Published online Sep 26, 2024. doi: 10.12998/wjcc.v12.i27.6057
Revised: June 25, 2024
Accepted: July 15, 2024
Published online: September 26, 2024
Processing time: 56 Days and 11.3 Hours
Although cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) offer the potential for long-term survival in peritoneal carcinomatosis, outcomes following CRS/HIPEC vary significantly.
To identify the clinical factors associated with progression-free survival (PFS) after complete CRS/HIPEC in patients with colorectal/high-grade appendiceal, ovarian, and gastric cancers.
We retrospectively evaluated the risk of recurrence within 1 year after CRS/HIPEC and its impact on overall survival (OS) in patients recruited between 2015 and 2020. Logistic regression models were used to assess the prognostic factors for the risk of recurrence within 1 year. Kaplan–Meier survival curves and Cox proportional hazards models were used to evaluate the association between recurrence and OS.
Of the 80 enrolled patients, 39 had an unfavorable PFS (< 1 year) and 41 had a favorable PFS (≥ 1 year). Simple logistic models revealed that the patients with a completeness of cytoreduction score of 0 (CC-0) or length of CRS ≤ 6 h had a favorable PFS [odds ratio (OR) = 0.141, P = 0.004; and OR = 0.361, P = 0.027, respectively]. In multiple logistic regression, achieving CC-0 was the strongest prognostic factor for a favorable PFS (OR = 0.131, P = 0.005). A peritoneal cancer index score > 12 was associated with a lower rate of achieving CC-0 (P = 0.027). The favorable PFS group had a significantly longer OS (median 81.7 mo vs 17.0 mo, P < 0.001).
Achieving CC-0 was associated with a lower early recurrence rate and improved long-term survival. This study underscores the importance of selecting appropriate candidates for CRS/HIPEC to manage peritoneal carcinomatosis.
Core Tip: Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) can extend survival in peritoneal carcinomatosis, but outcomes vary. This study examined factors affecting progression-free survival (PFS) after CRS/HIPEC in patients with colorectal, high-grade appendiceal, ovarian, and gastric cancers. Evaluating the results of 80 patients from 2015-2020 showed that those with a completeness of cytoreduction score of 0 (CC-0) or surgery duration ≤ 6 h had better PFS. Achieving CC-0 was the key predictor of favorable PFS and longer overall survival. The study highlights the importance of patient selection for optimal CRS/HIPEC outcomes.
- Citation: Chen CY, Huang TH, Lee LW, Lung J, Ou YC, Hung CH, Chuang HC, Chen MC, Wang TY. Prognostic factors of early recurrence after complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Clin Cases 2024; 12(27): 6057-6069
- URL: https://www.wjgnet.com/2307-8960/full/v12/i27/6057.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i27.6057
Peritoneal carcinomatosis is a devastating condition often managed with systemic therapy or supportive care. However, extensive cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has emerged as a promising treatment, offering the potential for long-term survival and even cure in select patients[1]. HIPEC involves the infusion of chemotherapeutic drugs into the peritoneal cavity at a temperature of 41 °C-43 °C for 60-90 min following optimal CRS. This localized administration targets microscopic residual cancer cells, with both chemotherapy and hyperthermia contributing to the therapeutic effect[2]. Nevertheless, CRS/HIPEC is an aggressive treatment associated with significant side effects[3]. Identifying patients who are likely to respond favorably to CRS/HIPEC and benefit from this intensive approach remains a major challenge.
Complete cytoreduction is considered to be the most crucial prognostic factor in CRS/HIPEC, and various clinical indicators have been investigated to determine optimal patient selection to achieve this goal. The completeness of cytoreduction score (CC score) is evaluated after CRS, with cytoreduction score of 0 (CC-0) indicating the absence of peritoneal seeding, CC-1 denoting nodules persisting after cytoreduction measuring less than 0.25 cm, CC-2 representing nodules between 0.25 cm and 2.50 cm, and CC-3 indicating nodules larger than 2.50 cm[4]. Advanced imaging techniques have been shown to enhance the preoperative evaluation of completeness of cytoreduction and predict surgical outcomes[5].
Even when achieving complete cytoreduction, the outcomes following CRS/HIPEC can still vary significantly. For example, 1-year mortality and recurrence rates of 13% and 35%, respectively, have been reported after CRS/HIPEC in patients with colon cancer[6,7]. In gastric cancer patients, a systematic review reported recurrence rates in those undergoing CRS/HIPEC ranging from 10% to 27%[8], and a meta-analysis of CRS/HIPEC in patients with epithelial ovarian cancer showed significant improvements in overall survival (OS) [hazard ratio (HR) = 0.5] and progression-free survival (PFS) (HR = 0.57)[9]. While CRS/HIPEC may contribute to longer survival in patients with peritoneal carcinomatosis, some patients fail to achieve favorable outcomes, and the outcomes cannot be reliably predicted even with complete cytoreduction. Several studies have explored preoperative predictors of recurrence after CRS/HIPEC, including well-established factors such as low peritoneal cancer index (PCI)[4,10]. A study involving 52 patients with colorectal cancer reported a median PFS of 229 d, with high-grade primary tumor (≥ 3) identified as an independent risk factor for worse outcomes[11]. Lymph node (LNs) metastasis or the number of positive LNs has also been associated with poorer outcomes in patients with appendiceal and colorectal cancers[12,13]. Predictors of worse PFS in patients with recurrent ovarian cancer include platinum resistance, more than one relapse prior to HIPEC, presence of ascites, ≥ 2 lines of prior chemotherapy, chemotherapy-free interval < 6 mo, and CA-125 level > 35 U/mL[14,15]. However, these studies have been limited by small sample sizes and variations in the degree of cytoreduction. Moreover, complete cytoreduction is often defined as achieving CC-0 or CC-1, with CC-1 tumor nodules believed to be penetrable by intracavitary chemotherapy, making them eligible for complete cytoreduction when HIPEC is used[4]. Therefore, there remains a need for comprehensive studies investigating PFS outcomes and relevant predictors in patients who have achieved complete cytoreduction.
The aim of this retrospective cohort study was to investigate the clinical factors associated with recurrence after complete cytoreduction and HIPEC in patients with colorectal/high-grade appendiceal, ovarian, and gastric cancers presenting with peritoneal carcinomatosis.
From April 2015 to August 2020, a total of 205 patients underwent HIPEC procedures at Chang Gung Memorial Hospital, Chiayi, Taiwan. All patients were discussed in a multidisciplinary team meeting prior to CRS/HIPEC. The study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (202001607A3). All personal information has been removed by de-identification, so that specific persons and their identities cannot be re-identified or be linked to other database. In accordance with the Declaration of Helsinki, this study did not increase the risk of participants, and the requirement for patient consent was waived by the IRB.
