Published online Sep 16, 2024. doi: 10.12998/wjcc.v12.i26.5983
Revised: July 2, 2024
Accepted: July 10, 2024
Published online: September 16, 2024
Processing time: 116 Days and 4 Hours
A sclerosing epithelioid fibrosarcoma (SEF) is a rare malignant fibroblastic soft tissue tumor that rarely occurs in intra-abdominal organs. A case of a SEF in the pancreatic head is reported herein, including its clinical manifestations, pre
A 33-year-old male patient was admitted to Peking Union Medical College Hospital in December 2023 due to a one-year history of intermittent upper abdominal pain and the discovery of a pancreatic mass. The patient underwent an enhanced computed tomography scan of the abdomen, which revealed a well-defined, round mass with clear borders and calcifications in the pancreatic head. The mass exhibited progressive, uneven mild enhancement, measuring approximately 6.6 cm × 6.3 cm. The patient underwent laparoscopic pylorus-preserving pancreaticoduodenectomy. Postoperative pathological examination revealed that the lesion was consistent with a SEF. At the 3-month postoperative follow-up, the patient did not report any short-term complications, and there were no signs of tumor recurrence.
SEFs are rare malignant fibrous soft tissue tumors. SEFs rarely develop in the pancreas, and its preoperative diagnosis depends on imaging findings, with confirmation depending on pathological examination and immunohistochemistry. Currently, only four cases of pancreatic SEF have been reported in studies written in English. This case is the first reported case of a pancreatic SEF by a clinical physician.
Core Tip: Sclerosing epithelioid fibrosarcoma (SEF) is a rare malignant fibroblastic soft tissue tumor, which is extremely rare in intra-abdominal organs. This article reports a case of SEF in the pancreatic head, presenting its clinical manifestations, preoperative imaging, gross specimen and pathological findings.
- Citation: Sun MQ, Guo LN, You Y, Qiu YY, He XD, Han XL. Sclerosing epithelioid fibrosarcoma of the pancreas: A case report. World J Clin Cases 2024; 12(26): 5983-5989
- URL: https://www.wjgnet.com/2307-8960/full/v12/i26/5983.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i26.5983
Sclerosing epithelioid fibrosarcoma (SEF) is a malignant fibrous soft tissue tumor that was first reported by Meis-Kindblom et al[1]. This disease is primarily diagnosed via pathological examination and immunohistochemical (IHC) staining. SEF is generally considered a low-grade malignant tumor, but some have proposed a more aggressive clinical course. The tumor can develop at any time during the life course in males and females alike. SEFs most commonly occur in the soft tissues of the limbs, followed by the trunk and head and neck. SEFs are extremely rare in intra-abdominal organs, with only a few cases reported in the pancreas. A case of SEF in the pancreatic head, including its clinical manifestations, preoperative imaging features, gross specimen and pathological findings, are presented herein.
A 33-year-old male patient was admitted to Peking Union Medical College Hospital in December 2023 due to a one-year history of intermittent upper abdominal pain and the discovery of a pancreatic mass.
The patient had been experiencing unexplained upper abdominal pain without fever, abdominal distension, diarrhea, nausea, or vomiting. A previous abdominal ultrasound at a local hospital revealed a mass in the pancreatic head, and the patient was given oral ibuprofen for symptomatic relief. An abdominal magnetic resonance imaging (MRI) in October 2022 revealed a round mass in the pancreatic head area measuring approximately 6.4 cm × 6.1 cm × 6.6 cm (no images available). The patient experienced episodic abdominal pain every three months and responded to symptomatic treatment. A repeat abdominal ultrasound at our hospital revealed a hypoechoic mass in the pancreatic head area measuring approximately 6.6 cm × 5.6 cm × 5.7 cm, with somewhat regular morphology and uneven internal echoes. The patient was admitted for surgical treatment of a suspected pancreatic head mass.
There was no significant medical history, personal history, or family history.
See above.
On admission, physical examination did not reveal any significant positive signs, and no obvious mass was palpated in the abdomen.
Preoperative tumor marker examination revealed a carbohydrate antigen 19-9 level of 5.0 U/mL and a carcinoembryonic antigen level of 1.3 ng/mL.
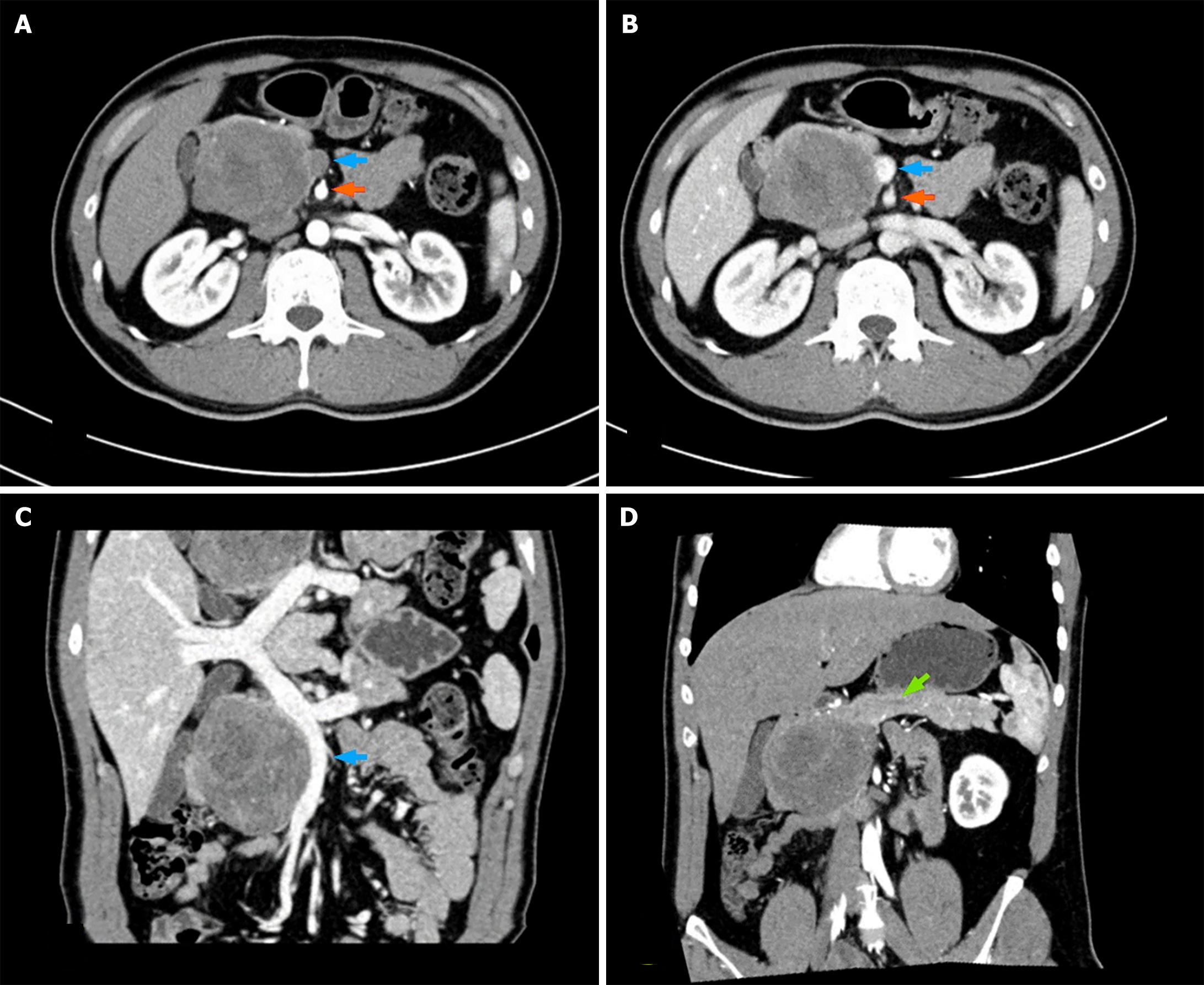
Following admission, the patient underwent an enhanced computed tomography (CT) scan of the abdomen, which revealed a well-defined, round mass with clear borders and calcifications in the pancreatic head. The mass exhibited progressive, uneven mild enhancement, measuring approximately 6.6 cm × 6.3 cm. Additionally, compression and displacement were observed in the distal duodenum, gallbladder, lower segment of the inferior vena cava, and proximal segment of the superior mesenteric vein. These imaging findings suggest a potential diagnosis of a solid pseudopapillary tumor (SPT) of the pancreas. Notably, the size and morphology of the body and tail of the pancreas appeared normal, with clear margins and homogeneous parenchymal density. No significant dilation of the pancreatic duct was evident (Figure 1).
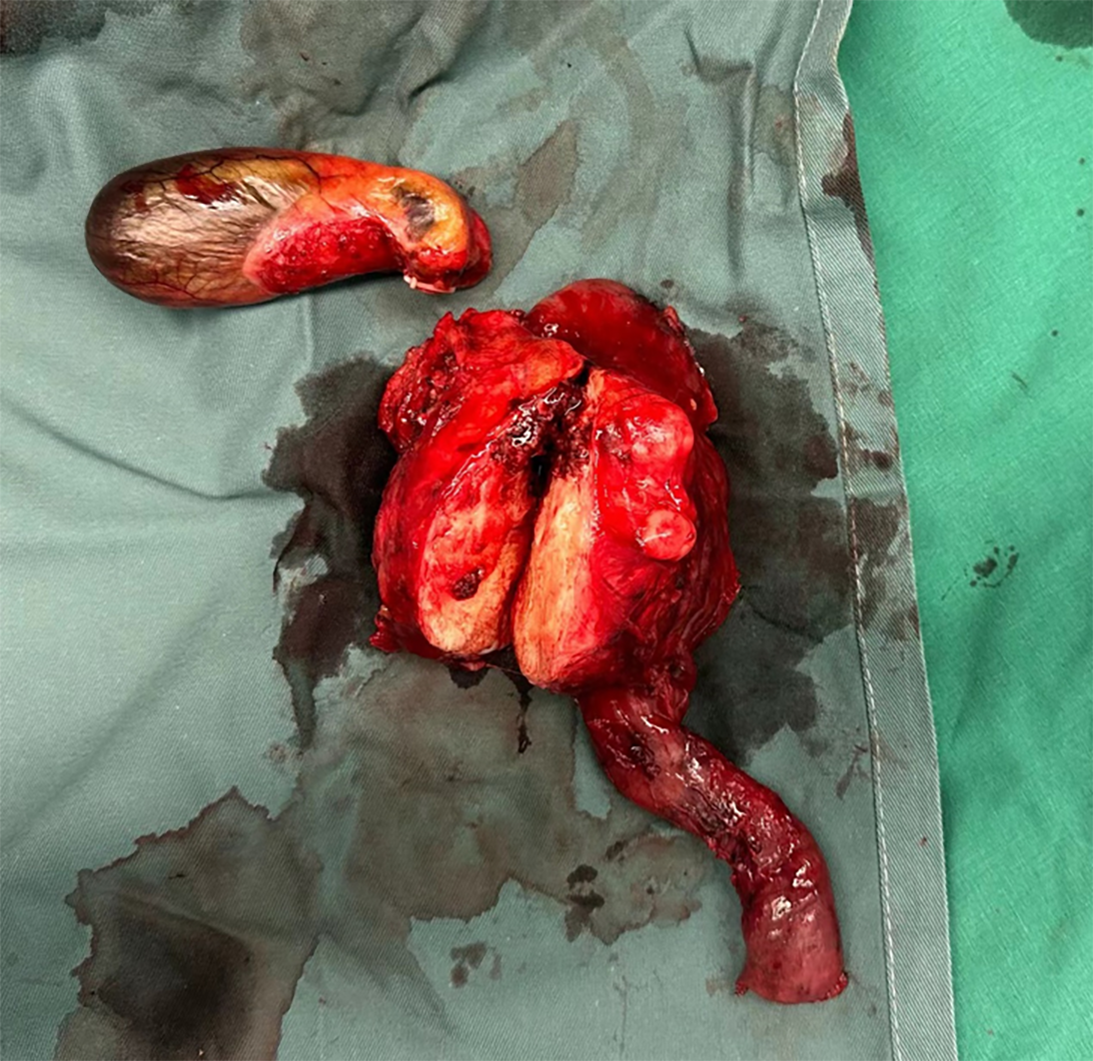
The patient subsequently underwent laparoscopic pylorus-preserving pancreaticoduodenectomy. Upon resection, the tumor exhibited solid characteristics, with a gray-white cut surface, hard texture, and well-defined borders, measuring 8 cm × 8 cm × 6 cm (Figure 2). Postoperatively, the patient had a smooth recovery, resumed an oral liquid diet on postoperative day (POD) 5, had all the abdominal drainage tubes removed on POD 7, and was discharged on POD 9.
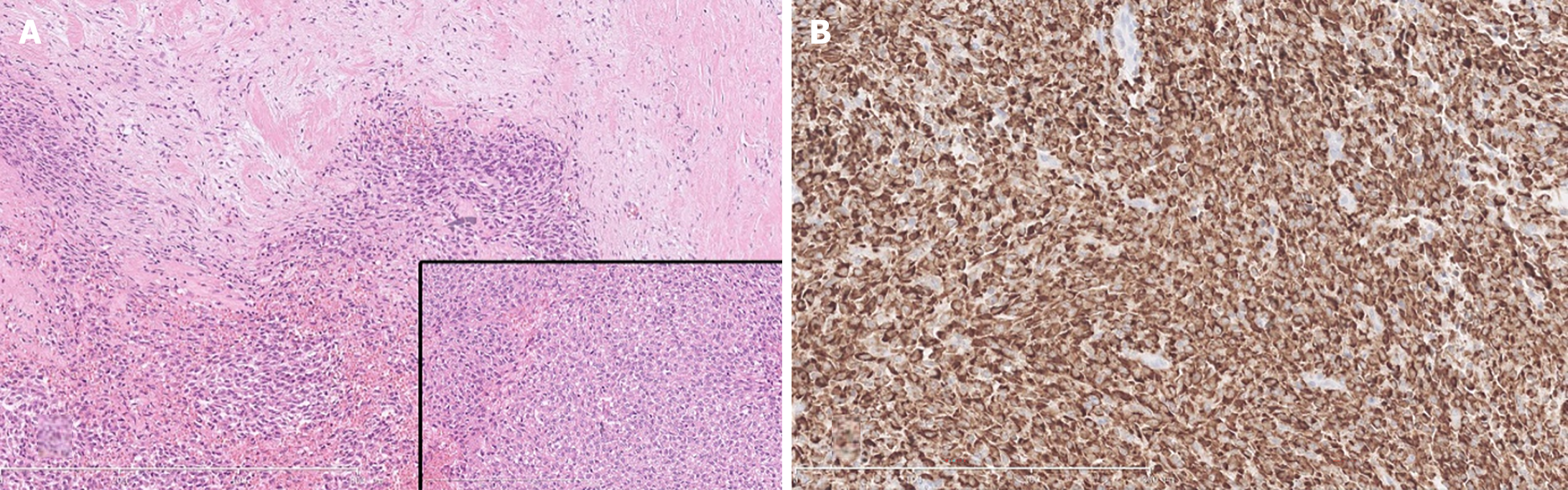
Postoperative pathological examination revealed that the lesion was consistent with a SEF, invading peripancreatic adipose tissue but not involving the duodenal muscular layer, common bile duct, or major duodenal papilla. The margins of the small intestine, bile duct, portal vein groove, and retroperitoneum were unremarkable, and no lymph node metastasis was observed. IHC analysis revealed positive staining for MUC4, vimentin, ATRX, SMARCA4, sporadic S-100 protein, occasional SATB2, β-catenin, partial cluster of differentiation (CD) 99, and vascular CD34 and negative staining for Cytokeratin AE1/AE3, CD10, chromogranin A, synaptophysin, B-cell lymphoma 10, tumor protein P53, SOX10, CD117, DOG-1, smooth muscle actin, desmin, Myo-D1, Myogenin, and P63, with a Ki-67 index of 30% in hot spots (Figure 3).
At the 3-month postoperative follow-up, the patient did not report any short-term complications, and there were no signs of tumor recurrence.
A SEF is a rare malignant fibroblastic soft tissue tumor that can at any age. The average age at onset, as reported in various studies, is approximately 38-55 years, with an equal incidence in both men and women[1-3]. A SEF can occur in almost any soft tissue, typically affecting the limbs or trunk, followed by the intra-abdominal organs such as the liver, gastrointestinal tract, ovaries, and kidneys[4-6]. The tumor tends to grow slowly, but the average size at the time of diagnosis is 7-8 cm, and it can metastasize to the lungs, bones, and other sites[2,3,7]. SEFs rarely develop in the pancreas, with only four cases reported in studies written in English to date[8-11]. These cases were reported by departments of radiology or pathology, and this case represents the first reported case of pancreatic SEF by clinicians. A summary of the reported cases of pancreatic SEF is presented in Table 1.
| No. | Year | Ref. | Gender | Age | Location | Size (cm) | Clinical presentation | Surgical procedure | IHC | Ki-67 index | Follow up (m) | Recurrence |
| 1 | 2013 | Bai et al[8] | M | 67 | Body | 1 | None | DP and splenectomy | Vimentin (+), EMA (-), SMA (-), S-100 protein (-) | < 5% | NM | NM |
| 2 | 2014 | Luo et al[11] | M | 42 | Head | 2.9 | Upper abdominal pain | PD | Vimentin (+), SMA (-), S-100 protein (-), bcl-2 (-) | 15% | 16 | None |
| 3 | 2020 | Xia et al[10] | M | 64 | Tail | 2 | None | DP and splenectomy | Vimentin (+), bcl-2 (+), EMA (+), SMA (+), CD34 (-), S-100 protein (-) | 8% (+) | 12 | None |
| 4 | 2020 | Kramer et al[9] | M | 58 | Head | 6 | None | PPPD | MUC4 (+) | NM | 3 | None |
| 5 | 2024 | Present case | M | 33 | Head | 8 | Upper abdominal pain | Laparoscopic PPPD | MUC4 (+), Vimentin (+), SMA (-), S-100 protein (scattered +), CD34 (vessel +) | 30% | 2 | None |
All 5 patients were male, with a median age of 58 years (range: 33-67). Two patients presented with nonspecific abdominal symptoms, such as upper abdominal pain, while the remaining patients did not report any tumor-related discomfort. SEFs can be found in the head, body, or tail of the pancreas, ranging from 1 to 8 cm in size at the time of discovery. None of the patients were diagnosed with a SEF preoperatively. In two patients with small tumors, the preoperative diagnosis was pancreatic neuroendocrine tumors[8,10], whereas in this case, the preoperative diagnosis was SPT of the pancreas. The preoperative diagnoses for the remaining two patients were not mentioned. All 5 patients underwent radical surgery (pancreaticoduodenectomy or distal pancreatectomy with splenectomy), and during the reported follow-up period, no tumor recurrence or metastasis was observed. However, one patient underwent right buttock soft tissue tumor resection 6 years prior, and upon reviewing the histological slides of the tumor, it was also considered to be a SEF. Therefore, the pancreatic lesion in this patient was considered to be a metastatic SEF[11].
SEFs are mainly diagnosed via imaging, especially enhanced CT and MRI. Imaging can provide information on the location, size, and borders of the tumor, as well as the tumor's relationship with blood vessels, bile ducts, and the main pancreatic duct and the presence of lymph node or distant metastases. On enhanced CT, SEFs appear as solid masses in the pancreas, with larger SEFs possibly showing uneven density. The enhancement pattern is characterized by gradual centripetal and nonuniform enhancement toward the center of the lesion[10]. Previously reported pancreatic SEFs often exhibit expansive growth, causing compressive and space-occupying changes to adjacent organ tissues, with direct invasion being less common. Apart from one patient with pancreatic duct dilation, the remaining patients did not show any signs of vascular invasion or pancreatic duct or bile duct dilation[11]. In this case, the large tumor caused dis
There are no studies on the value of preoperative biopsy. In this case, the patient did not undergo preoperative biopsy, mainly because the diagnosis of the pancreatic head mass was relatively clear, with preoperative images showing a SPT clearly indicated for surgical intervention. Preoperative biopsy does not alter the surgical approach and carries risks of bleeding, pancreatitis, and other complications. Pancreatic SEFs are extremely rare; thus, is it necessary to perform preoperative biopsy for differentiation? We believe that the answer to this question is complex.
The gross appearance of SEFs is typically nodular or lobulated, with a gray-white cut surface, firm or elastic consistency, and potential cystic changes and calcifications. Histologically, it is characterized by epithelioid tumor cells arranged in nests or cords that are uniformly embedded in a dense collagenous stroma. However, its tissue morphology can be easily confused with that of primary or metastatic sarcomas, SPTs of the pancreas, and other tumors. Therefore, it is typically diagnosed via immunohistochemistry. IHC staining for SEFs typically shows strong positivity for MUC4 and vimentin, whereas epithelial membrane antigen, CD34, S100 protein, and bcl-2 are weakly positive or negative[7,12]. Most cases (75%) exhibit EWSR1 gene rearrangement[7,13]. In this case, the gross specimen had a hard, gray-white cut surface, and histological examination showed epithelioid tumor cells arranged in nests and cords, with visible nuclear division. Immunohistochemistry revealed MUC4 (+), vimentin (+), S-100 (scattered +), and CD34 (vascular +), which is consistent with the above characteristics of this disease.
Although a SEF is a low-grade tumor, it typically presents aggressiveness, with a risk of local recurrence and distant metastasis. These recurrences and metastases often occur several years after initial treatment. While some patients with SEFs undergo postoperative chemotherapy, existing radiotherapy and chemotherapy have shown poor efficacy for this disease[7]. There have been reports exploring molecular targeted therapy, immunotherapy, and other potential future treatment approaches[14,15]. At present, the patient in this report has been followed for a relatively short period and has not shown signs of recurrence or metastasis, but the long-term prognosis requires further assessment. In conclusion, pancreatic SEFs are rare malignant soft tissue tumors of the pancreas with nonspecific clinical manifestations. Preoperative diagnosis is primarily based on imaging, with confirmation depending on the results of pathological examination and immunohistochemistry. Radical surgical resection is currently the main treatment method.
In conclusion, pancreatic SEFs are rare malignant soft tissue tumors of the pancreas with nonspecific clinical manifestations. Preoperative diagnosis is primarily based on imaging, with confirmation depending on the results of pathological examination and immunohistochemistry. Radical surgical resection is currently the main treatment method.
| 1. | Meis-Kindblom JM, Kindblom LG, Enzinger FM. Sclerosing epithelioid fibrosarcoma. A variant of fibrosarcoma simulating carcinoma. Am J Surg Pathol. 1995;19:979-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 190] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Blay JY, Tlemsani C, Toulmonde M, Italiano A, Rios M, Bompas E, Valentin T, Duffaud F, Le Nail LR, Watson S, Firmin N, Dubray-Longeras P, Ropars M, Perrin C, Hervieu A, Lebbe C, Saada-Bouzid E, Soibinet P, Fiorenza F, Bertucci F, Boudou P, Vaz G, Bonvalot S, Honoré C, Marec-Berard P, Minard V, Cleirec M, Biau D, Meeus P, Babinet A, Dumaine V, Carriere S, Fau M, Decanter G, Gouin F, Ngo C, Le Loarer F, Karanian M, Meurgey A, Dufresne A, Brahmi M, Chemin-Airiau C, Ducimetiere F, Penel N, Le Cesne A; NETSARC/REPPS/RESOS and French Sarcoma Group- Groupe d′Etude des Tumeurs Osseuses (GSF-GETO) networks. Sclerosing Epithelioid Fibrosarcoma (SEF) versus Low Grade Fibromyxoid Sarcoma (LGFMS): Presentation and outcome in the nationwide NETSARC+ series of 330 patients over 13 years. Eur J Cancer. 2024;196:113454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 3. | Peng Y, Zhang D, Lei T, Xie J, Wu C, Wang H, Shi Y, Li Q, Wang J. The clinicopathological spectrum of sclerosing epithelioid fibrosarcoma: report of an additional series with review of the literature. Pathology. 2023;55:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Wu KH, Huang YS, Li CC. Primary sclerosing epithelioid fibrosarcoma of the kidney: A rare case report. Urol Case Rep. 2024;53:102657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 5. | Armstrong A, Boulos F, Kulkarni S, Stoll J, Doyle MBM, Khan A, He M. Sclerosing Epithelioid Fibrosarcoma of the Liver in a Pediatric Patient. Pediatr Dev Pathol. 2023;26:153-160. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Pathak N, Kakkar A, Kaushal S, Batra A. Sclerosing epithelioid fibrosarcoma of the kidney. BMJ Case Rep. 2023;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Warmke LM, Yu W, Meis JM. Sclerosing Epithelioid Fibrosarcoma. Surg Pathol Clin. 2024;17:119-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Bai S, Jhala N, Adsay NV, Wei S. Sclerosing epithelioid fibrosarcoma of the pancreas. Ann Diagn Pathol. 2013;17:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Kramer SP, Bowman CJ, Wang ZJ, Sheahon KM, Nakakura EK, Cho SJ, Umetsu SE, Behr SC. Hybrid Low-Grade Fibromyxoid Sarcoma and Sclerosing Epithelioid Fibrosarcoma of the Pancreas. J Gastrointest Cancer. 2020;51:1025-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Xia W, Yang Y, Huang Y. Imaging Features of Sclerosing Epithelioid Fibrosarcoma of the Pancreas: A Case Report. Front Oncol. 2020;10:901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Luo Y, Hu W, Wu H, Xue H, Huo L, Li F, Zhao Y, Dai M. 18F-fluorodeoxyglucose PET/CT features and correlations with histopathologic characteristics in sclerosing epithelioid fibrosarcoma. Int J Clin Exp Pathol. 2014;7:7278-7285. [PubMed] |
| 12. | Hassan U, Saeed SM Sr, Mushtaq S, Hussain M, Hameed M. The Expression of Mucin-4 (MUC4) in Sarcomas Apart From Sclerosing Epithelioid Fibrosarcoma and Low-Grade Fibromyxoid Sarcoma. Cureus. 2023;15:e49546. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Zhang M, Yu Y, Guan X, Yao X, Jia C, Hong E, Guo Y, He L. A group of sclerosing epithelioid fibrosarcomas with low-level amplified EWSR1-CREB3L1 fusion gene in children. Pathol Res Pract. 2022;230:153754. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Koerner AS, Zhou M, Brook A, Yoon SS, Ganjoo KN. Response to Immunotherapy in Sclerosing Epithelioid Fibrosarcoma: Case Report and Literature Review. Cureus. 2023;15:e50967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 15. | Badran A, Steele C, Alquaydheb H, Ba Theeb A, Bawazir A, Elshenawy MA, Atallah JP. The Use of Crizotinib in Sclerosing Epithelioid Fibrosarcoma with ALK Mutation: A Case Report. Case Rep Oncol. 2023;16:746-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |