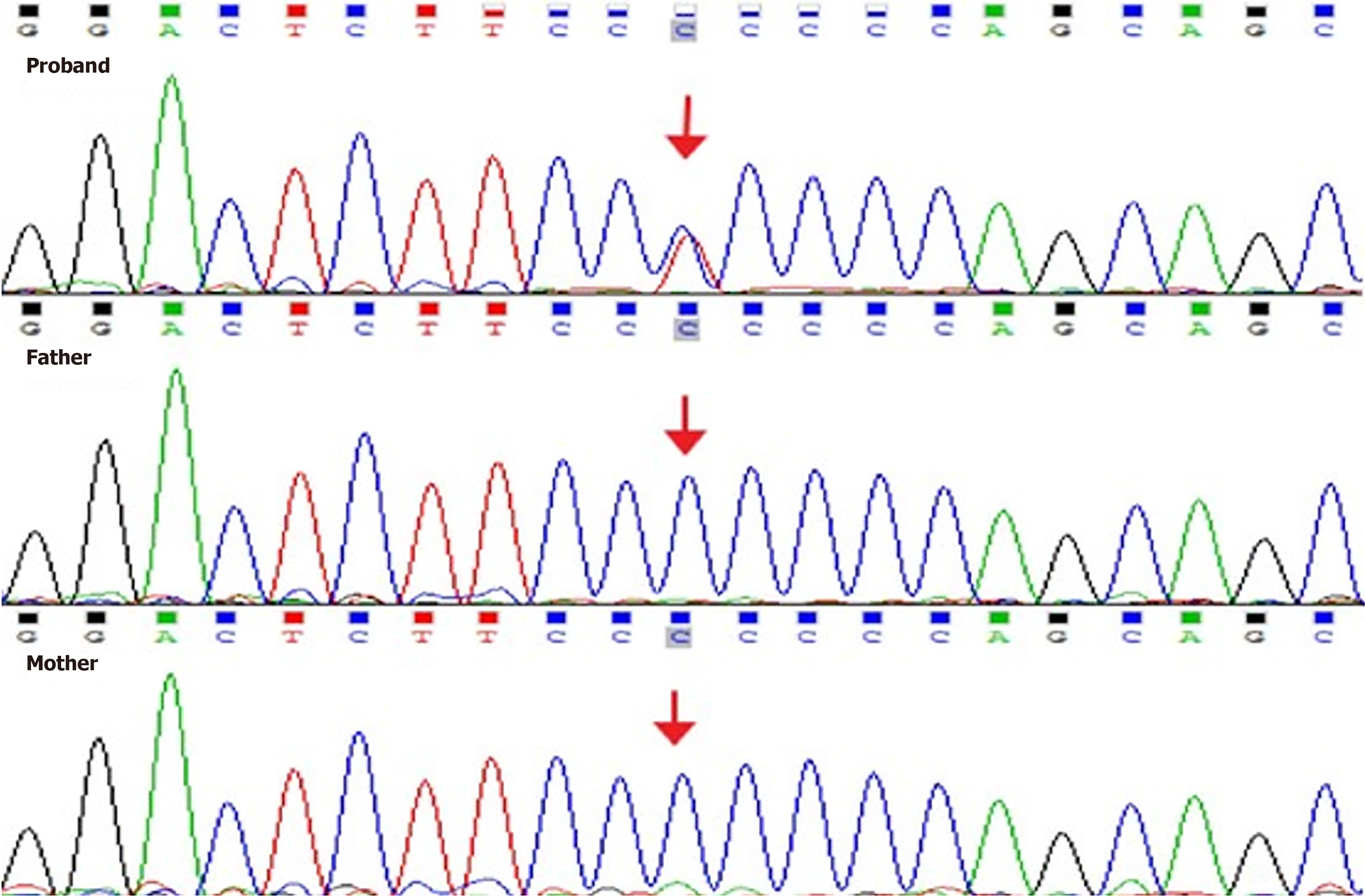
Genetic analysis was performed using next-generation sequencing at the genetics laboratories of the MyGenostics biotechnology company in China, using “the inherited renal diseases panel”, which covers genes strongly correlated with this disorder. The results showed that the boy carried a hemizygous variant, c.704C>T (p.Pro235 Leu), in exon 3 of the GATA3 gene, and his parents were both wild type (Figure 1). The variant was not found in the Human Gene Mutation Database or the SNP databases including ALFA, ExAC, GnomAD and TOPMED. PolyPhen-2, Variant Taster and GERP++ analysis showed that the variant was pathogenic, while SIFT analysis indicated that the variant might be benign (ACMG guideline: PS2 + PM2_Supporting).
Due to the presence of growth retardation, early onset nephrotic syndrome, microscopic hematuria, sensorineural deafness, abnormal immune functions, congenital heart disease and c.704C>T (p.Pro235 Leu) in the GATA3 gene, HDR syndrome was diagnosed.
Following the diagnosis of HDR syndrome caused by GATA3 variant, there was no specific treatments.
The patient did not receive any specific treatment after the diagnosis of HDR syndrome caused by a GATA3 variant. Over a follow-up of 3 months, there was no obvious change in proteinuria (urinary microalbumin: 4695-5739 mg/L, urine protein/creatinine: 3.05-6.21), microscopic hematuria (urine red blood cells: 5-10/HP) or growth retardation.
Renal disorders of HDR mainly involve developmental abnormalities. However, renal functional abnormalities can also be observed. Here, we report the case of a Chinese infant with HDR syndrome and renal disease, including early-onset nephrotic syndrome and microscopic hematuria.
To date, approximately 180 HDR syndrome cases have been reported worldwide. Over 90% of patients present with hypoparathyroidism and sensorineural deafness, and more than 80% of patients exhibit urinary tract and renal abnor
Renal disorders of HDR syndrome include both developmental abnormalities (such as renal hypoplasia, dysplasia, aplasia, cystic kidney disease, pelvicalyceal deformity, and vesicoureteral reflux) and functional abnormalities (such as proteinuria, hematuria, glomerulonephritis, proximal or distal renal tubular acidosis, and nephrocalcinosis)[1-3,10]. Nephrotic syndrome and nephrotic-level proteinuria are rare in HDR syndrome patients. Chenouard et al[11] reported a child with HDR syndrome with nephrotic syndrome as a novel finding. The first renal biopsy at 3 years revealed tubuloi
Overall, 10% of HDR patients progress to end stage renal disease[14]. The age at which renal dysfunction occurs in HDR syndrome patients is variable. In the report of Chenouard et al[11], renal failure was detected with a serum crea
The mechanism of GATA-3 pathogenesis in the renal involvement of HDR syndrome remains unclear, and to date, no potential target of this transcription factor has been identified. However, GATA3 is expressed in mouse kidney mesangial cells, and is markedly increased in rodent models of mesangial proliferative glomerulonephritis, suggesting that GATA3 plays a critical role in normal glomerular development and might be a useful nuclear marker of human mesangial cells[17]. In the last 20 years, 133 GATA3 variations have been reported; patients have shown great clinical variability, and the penetrance of each HDR defect increases with age[18]. However, no clear genotype and phenotype correlation has been determined[19].
Here, we report a Chinese infant with HDR syndrome who presented with early-onset nephrotic syndrome, microscopic hematuria, sensorineural deafness, growth retardation, abnormal immune function and congenital heart disease. We suggest that variants in the GATA3 gene might be associated with infant-onset nephrotic syndrome, which extends the spectrum of phenotypes of GATA3 disorders. Screening for GATA3 variations is therefore relevant for patients with either two or three of the phenotypic manifestations of HDR syndrome. Further studies of GATA3 are needed to improve our knowledge of the involvement and phenotype of this transcription factor in human development, particularly in the kidneys.
| 1. | Ali A, Christie PT, Grigorieva IV, Harding B, Van Esch H, Ahmed SF, Bitner-Glindzicz M, Blind E, Bloch C, Christin P, Clayton P, Gecz J, Gilbert-Dussardier B, Guillen-Navarro E, Hackett A, Halac I, Hendy GN, Lalloo F, Mache CJ, Mughal Z, Ong AC, Rinat C, Shaw N, Smithson SF, Tolmie J, Weill J, Nesbit MA, Thakker RV. Functional characterization of GATA3 mutations causing the hypoparathyroidism-deafness-renal (HDR) dysplasia syndrome: insight into mechanisms of DNA binding by the GATA3 transcription factor. Hum Mol Genet. 2007;16:265-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Fukami M, Muroya K, Miyake T, Iso M, Kato F, Yokoi H, Suzuki Y, Tsubouchi K, Nakagomi Y, Kikuchi N, Horikawa R, Ogata T. GATA3 abnormalities in six patients with HDR syndrome. Endocr J. 2011;58:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Chiu WY, Chen HW, Chao HW, Yann LT, Tsai KS. Identification of three novel mutations in the GATA3 gene responsible for familial hypoparathyroidism and deafness in the Chinese population. J Clin Endocrinol Metab. 2006;91:4587-4592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Lin YH, Wu CC, Hsu TY, Chiu WY, Hsu CJ, Chen PL. Identification of a novel GATA3 mutation in a deaf Taiwanese family by massively parallel sequencing. Mutat Res. 2015;771:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Cheon CK, Kim GH, Yoo HW. The first Korean case of HDR syndrome confirmed by clinical and molecular investigation. Yonsei Med J. 2015;56:300-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Muroya K, Hasegawa T, Ito Y, Nagai T, Isotani H, Iwata Y, Yamamoto K, Fujimoto S, Seishu S, Fukushima Y, Hasegawa Y, Ogata T. GATA3 abnormalities and the phenotypic spectrum of HDR syndrome. J Med Genet. 2001;38:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Ferraris S, Del Monaco AG, Garelli E, Carando A, De Vito B, Pappi P, Lala R, Ponzone A. HDR syndrome: a novel "de novo" mutation in GATA3 gene. Am J Med Genet A. 2009;149A:770-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Hernández AM, Villamar M, Roselló L, Moreno-Pelayo MA, Moreno F, Del Castillo I. Novel mutation in the gene encoding the GATA3 transcription factor in a Spanish familial case of hypoparathyroidism, deafness, and renal dysplasia (HDR) syndrome with female genital tract malformations. Am J Med Genet A. 2007;143A:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Lichtner P, König R, Hasegawa T, Van Esch H, Meitinger T, Schuffenhauer S. An HDR (hypoparathyroidism, deafness, renal dysplasia) syndrome locus maps distal to the DiGeorge syndrome region on 10p13/14. J Med Genet. 2000;37:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Al-Shibli A, Al Attrach I, Willems PJ. Novel DNA mutation in the GATA3 gene in an Emirati boy with HDR syndrome and hypomagnesemia. Pediatr Nephrol. 2011;26:1167-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Chenouard A, Isidor B, Allain-Launay E, Moreau A, Le Bideau M, Roussey G. Renal phenotypic variability in HDR syndrome: glomerular nephropathy as a novel finding. Eur J Pediatr. 2013;172:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Maleki N, Bashardoust B, Iranparvar Alamdari M, Tavosi Z. Seizure, deafness, and renal failure: a case of barakat syndrome. Case Rep Nephrol. 2013;2013:261907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Barakat AY, D'Albora JB, Martin MM, Jose PA. Familial nephrosis, nerve deafness, and hypoparathyroidism. J Pediatr. 1977;91:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Belge H, Dahan K, Cambier JF, Benoit V, Morelle J, Bloch J, Vanhille P, Pirson Y, Demoulin N. Clinical and mutational spectrum of hypoparathyroidism, deafness and renal dysplasia syndrome. Nephrol Dial Transplant. 2017;32:830-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Horta M, Lino C, Lemos MC. Hypoparathyroidism, deafness and renal dysplasia (HDR) syndrome and GATA3. QJM. 2017;110:837-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Joseph ADD, Sirisena ND, Kumanan T, Sujanitha V, Strelow V, Yamamoto R, Wieczorek S, Dissanayake VHW. Hypoparathyroidism, Sensorineural deafness and renal disease (Barakat syndrome) caused by a reduced gene dosage in GATA3: a case report and review of literature. BMC Endocr Disord. 2019;19:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Grigorieva IV, Oszwald A, Grigorieva EF, Schachner H, Neudert B, Ostendorf T, Floege J, Lindenmeyer MT, Cohen CD, Panzer U, Aigner C, Schmidt A, Grosveld F, Thakker RV, Rees AJ, Kain R. A Novel Role for GATA3 in Mesangial Cells in Glomerular Development and Injury. J Am Soc Nephrol. 2019;30:1641-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Lemos MC, Thakker RV. Hypoparathyroidism, deafness, and renal dysplasia syndrome: 20 Years after the identification of the first GATA3 mutations. Hum Mutat. 2020;41:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Upadhyay J, Steenkamp DW, Milunsky JM. The syndrome of hypoparathyroidism, deafness, and renal anomalies. Endocr Pract. 2013;19:1035-1042. [PubMed] [DOI] [Full Text] |