The inclusion criteria were as follows: (1) Patients with primary or recurrent colorectal/high-grade appendiceal cancer, gastric cancer, or ovarian cancer with peritoneal carcinomatosis who underwent curative-intent salvage CRS/HIPEC; (2) patients in whom CRS achieved CC-0 or CC-1; and (3) patients with resectable extraperitoneal oligometastasis. The exclusion criteria were as follows: (1) Patients undergoing palliative HIPEC to control ascites without curative intent; (2) patients receiving adjuvant HIPEC who did not have peritoneal carcinomatosis; (3) patients with primary ovarian cancer who achieved a clinical response after neoadjuvant chemotherapy; (4) patients who underwent repeated CRS/HIPEC procedures (≥ 2 times); (5) patients planned for curative-intent CRS/HIPEC but who had a CC score ≥ 2; and (6) patients who were lost to follow-up.
The surgical procedures and HIPEC were performed by members of the multidisciplinary team. Preoperative PCI scores were evaluated using multidetector computed tomography (CT) and/or magnetic resonance imaging (MRI), and intraoperative assessments were performed using laparoscopy or laparotomy exploration. Extraperitoneal LNs were removed if preoperative imaging indicated positive involvement. After CRS, the CC score was assessed. HIPEC was delivered using the closed method with a PerformerTM HT (RanD Biotech, Medolla, Italy). The perfusate used for HIPEC included a mixture of normal saline and pentastarch (Haes-steril, 60 mg/mL, Meda, Sweden) 10% (3:1), or Dianeal® PD4 peritoneal dialysis solution 1.5% dextrose (Baxter) when using oxaliplatin-based regimens. The perfusate was administered at a dose of 2 L/m2 of body surface area. Chemotherapy was initiated after achieving an intra-abdominal temperature of 43 °C, and the duration of HIPEC was 30, 60, or 90 min depending on the regimen. The choices of chemotherapy regimen and HIPEC duration were based on the specific cancer type, disease status, and relevant references. The HIPEC regimens were as follows: (1) For colorectal cancer or pseudomyxoma peritonei: mitomycin 40 mg (30 mg at time 0; 10 mg at 60 min) over 90 min; or intraperitoneal oxaliplatin (460 mg/m2) and intravenous 5-FU 1 h before, for 30 min; (2) For platinum- sensitive recurrent ovarian cancer: cisplatin 50-100 mg/m2 and/or paclitaxel 175 mg/m2 over 60 min; (3) For platinum-resistant recurrent ovarian cancer: doxorubicin 35 mg/m2 and mitomycin 15 mg/m2 over 60 min; and (4) For gastric cancer: mitomycin 15 mg/m2 and cisplatin 50 mg/m2 over 60 min; doxorubicin 12.5 mg/m2 and cisplatin 50 mg/m2 over 60 min; or mitomycin 40 mg (30 mg at time 0; 10 mg at 60 min) over 90 min. The intraperitoneal chemotherapy drugs were drained out after completing HIPEC[16].
Data on patient characteristics, operative details, postoperative outcomes, and pathology were recorded by the case manager and evaluated by the multidisciplinary team committee. Postoperative complications were classified using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0.
Follow-up evaluations were conducted at our institution or the patient’s referring outside institution, with outside medical records obtained and reviewed. Follow-up CT/MRI scans were performed at 3-6 mo after surgery, upon recurrence of clinical symptoms, or when tumor marker levels increased. Survival and relapse information was recorded from medical records and/or the case manager’s record system. The patients were followed from the date of CRS/HIPEC until disease progression. If no progression occurred during the study period, the patients were censored at their last follow-up appointment.
Descriptive statistics were reported as mean ± SD, median with minimum and maximum, or frequency with percentage, as appropriate. Potential prognostic variables included age, sex, primary cancer site, LN involvement, CC score, PCI, and number of visceral resections. LN involvement was defined as the involvement of extraperitoneal LNs and recorded dichotomously as wither present or absent. If no LNs were removed, the number of positive LNs was considered to be zero. The association between the receipt of systemic chemotherapy and PFS was also examined. Both pre-HIPEC salvage chemotherapy (within 6 mo before HIPEC) and post-HIPEC salvage chemotherapy (within 6 mo after HIPEC) were recorded as either having received or not received.
The follow-up period was from 1 April 2015 to 31 March 2023. The primary outcome was PFS < 1 year or PFS ≥ 1 years, where PFS was defined as the time from CRS/HIPEC to the first radiographic or pathologic evidence of new or enlarging intra- or extraperitoneal lesions or death, whichever occurred first. Differences in patient characteristics between the favorable and unfavorable groups were compared using a two-sample t-test, Mann–Whitney U-test or χ2 test. Crude odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were calculated for all variables using simple logistic regression, and explanatory variables with a P value < 0.1 were considered in multiple logistic regression analysis. The final model included baseline characteristics such as age and sex, and significant explanatory variables selected by sequentially removing covariates with a P value > 0.05. To examine the impact of recurrence on OS, which was defined as the time from CRS/HIPEC to death, we used Kaplan–Meier curves, log rank tests, and adjusted HRs calculated from Cox proportional hazards models. All tests were two-sided, and a P value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS statistical software (IBM SPSS Statistics for Windows, version 23.0, IBM Corp., Armonk, NY, United States).
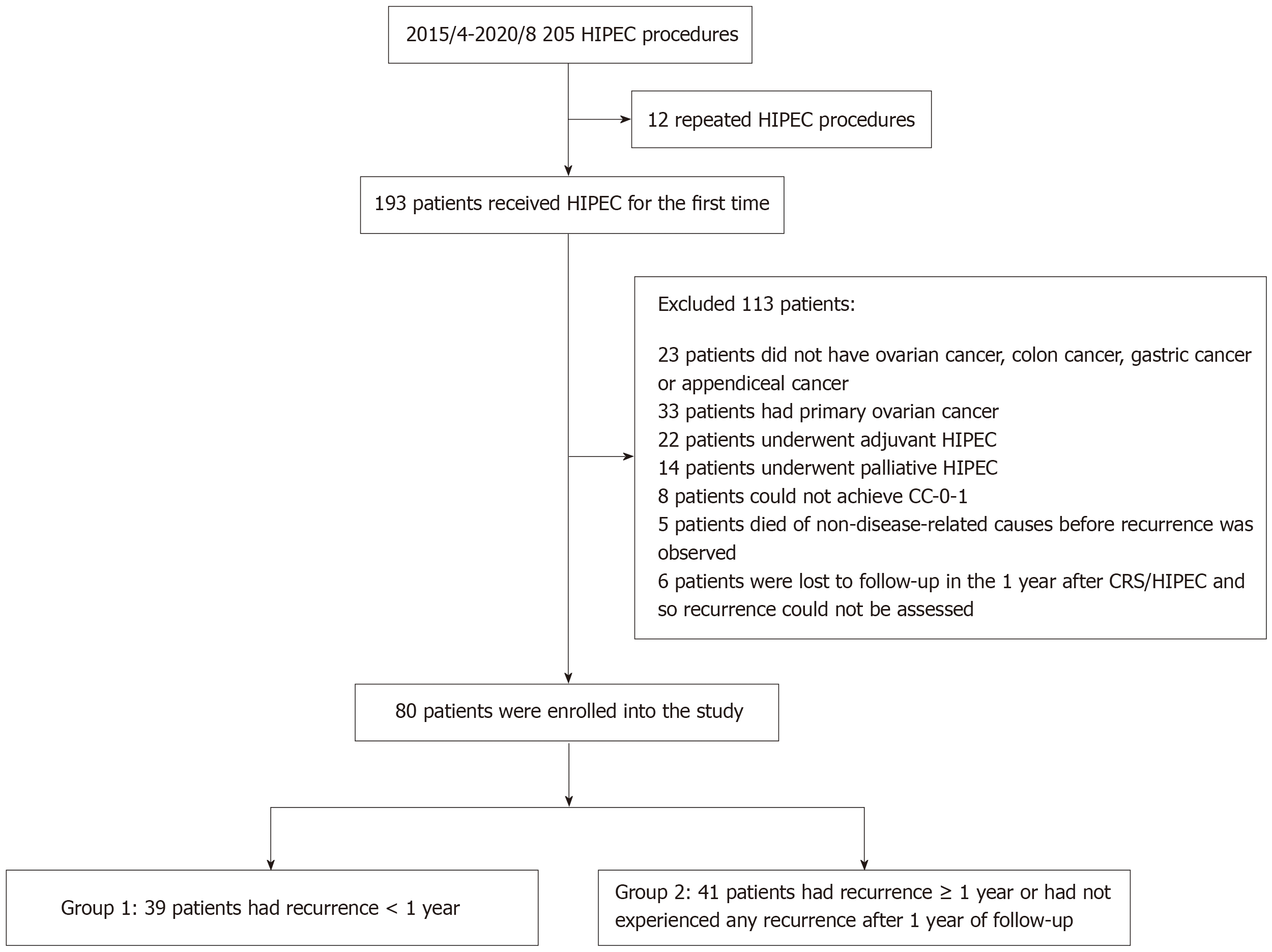
The patient enrollment flowchart is shown in Figure 1. Of the initial 205 procedures performed at the hospital from April 2015 to August 2020, 12 were excluded due to having undergone repeated HIPEC procedures. Among the remaining 193 patients, 113 were further excluded from the analysis for the reasons shown in Figure 1, including not having the specified cancer type, receiving adjuvant or palliative HIPEC, not achieving CC-0/1, non-disease-related deaths before recurrence, and loss to follow-up. As a result, the final analysis included 80 patients. Of these patients, 42 had colorectal cancer, 22 had recurrent ovarian cancer (11 platinum-sensitive and 11 platinum-resistant), 12 had gastric cancer, and 1 had appendiceal high-grade adenocarcinoma. These 80 patients formed the basis for further analysis and investigation of outcomes related to the CRS/HIPEC procedure.
The patients were divided into two groups based on their PFS outcomes: a favorable PFS group (PFS ≥ 1 year; n = 41) and an unfavorable PFS group (PFS ≤ 1 year; n = 39). The demographics of the patients are shown in Table 1. The distributions of cancer type, previous chemotherapy, and extraperitoneal lesions did not differ significantly between the two groups. However, there was a significant difference in histological grade (P = 0.02), with the unfavorable PFS group having a higher proportion of grade 3 disease [24/39 (61.5%) vs 21/41 (51.2%)].
| Characteristic | All | < 1 yr group, n = 39 | ≥ 1 yr group, n = 41 | P value |
| Age in yr | ||||
| mean ± SD | 55.21 ± 11.48 | 52.74 ± 12.36 | 57.56 ± 10.18 | 0.060 |
| Median (mix, max) | 58 (22, 74) | 56 (22, 73) | 59 (36, 74) | 0.102 |
| Sex | ||||
| Male | 27 (33.7) | 15 (38.5) | 12 (29.3) | 0.480 |
| Female | 53 (66.3) | 24 (61.5) | 29 (70.7) | |
| BMI in kg/m2 | ||||
| mean ± SD | 24.20 ± 3.98 | 24.05 ± 4.22 | 24.34 ± 3.77 | 0.746 |
| Hypertension | 23 (28.8) | 11 (28.2) | 12 (29.3) | 1.000 |
| Diabetes mellitus | 16 (20.0) | 8 (20.5) | 8 (19.5) | 1.000 |
| Clinical presentation | ||||
| Primary | 35 (43.7) | 18 (46.2) | 17 (41.5) | 0.822 |
| Recurrence | 45 (56.3) | 21 (53.8) | 24 (58.5) | |
| Ascites presentation | 2 (2.5) | 1 (2.6) | 1 (2.4) | 1.000 |
| Previous systemic therapy | ||||
| Never | 20 (25.0) | 8 (20.5) | 12 (29.3) | 0.443 |
| 1st line or more | 60 (75.0) | 31 (79.5) | 29 (70.7) | |
| Extraperitoneal oligometastasis | ||||
| Liver | 13 (16.3) | 7 (17.9) | 6 (14.6) | 0.767 |
| Lung | 3 (3.8) | 1 (2.6) | 2 (4.9) | 1.000 |
| Extraperitoneal LN | 3 (3.8) | 1 (2.6) | 2 (4.9) | 1.000 |
| Skin | 4 (5.0) | 3 (7.7) | 1 (2.4) | 0.353 |
| Vagina | 2 (2.5) | 1 (2.6) | 1 (2.4) | 1.000 |
| Cancer types | 0.540 | |||
| Colorectal | 42 (52.5) | 22 (56.4) | 20 (48.8) | |
| Ovary | 22 (27.5) | 9 (23.1) | 13 (31.7) | |
| Platinum-sensitive | 11 (13.8) | 3 (7.7) | 8 (19.5) | |
| Platinum-resistance | 11 (13.8) | 6 (15.4) | 5 (12.2) | |
| Gastric | 12 (15.0) | 7 (17.9) | 5 (12.2) | |
| Appendix, high grade | 4 (5.0) | 1 (2.6) | 3 (7.3) | |
| Histology grade | 0.020a | |||
| 1 | 10 (12.4) | 1 (2.6) | 9 (22.0) | |
| 2 | 25 (31.3) | 14 (35.9) | 11 (26.8) | |
| 3 | 45 (56.3) | 24 (61.5) | 21 (51.2) |
Comparisons between the two groups regarding perioperative factors are shown in Table 2. In terms of CC score, only 25 of the 39 patients (64%) in the unfavorable PFS group achieved CC-0, compared to 38 of the 41 patients (93%) in the favorable PFS group, and the difference was significant (P = 0.002). Analysis of recurrent patterns also revealed a significant difference between the two groups, with a higher incidence of extraperitoneal recurrence (30/39, 76.9%) in the unfavorable PFS group compared to the favorable PFS group (10/41, 24.4%) (P < 0.001).
| Characteristic | All | < 1 yr group, n = 39 | ≥ 1 yr group, n = 41 | P value |
| Surgical method | ||||
| Laparoscopy | 11 (13.7) | 5 (12.8) | 6 (14.6) | 1.000 |
| Laparotomy | 69 (86.3) | 34 (87.2) | 35 (86.4) | |
| Preoperative PCI | 7.99 ± 5.97 | 9.08 ± 6.52 | 6.95 ± 5.26 | 0.112 |
| CC score | ||||
| 0 | 63 (78.8) | 25 (64.1) | 38 (92.7) | 0.002a |
| 1 | 17 (21.2) | 14 (35.9) | 3 (7.3) | |
| Residual lesion | ||||
| Small bowel | 6 (7.5) | 4 (10.3) | 2 (4.9) | |
| Large bowel | 1 (1.3) | 1 (2.6) | 0 | |
| Major vessels | 3 (3.8) | 3 (7.7) | 0 | |
| Peritoneum | 7 (8.8) | 6 (15.4) | 1 (2.4) | |
| Pre-HIPEC chemotherapy1 | 41 (51.3) | 24 (61.5) | 17 (41.5) | 0.080 |
| Response to pre-HIPEC chemotherapy | 0.121 | |||
| Complete remission | 0 | 0 | 0 | |
| Partial remission | 15 (18.8) | 8 (20.5) | 7 (17.1) | |
| Stable disease | 6 (7.5) | 1 (2.6) | 5 (12.2) | |
| Progression disease | 16 (20.0) | 11 (28.2) | 5 (12.2) | |
| CRS time in min | 338.60 ± 144.80 | 365.50 ± 135.10 | 313.10 ± 150.80 | 0.106 |
| Visceral organ resections | ||||
| Liver | 6 (7.5) | 3 (7.7) | 3 (7.3) | 1.000 |
| Lung wedge resection | 3 (3.8) | 1 (2.6) | 2 (4.9) | 1.000 |
| Small bowel | 23 (28.8) | 13 (33.3) | 10 (24.4) | 0.461 |
| Large bowel | 45 (56.3) | 25 (64.1) | 20 (48.8) | 0.184 |
| Uterus and adnexa2 | ||||
| TH-BSO | 7 (13.2) | 2 (8.3) | 5 (17.2) | 0.433 |
| BSO | 3 (5.6) | 1 (4.2) | 2 (6.9) | 1.000 |
| HIPEC duration in min | 0.603 | |||
| 30, oxaliplatin-based | 18 (22.4) | 10 (25.6) | 8 (19.5) | |
| 60 | 31 (38.8) | 16 (41.0) | 15 (36.6) | |
| 90, mitomycin c | 31 (38.8) | 13 (33.3) | 18 (43.9) | |
| HIPEC regimens | ||||
| Cisplatin-based | 22 (27.5) | 13 (33.3) | 9 (22.0) | 0.319 |
| Non-cisplatin-based | 58 (72.5) | 26 (66.7) | 32 (78.0) | |
| Post-HIPEC chemotherapy3 | 70 (87.5) | 35 (89.7) | 35 (85.4) | 0.738 |
| Any postoperative complication ≥ grade 3 | 7 (8.8) | 4 (10.3) | 3 (7.3) | 0.709 |
| AKI | 1 (1.3) | 0 | 1 (2.4) | |
| Bowel perforation | 4 (5.0) | 4 (10.3) | 0 | |
| Others | 2 (2.5) | 0 | 2 (4.9) | |
| Recurrent site | < 0.001a | |||
| Extraperitoneal | 23 (28.8) | 18 (46.2) | 5 (12.2) | |
| Intraperitoneal | 26 (32.5) | 9 (23.1) | 17 (41.5) | |
| Both | 17 (21.3) | 12 (30.8) | 5 (12.2) | |
| No recurrence | 14 (17.5) | 0 | 14 (34.1) |
Simple logistic regression was conducted to identify the potential prognostic factors for the likelihood of recurrence within 1 year. As presented in Table 3, the patients who achieved a CC score of 0 and those who had a length of CRS of ≤ 6 h had the lower risk of recurrence within 1 year (OR = 0.141, P = 0.004; and OR = 0.361, P = 0.027, respectively). For the non-significant covariates, there was a trend toward a favorable PFS with a PCI score of ≤ 7 (OR = 0.444, P = 0.078) and no pre-HIPEC chemotherapy (OR = 2.529, P = 0.075). Cancer type, previous systemic chemotherapy, extraperitoneal metastasis, surgical method, and bowel resection were not associated with recurrence. Furthermore, the multiple logistic regression model revealed that achieving a CC score of 0 during CRS was significantly associated with a favorable PFS (adjusted OR = 0.130, P = 0.005) (Table 4). We further analyzed the association between PCI score and CC score, and found that 85% (51/60) of the patients with a PCI score ≤ 12 achieved CC-0, compared to only 60% (12/20) of those with a PCI score > 12 (P = 0.027). Given that HIPEC procedures were more frequently performed for gastrointestinal cancers than for recurrent ovarian cancer in clinical practice, and given the distinct behaviors of these cancer types, we stratified the cancer types into two subgroups: recurrent ovarian cancer and gastrointestinal cancers (including gastric cancer, colorectal cancer, and appendiceal cancer). These subgroups were incorporated into the multiple logistic regression model. The subgroup analysis of gastrointestinal cancers demonstrated that achieving a CC score of 0 during CRS was significantly associated with favorable PFS (adjusted OR = 0.046, P = 0.008). However, in the subgroup of recurrent ovarian cancer, the use of pre-HIPEC chemotherapy was significantly associated with unfavorable outcomes (adjusted OR = 22.932, P = 0.042), whereas achieving a CC score of 0 was not significantly associated with outcomes (adjusted OR = 0.239, P = 0.332).
| Parameter | Crude OR (95%CI) | P value |
| Age in yr | ||
| ≥ 55 | 0.546 (0.222, 1.343) | 0.188 |
| < 55 | 1.000 | |
| Sex | ||
| Female | 0.662 (0.261, 1.681) | 0.386 |
| Male | 1.000 | |
| Previous systemic therapy | ||
| Any | 1.603 (0.574, 4.482) | 0.368 |
| Never | 1.000 | |
| Clinical presentation | ||
| Primary | 1.210 (0.500, 2.931) | 0.673 |
| Recurrence | 1.000 | |
| Cancer type | ||
| Ovary | 0.646 (0.239, 1.746) | 0.389 |
| GI | 1.000 | |
| CC score | ||
| 0 | 0.141 (0.037, 0.541) | 0.004a |
| 1 | 1.000 | |
| PCI score | ||
| 1-7 | 0.444 (0.180, 1.095) | 0.078 |
| 8-39 | 1.000 | |
| HIPEC regimen | ||
| Cisplatin | 1.778 (0.657, 4.809) | 0.257 |
| Non-cisplatin | 1.000 | |
| HIPEC duration in min | ||
| ≤ 60 | 1.565 (0.632, 3.879) | 0.333 |
| 90 | 1.000 | |
| Extraperitoneal oligometastasis | ||
| Any | 1.071 (0.401, 2.830) | 0.890 |
| None | 1.000 | |
| Surgical method | ||
| Laparoscopy | 0.858 (0.239, 3.077) | 0.814 |
| Laparotomy | 1.000 | |
| Histology grade | ||
| Grade 1/2 | 0.656 (0.270, 1.597) | 0.353 |
| Grade 3 | 1.000 | |
| Pre-HIPEC chemotherapy1 | ||
| Yes | 2.259 (0.922, 5.532) | 0.075 |
| No | 1.000 | |
| Post-HIPEC chemotherapy2 | ||
| Yes | 1.500 (0.389, 5.781) | 0.556 |
| No | 1.000 | |
| CRS time in h | ||
| ≤ 6 | 0.361 (0.146, 0.892) | 0.027a |
| > 6 | 1.000 | |
| Bowel resection | ||
| Yes | 2.143 (0.858, 5.351) | 0.103 |
| No | 1.000 |
| Parameters | Overall | P value | Ovarian cancer | P value | GI cancers | P value |
| Adjusted OR | Adjusted OR | Adjusted OR | ||||
| Age in yr | ||||||
| ≥ 55 | 0.496 | 0.157 | 0.097 | 0.114 | 0.528 | 0.293 |
| < 55 | 1.000 | 1.000 | 1.000 | |||
| Sex | ||||||
| Female | 1.091 | 0.872 | - | 0.566 | 0.375 | |
| Male | 1.000 | - | 1.000 | |||
| CC score | ||||||
| 0 | 0.130 | 0.005a | 0.239 | 0.332 | 0.046 | 0.008 |
| 1 | 1.000 | 1.000 | 1.000 | |||
| Pre-HIPEC chemotherapy | ||||||
| Yes | 22.932 | 0.042a | - | |||
| No | 1.000 | - | ||||
| Platinum-response1 | ||||||
| Resistant | 7.133 | 0.153 | - | |||
| Sensitive | 1.000 | - |
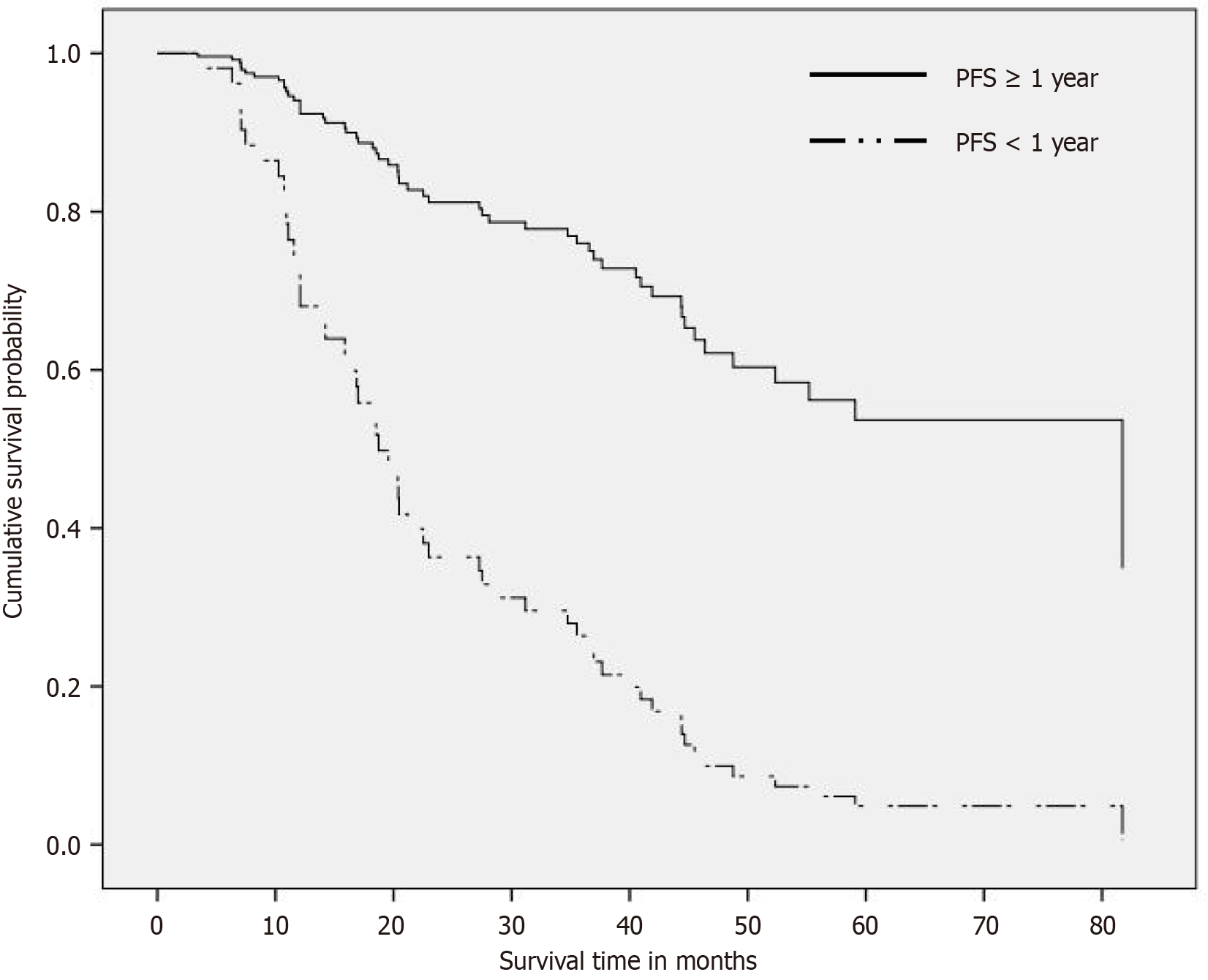
The median follow-up duration was 44.5 mo. In the overall cohort, the median PFS and OS after primary CRS/HIPEC were 12.3 mo (95%CI: 9.2-15.5 mo) and 37.0 mo (95%CI: 26.1-47.9 mo), respectively. The favorable PFS group had a significantly longer OS compared to the unfavorable PFS group (81.7 mo vs 17.0 mo, P < 0.001) (Figure 2). This was confirmed in a Cox model, which revealed that the unfavorable PFS group had a significantly shorter survival time than the favorable group after adjusting for age, sex, and CC score (adjusted HR = 4.853, P < 0.001).
Among the patients with a potential 3-year follow-up period (those who underwent CRS/HIPEC before 31 March 2020, if alive), 23 were still alive, of whom 9 were alive with disease (AWD) and 14 had no evidence of disease (NED). Among the 9 patients who were AWD, only 3 belonged to the unfavorable PFS group (1 had platinum-sensitive recurrent ovarian cancer, and 2 had colorectal cancer). Among the 14 patients with NED, 10 remained recurrence-free after CRS/HIPEC, including 5 with recurrent ovarian cancer (3 patients were platinum-sensitive and had one episode of recurrence, while 2 patients were platinum-resistant and had four and five episodes of recurrences prior to this CRS/HIPEC procedure), and the other 5 had colon cancer. Notably, all 10 patients without recurrence (among the 14 patients with NED) achieved CC-0 CRS. The 3-year PFS rate was 12.98%, and the 3-year OS rate was 29.87% (23/77). In contrast, in the unfavorable PFS group, 22 patients experienced recurrence within 6 mo, and 15 patients died of the disease within 1 year.
In this study, we aimed to identify the prognostic factors for PFS in patients who underwent CRS/HIPEC. The results showed that 51.25% (41/80) of the patients with peritoneal carcinomatosis had a PFS ≥ 1 year, and the 3-year PFS rate was 12.98% (10/77) after strict cytoreduction and HIPEC. The patients with a better PFS also had a significantly better OS. CC-0 was found to be a major prognostic factor for prolonged PFS.
To ensure that the study population was primarily comprised of those with stage IV peritoneal carcinomatosis, patients with primary ovarian cancer and carcinomatosis were excluded due to their relatively good response to systemic chemotherapy and the lack of convincing randomized control trials regarding the indication for CRS/HIPEC[17]. Peritoneal carcinomatosis is associated with a poor prognosis and worse outcomes compared to other metastatic sites. A median OS of 5-11 mo has been reported in patients with gastric cancer and peritoneal carcinomatosis[18], and a median OS of 15-24 mo has been reported in patients with colon cancer and peritoneal carcinomatosis, even in those who receive systemic chemotherapy[19]. In addition, OS of around 5-17 mo has been reported in patients with recurrent ovarian cancer depending on the line of therapy and chemotherapy-sensitive or resistant status[20]. Long-term survival is rare in patients with peritoneal carcinomatosis. However, the OS rate in the present study was better compared to previous studies that only included patients receiving systemic therapy. This suggests that aggressive CRS/HIPEC in selected patients has clinical value in the treatment of this challenging disease.
Previous studies on CRS/HIPEC outcomes have often included patients with varying cytoreduction statuses (CC-0 to CC-3), and concluded that CC-0 and CC-1 represent complete cytoreduction. While CC score is a common factor associated with CRS/HIPEC outcomes[4], some studies have suggested that CC score is not a strong prognostic factor[12,21]. In the present study, strict cytoreduction to achieve CC-0 was the strongest prognostic factor, particularly in gastrointestinal cancers, consistent with findings from the CYTO-CHIP study[10]. A PCI score ≤ 7 showed a trend toward a favorable PFS in simple regression analysis, but it was not a significant predictor in the multiple logistic regression model, due to its correlation with the CC score. Although the PCI score did not directly affect patient prognosis, a PCI score > 12 was associated with a lower rate of achieving CC-0. Furthermore, better PFS was associated with better OS. Notably, all 10 patients who were progression-free for more than 3 years without any recurrence events had a CC score of 0. These findings suggest that all efforts should be made to achieve CC-0, and that peritoneal minimal residual tumors on non-vital organs should not be left to chance. Considering our results, we further suggest that in patients with a preoperative imaging PCI score > 12, clinicians should be aware of the potential challenges in achieving CC-0 and may consider incorporating diagnostic laparoscopy before proceeding with CRS. For patients with potential CC-1, especially those with diffuse small bowel lesions or critical major vessel invasion, prioritizing systemic therapy to reduce tumor burden or considering novel therapy combinations (such as immunotherapy or pressurized intraperitoneal aerosolized chemotherapy) may be beneficial, particularly if they have received multiple lines of treatment before CRS/HIPEC. When involving vital organs, repeated HIPEC for CC-1 patients can also be considered[22,23]. Of the 17 patients with CC-1 in this study, 9 had unavoidable diffuse small miliary seeding of the bowel and major vessel involvement. However, the remaining 8 patients with large bowel or peritoneal miliary residual tumors should have been approached more proactively. These 8 patients were early cases at our institution, when the surgical experience and peritonectomy skills were not as advanced.
Approximately 5%-15% of patients with colorectal, gastric, and ovarian cancers present with oligometastasis. Resection of extraperitoneal lung or liver oligometastases and extraperitoneal LNs may improve survival and achieve curative intent[24]. A systematic review of patients with colorectal cancer with peritoneal and limited liver metastasis reported a 3-year survival rate of 34% with CRS/HIPEC and local liver treatment[25]. Resection of oligometastases and lymphadenectomy have also been associated with improved OS, with a reported survival of 35.2 mo and 5-year survival rate of 22% in patients with gastric cancer[26,27]. For patients with a low PCI score who have the potential to achieve CC-0, peritoneal carcinomatosis can be treated as a local disease with CRS/HIPEC, and vigorous resection of extraperitoneal metastases may be feasible. In this study, 12 patients underwent resection of extraperitoneal metastases during CRS/HIPEC, and all had a PFS > 1 year. However, due to distant metastatic disease, perioperative systemic therapy was encouraged to control the disease[25,27].
Systemic chemotherapy has been demonstrated to have limited efficacy in treating peritoneal dissemination compared to hematogenous spread[28]. In the present study, the use of post-HIPEC chemotherapy did not show a significant association with PFS in simple regression analysis. Similarly, the association between pre-HIPEC chemotherapy and PFS in overall population was not statistically significant, although there was a trend indicating potentially worse PFS with the use of chemotherapy before HIPEC. However, the use of pre-HIPEC chemotherapy was significantly associated with unfavorable outcomes in recurrent ovarian cancer. Recurrent ovarian cancer is characterized by repeated relapses[29]. Patients who received pre-HIPEC chemotherapy often had a more extensive tumor burden, which may be associated with shorter subsequent PFS. Therefore, postoperative chemotherapy and maintenance therapy are particularly important[29]. Notably, for patients with peritoneal carcinomatosis resulting from LN-positive colorectal carcinoma, perioperative systemic chemotherapy has been associated with increased OS and PFS[30]. It has been hypothesized that LN metastasis may arise from hematogenous spread[31].
In selected patients, minimally invasive procedures are equally effective and more tolerable. For patients with limited peritoneal disease (PCI < 10), laparoscopic CRS/HIPEC has been reported as a feasible and safe approach for curative treatment, potentially reducing postoperative complications[32]. In ovarian cancer, the laparoscopic approach is also considered safe, despite ongoing debate regarding its oncologic advantages[33]. In the present study, laparoscopic CRS/HIPEC did not impact PFS, consistent with previous evidence.
There are several strengths to this study. First, the study population was restricted to patients with curative intent and complete cytoreduction only, which reduced heterogeneity arising from incomplete surgery or potentially palliative cases. While CC score is commonly associated with prognosis, we found that achieving CC-0 was the only significant factor contributing to prolonged PFS. Second, the long follow-up period enhances the validity of our PFS results. Finally, the study significantly benefited from the presence of a well-established multidisciplinary program specifically designed for peritoneal malignancies. This program ensured consistent and standardized quality of care during the perioperative period.
Achieving CC-0 was a significant prognostic factor of recurrence in patients undergoing CRS/HIPEC. A PCI score > 12 was associated with a lower likelihood of achieving CC-0. Our results show that a favorable PFS can have a substantial impact on OS and long-term prognosis for patients with challenging peritoneal malignancies. It is crucial to explore novel therapeutic strategies for managing potential residual disease after CRS. Our findings offer valuable guidance for clinicians in decision-making regarding patient management. Future research to improve preoperative evaluations and the selection of patients who may be able to achieve CC-0 is also warranted.
The authors are grateful to the members of the Peritoneal Malignancy Program of the Cancer Center Chang Gung Memorial Hospital, Chiayi, and the case manager, Tzu-Ting Liao.
| 1. | Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: Progress toward a new standard of care. Cancer Treat Rev. 2016;48:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Sugarbaker PH. Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int J Hyperthermia. 2007;23:431-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Bartlett EK, Meise C, Roses RE, Fraker DL, Kelz RR, Karakousis GC. Morbidity and mortality of cytoreduction with intraperitoneal chemotherapy: outcomes from the ACS NSQIP database. Ann Surg Oncol. 2014;21:1494-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol. 2005;2:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Lin CN, Huang WS, Huang TH, Chen CY, Huang CY, Wang TY, Liao YS, Lee LW. Adding Value of MRI over CT in Predicting Peritoneal Cancer Index and Completeness of Cytoreduction. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 6. | Simkens GA, van Oudheusden TR, Braam HJ, Luyer MD, Wiezer MJ, van Ramshorst B, Nienhuijs SW, de Hingh IH. Treatment-Related Mortality After Cytoreductive Surgery and HIPEC in Patients with Colorectal Peritoneal Carcinomatosis is Underestimated by Conventional Parameters. Ann Surg Oncol. 2016;23:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Simkens GA, van Oudheusden TR, Luyer MD, Nienhuijs SW, Nieuwenhuijzen GA, Rutten HJ, de Hingh IH. Serious Postoperative Complications Affect Early Recurrence After Cytoreductive Surgery and HIPEC for Colorectal Peritoneal Carcinomatosis. Ann Surg Oncol. 2015;22:2656-2662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Martins M, Santos-Sousa H, Araújo F, Nogueiro J, Sousa-Pinto B. Impact of Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy in the Treatment of Gastric Cancer with Peritoneal Carcinomatosis: A Systematic Review and Meta-analysis. Ann Surg Oncol. 2022;29:7528-7537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 9. | Wu Q, Wu Q, Xu J, Cheng X, Wang X, Lu W, Li X. Efficacy of hyperthermic intraperitoneal chemotherapy in patients with epithelial ovarian cancer: a meta-analysis. Int J Hyperthermia. 2019;36:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Bonnot PE, Piessen G, Kepenekian V, Decullier E, Pocard M, Meunier B, Bereder JM, Abboud K, Marchal F, Quenet F, Goere D, Msika S, Arvieux C, Pirro N, Wernert R, Rat P, Gagnière J, Lefevre JH, Courvoisier T, Kianmanesh R, Vaudoyer D, Rivoire M, Meeus P, Passot G, Glehen O; FREGAT and BIG-RENAPE Networks. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J Clin Oncol. 2019;37:2028-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 11. | Königsrainer I, Horvath P, Struller F, Forkl V, Königsrainer A, Beckert S. Risk factors for recurrence following complete cytoreductive surgery and HIPEC in colorectal cancer-derived peritoneal surface malignancies. Langenbecks Arch Surg. 2013;398:745-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Baumgartner JM, Tobin L, Heavey SF, Kelly KJ, Roeland EJ, Lowy AM. Predictors of progression in high-grade appendiceal or colorectal peritoneal carcinomatosis after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2015;22:1716-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Turner KM, Morris MC, Delman AM, Hanseman D, Johnston FM, Greer J, Walle KV, Abbott DE, Raoof M, Grotz TE, Fournier K, Dineen S, Veerapong J, Maduekwe U, Kothari A, Staley CA, Maithel SK, Lambert LA, Kim AC, Cloyd JM, Wilson GC, Sussman JJ, Ahmad SA, Patel SH. Do Lymph Node Metastases Matter in Appendiceal Cancer with Peritoneal Carcinomatosis? A US HIPEC Collaborative Study. J Gastrointest Surg. 2022;26:2569-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Ayhan A, Akilli H, Abasiyanik MA, Taskiran C. Hyperthermic intraperitoneal chemotherapy in the treatment of recurrent ovarian cancer: When, and for whom? J Surg Oncol. 2023;127:457-464. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Chen WC, Huang HJ, Yang LY, Pan YB, Huang KG, Lin CT, Chen MY, Tang YH, Chang TC, Lai CH, Chou HH. Hyperthermic intraperitoneal chemotherapy for recurrent epithelial ovarian cancer. Biomed J. 2022;45:821-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Wang TY, Chen CY, Lu CH, Chen MC, Lee LW, Huang TH, Hsieh MC, Chen CJ, Yu CM, Chuang HC, Liao TT, Tseng CW, Huang WS. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal malignancy: preliminary results of a multi-disciplinary teamwork model in Asia. Int J Hyperthermia. 2018;34:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Gadducci A, Cosio S, Lippolis PV. Hyperthermic Intraperitoneal Chemotherapy in the Management of Primary Epithelial Ovarian Cancer: A Debated Issue for Gynecologic Oncologists. Anticancer Res. 2022;42:4659-4665. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Sun BJ, Lee B. Review of Regional Therapies for Gastric Cancer with Peritoneal Metastases. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Nadler A, McCart JA, Govindarajan A. Peritoneal Carcinomatosis from Colon Cancer: A Systematic Review of the Data for Cytoreduction and Intraperitoneal Chemotherapy. Clin Colon Rectal Surg. 2015;28:234-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Hanker LC, Loibl S, Burchardi N, Pfisterer J, Meier W, Pujade-Lauraine E, Ray-Coquard I, Sehouli J, Harter P, du Bois A; AGO and GINECO study group. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. 2012;23:2605-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 21. | Simkens GA, van Oudheusden TR, Nieboer D, Steyerberg EW, Rutten HJ, Luyer MD, Nienhuijs SW, de Hingh IH. Development of a Prognostic Nomogram for Patients with Peritoneally Metastasized Colorectal Cancer Treated with Cytoreductive Surgery and HIPEC. Ann Surg Oncol. 2016;23:4214-4221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Choudry HA, Bednar F, Shuai Y, Jones HL, Pai RK, Pingpank JF, Ahrendt SS, Holtzman MP, Zeh HJ, Bartlett DL. Repeat Cytoreductive Surgery-Hyperthermic Intraperitoneal Chemoperfusion is Feasible and Offers Survival Benefit in Select Patients with Peritoneal Metastases. Ann Surg Oncol. 2019;26:1445-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Votanopoulos KI. Repeat CRS/HIPEC: It Comes Down to Tumor Biology and Ability to Achieve a Complete CRS. Ann Surg Oncol. 2022;29:3366-3368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 24. | Ottaiano A, Santorsola M, Circelli L, Trotta AM, Izzo F, Perri F, Cascella M, Sabbatino F, Granata V, Correra M, Tarotto L, Stilo S, Fiore F, Martucci N, Rocca A, Picone C, Muto P, Borzillo V, Belli A, Patrone R, Mercadante E, Tatangelo F, Ferrara G, Di Mauro A, Scognamiglio G, Berretta M, Capuozzo M, Lombardi A, Galon J, Gualillo O, Pace U, Delrio P, Savarese G, Scala S, Nasti G, Caraglia M. Oligo-Metastatic Cancers: Putative Biomarkers, Emerging Challenges and New Perspectives. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Polderdijk MCE, Brouwer M, Haverkamp L, Ziesemer KA, Tenhagen M, Boerma D, Kok NFM, Versteeg KS, Sommeijer DW, Tanis PJ, Tuynman JB. Outcomes of Combined Peritoneal and Local Treatment for Patients with Peritoneal and Limited Liver Metastases of Colorectal Origin: A Systematic Review and Meta-Analysis. Ann Surg Oncol. 2022;29:1952-1962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Yamaguchi T, Takashima A, Nagashima K, Kumagai K, Yamada T, Terashima M, Yabusaki H, Nishikawa K, Tanabe K, Yunome G, Kawachi Y, Yamada T, Fukagawa T, Kinoshita T, Watanabe M, Ishiyama K, Inoue K, Boku N. Evaluating the efficacy of post-operative chemotherapy after curative resection of stage IV gastric cancer with synchronous oligo metastasis: a multicenter retrospective study. Gastric Cancer. 2023;26:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Montagnani F, Crivelli F, Aprile G, Vivaldi C, Pecora I, De Vivo R, Clerico MA, Fornaro L. Long-term survival after liver metastasectomy in gastric cancer: Systematic review and meta-analysis of prognostic factors. Cancer Treat Rev. 2018;69:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Foster JM, Zhang C, Rehman S, Sharma P, Alexander HR. The contemporary management of peritoneal metastasis: A journey from the cold past of treatment futility to a warm present and a bright future. CA Cancer J Clin. 2023;73:49-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 29. | Richardson DL, Eskander RN, O’Malley DM. Advances in Ovarian Cancer Care and Unmet Treatment Needs for Patients With Platinum Resistance: A Narrative Review. JAMA Oncol. 2023;9:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 95] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 30. | Kuijpers AM, Mehta AM, Boot H, van Leerdam ME, Hauptmann M, Aalbers AG, Verwaal VJ. Perioperative systemic chemotherapy in peritoneal carcinomatosis of lymph node positive colorectal cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Oncol. 2014;25:864-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Coste A, Karagiannis GS, Wang Y, Xue EA, Lin Y, Skobe M, Jones JG, Oktay MH, Condeelis JS, Entenberg D. Hematogenous Dissemination of Breast Cancer Cells From Lymph Nodes Is Mediated by Tumor MicroEnvironment of Metastasis Doorways. Front Oncol. 2020;10:571100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Passot G, Bakrin N, Isaac S, Decullier E, Gilly FN, Glehen O, Cotte E. Postoperative outcomes of laparoscopic vs open cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for treatment of peritoneal surface malignancies. Eur J Surg Oncol. 2014;40:957-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Ronsini C, Pasanisi F, Greco P, Cobellis L, De Franciscis P, Cianci S. Mininvasive Cytoreduction Surgery plus HIPEC for Epithelial Ovarian Cancer: A Systematic Review. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |