Published online Sep 16, 2024. doi: 10.12998/wjcc.v12.i26.5974
Revised: June 7, 2024
Accepted: July 10, 2024
Published online: September 16, 2024
Processing time: 118 Days and 3.8 Hours
Organizing pneumonia secondary to pulmonary tuberculosis is rare. Moreover, the temporal boundary between pulmonary tuberculosis and secondary orga
A 54 years old man, previously diagnosed with pulmonary tuberculosis and tuberculous pleurisy, underwent nine months of antituberculosis treatment. Follow-up lung computed tomography revealed multiple new subpleural ground-glass opacities in both lungs, and a lung biopsy confirmed organizing pneumonia. Treatment continued with anti-tuberculosis agents and hormone therapy, and subsequent dynamic pulmonary computed tomography exams demonstrated improvement in lesion absorption. No disease recurrence was observed after corticosteroid therapy discontinuation.
When treating patients with active pulmonary tuberculosis, if an increase in lesions is observed during anti-tuberculosis treatment, it is necessary to consider the possibility of tuberculosis-related secondary organizing pneumonia, timely lung biopsy is essential for early intervention.
Core Tip: We report a case of secondary organizing pneumonia associated with pulmonary tuberculosis occurring after nine months of antituberculosis treatment. So when treating patients with active pulmonary tuberculosis, if an increase in lesions is observed during antituberculosis treatment, it is necessary to consider the possibility of tuberculosis-related secondary organizing pneumonia, and timely lung biopsy is essential for early intervention.
- Citation: Liu M, Dong XY, Ding ZX, Wang QH, Li DH. Organizing pneumonia secondary to pulmonary tuberculosis: A case report. World J Clin Cases 2024; 12(26): 5974-5982
- URL: https://www.wjgnet.com/2307-8960/full/v12/i26/5974.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i26.5974
Organizing pneumonia (OP) is a form of lung tissue repair following injury, characterized pathologically by the infiltration of inflammatory fragments into the alveoli, spreading to the alveolar ducts and terminal bronchioles, accompanied by the typical intraluminal granulation tissue, forming Masson bodies[1]. OP is categorized into secondary OP (SOP) and cryptogenic OP (COP). COP is a form of idiopathic interstitial pneumonia that results from the pulmonary reactions to various unidentified injuries[2]. SOP, differing from COP, is attributed to specific causes including connective tissue disease, infections, tumors and drugs[3]. Among these, SOP caused by infections presents diagnostic challenges and there are limited reports both domestically and internationally on SOP caused by Mycobacterium tuberculosis (MTB) infection. Herein, we report a case of pulmonary tuberculosis with subsequent SOP treated in our department of tuberculosis, and review the relevant literatures.
Intermittent chest pain and fever for one month.
He experienced paroxysmal and pinprick right-sided chest pain a month earlier without any obvious triggers that worsened with physical activity. He also experienced fever, which was more pronounced at night and in the afternoon, with a maximum temperature of 39 °C, accompanied by dizziness and fatigue. He self-administered cephalosporin antibiotics orally for five days, but his symptoms did not significantly improve. A chest radiograph taken at a local hospital showed right-sided pleural effusion and chronic bronchitis changes. Routine blood analysis revealed a slightly increased white blood cell count mainly composed of neutrophils. Additional lung computed tomography (CT) imaging performed at our hospital revealed multiple bilateral nodules and cavitary lesions, which were considered infectious lesions caused by tuberculosis.
The patient has exhibited a history of robust health, he had no history of respiratory, heart, liver or kidney diseases.
The patient has no history of smoking or drinking; no cases of similar diseases have been reported in the patient's family.
Physical examination revealed decreased breathing sounds in the lower right lung and dullness on percussion.
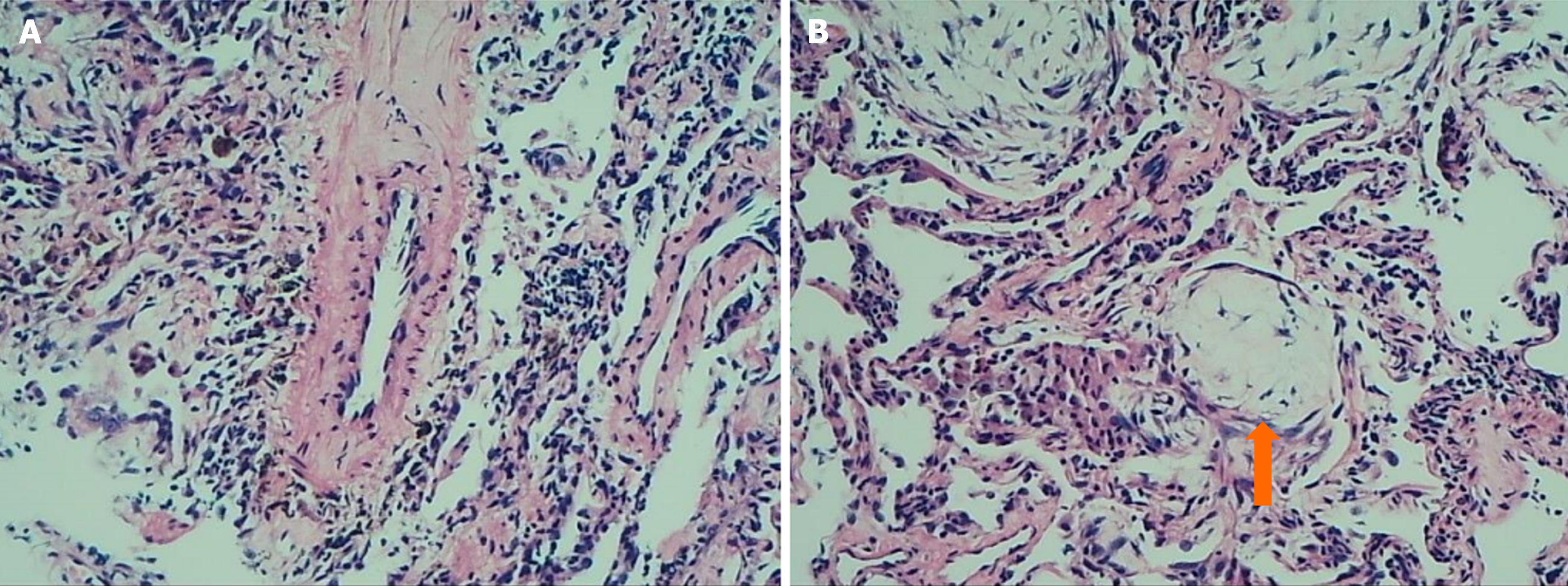
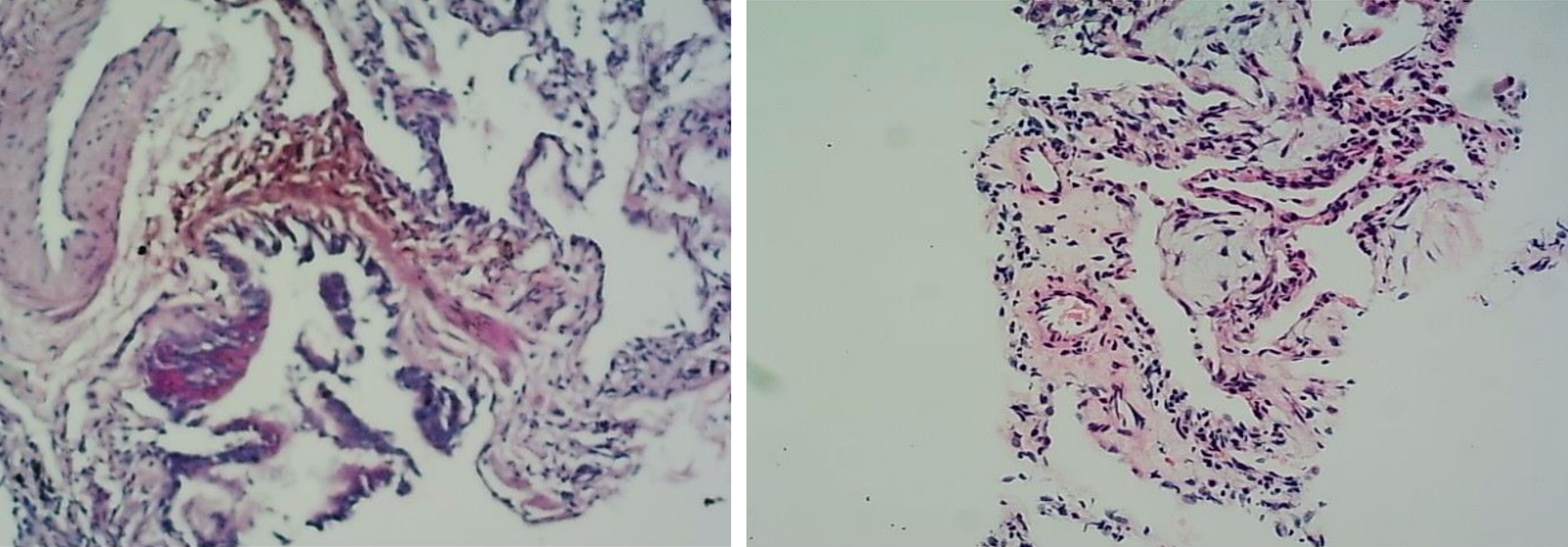
(1) Blood tests analysis (A local hospital): Indicated a slight increase in white blood cell count, predominantly neutrophils; (2) An adenosine deaminase level of the pleural fluid was 35.0 U/L; An erythrocyte sedimentation rate of 78 mm/hour; (3) The levels of tumor markers, myocardial enzymes, electrolytes, blood glucose, lipids, and bicarbonate were normal; (4) Two sputum specimens positive for acid-fast bacillus confirmed mycobacterial growth; and (5) The lung biopsy of new lesions showed alveolar atrophy, interstitial fibrosis, scattered inflammatory cell infiltration, and focal organizing pneumonia changes (Figure 1). Special staining, including acid-fast, periodic acid shiff, and hexamine-silver, did not detect pathogenic bacteria (Figure 2).
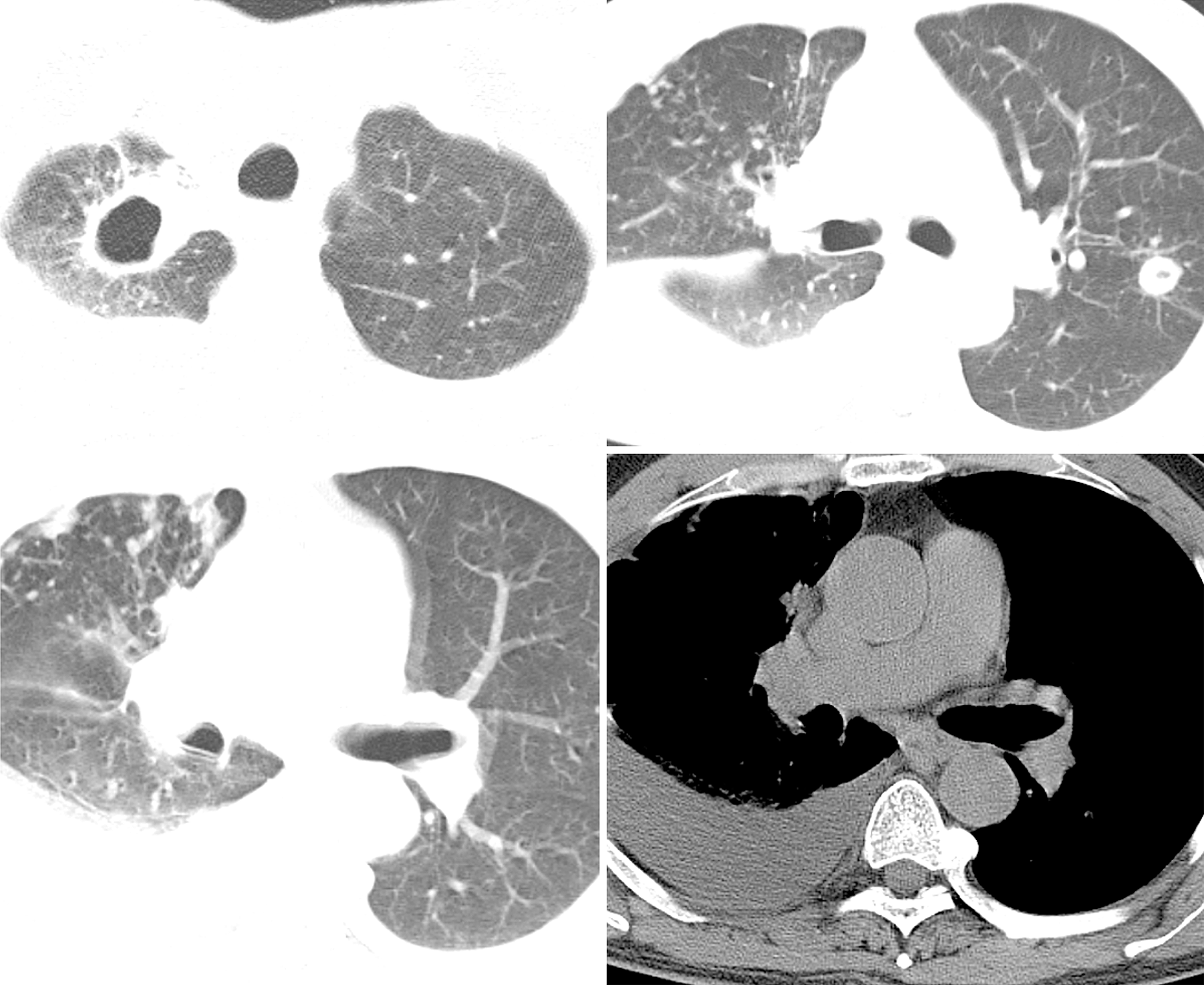
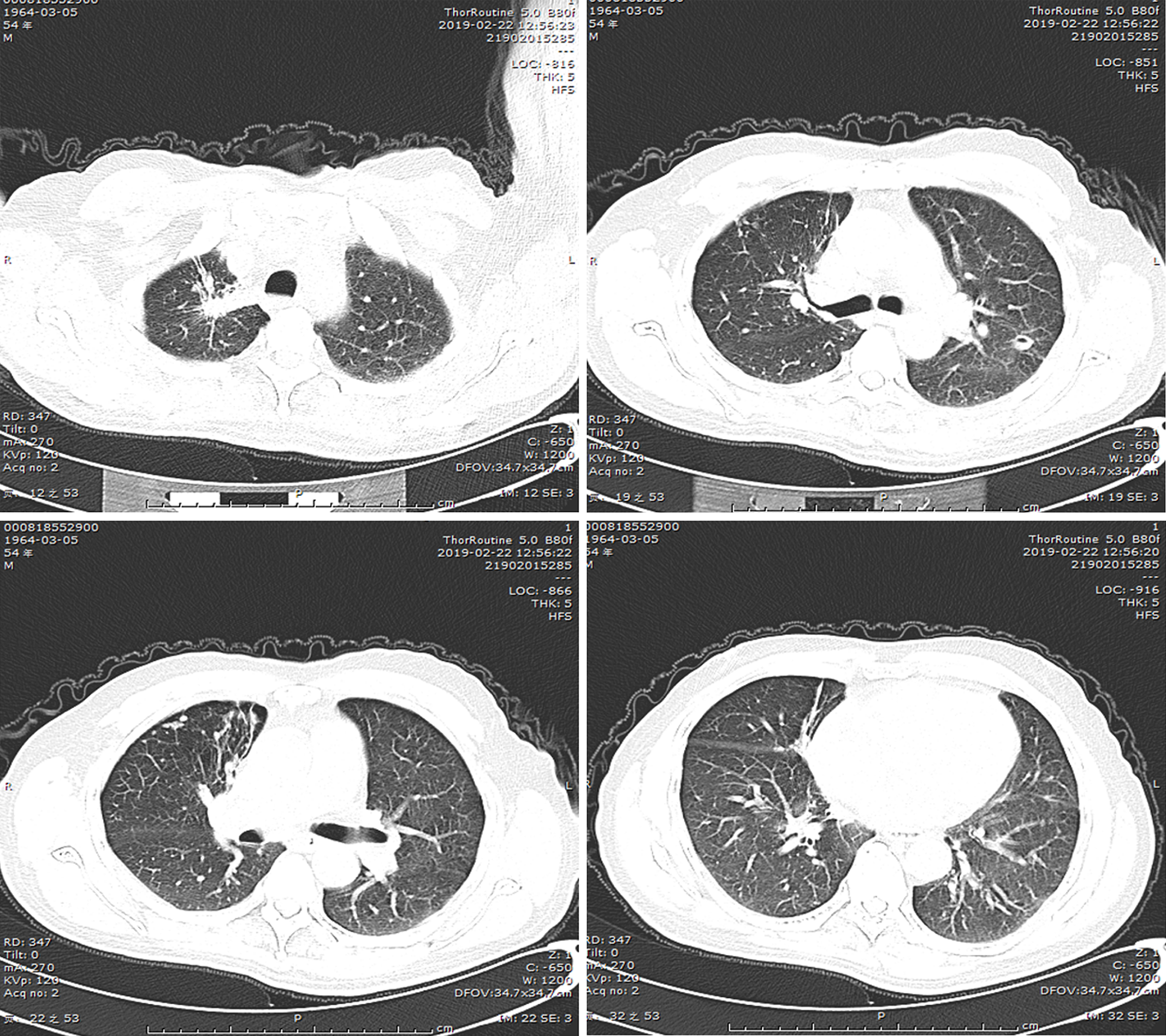
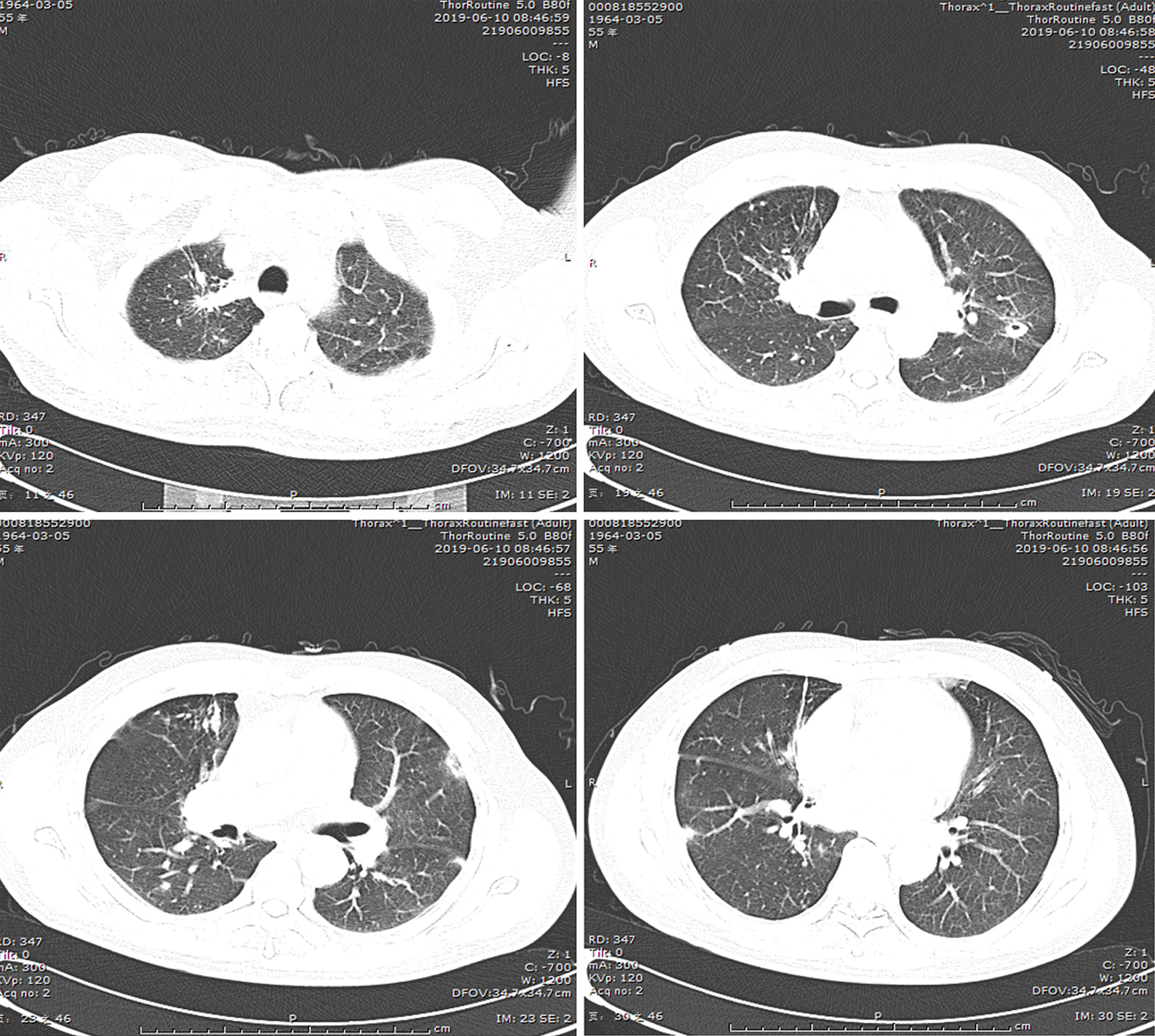
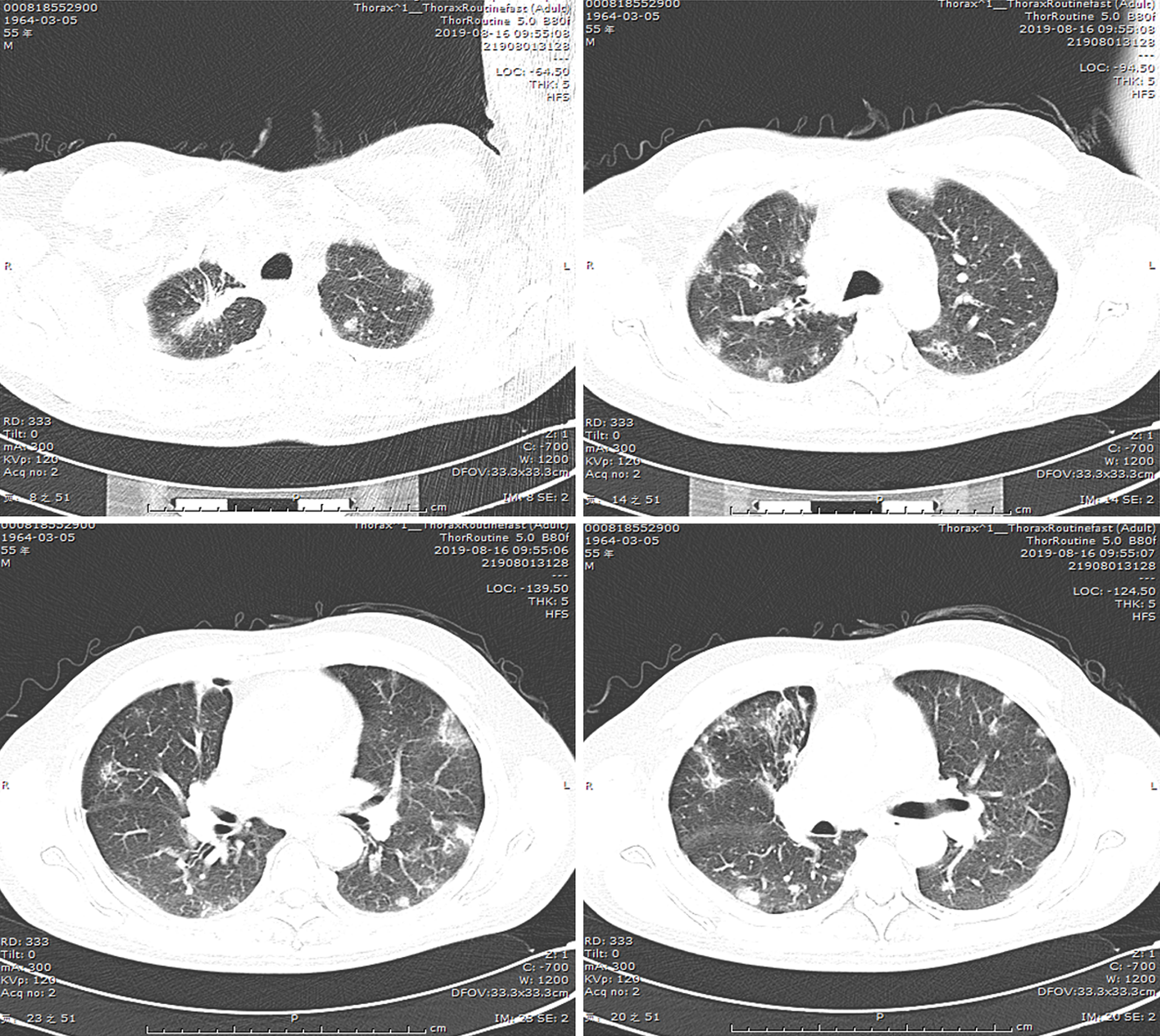
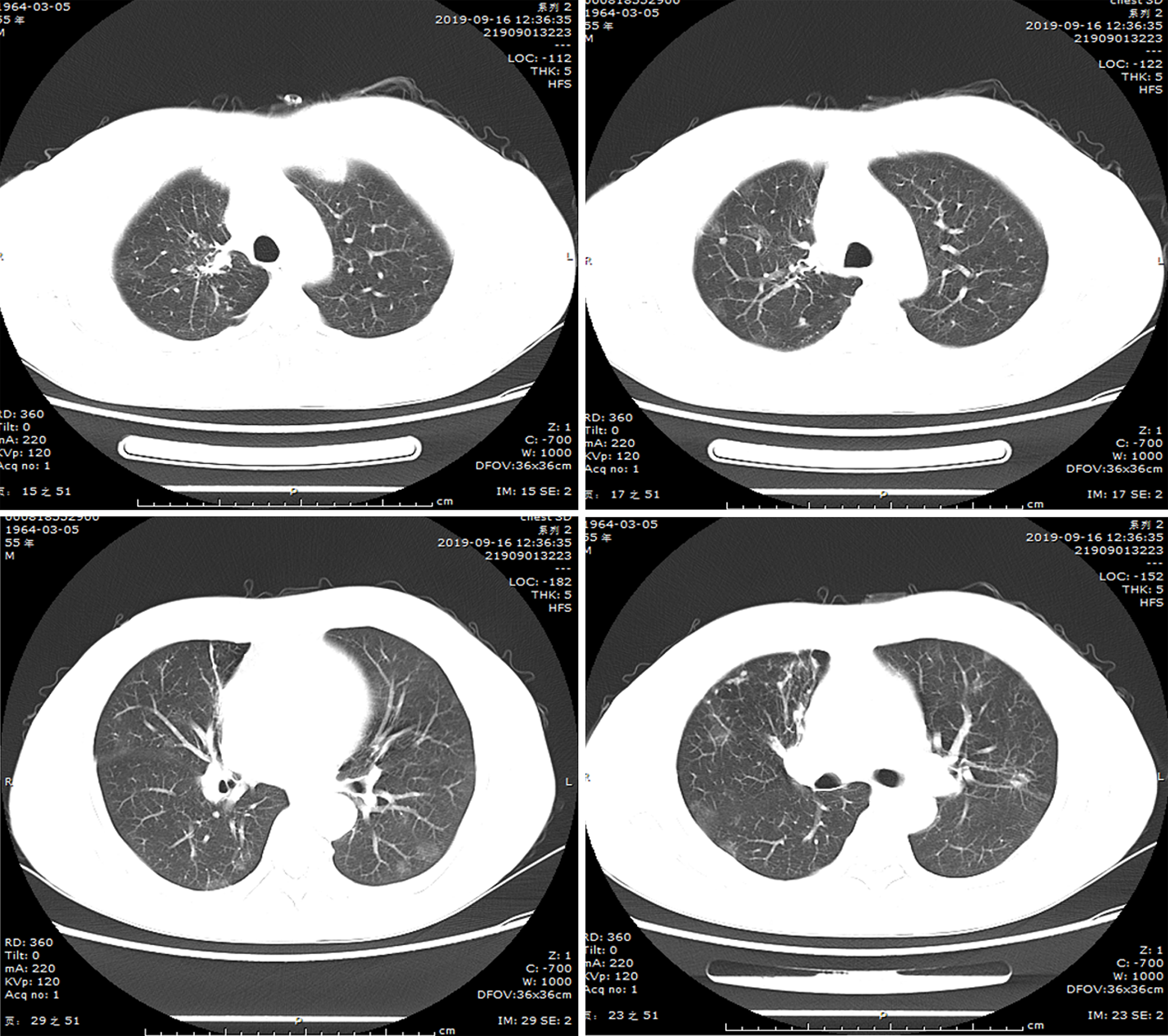
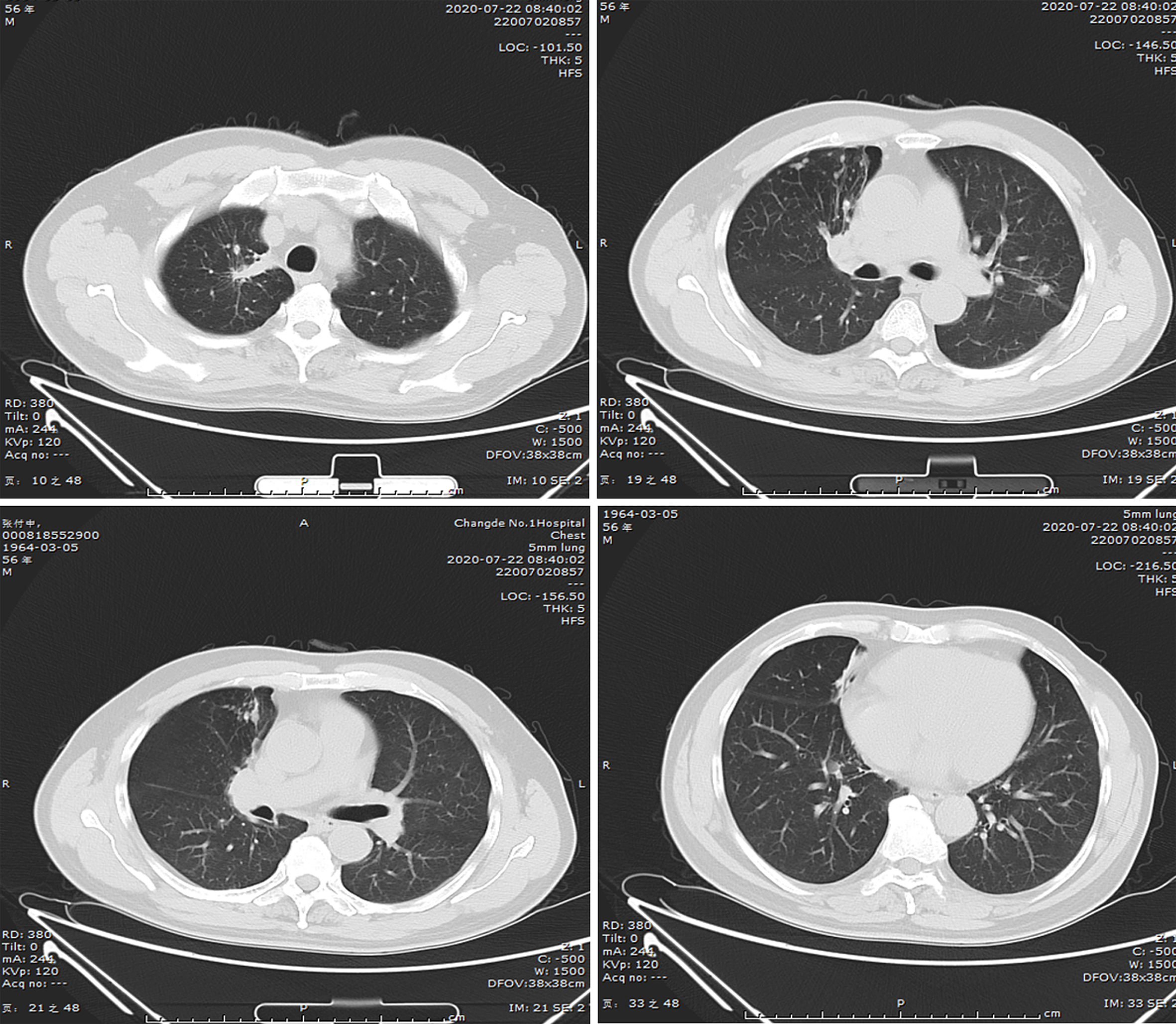
(1) A local hospital's chest radiograph showed right-sided pleural effusion and chronic bronchitis changes; (2) Lung CT imaging at our hospital (September 10, 2018): Showed multiple bilateral nodules and cavitary lesions, suggesting infection particularly due to tuberculosis (Figure 3); (3) Following a 5-month course of anti-tuberculosis treatment, the subsequent CT scan revealed complete closure of the cavity in the right upper lung, absorbtion of pleural effusion in the right hemithorax, and significant reduction in size of the cavity in the lower left lung (Figure 4); (4) A follow-up lung CT scan in June 2019 revealed new multifocal subpleural ground-glass opacities in both lungs (Figure 5); (5) Another lung CT reexamination on August 16, 2019, indicated a significant increase in ground-glass opacities in both lungs (Figure 6); (6) On August 21, 2019, bronchoscopy and bronchoalveolar lavage (BAL) of the upper lobes of both lungs were performed and no significant abnormalities were observed; (7) On September 16, 2019, after undergoing hormone therapy, subsequent dynamic pulmonary CT exams showed improvement in lesion absorption and gradual closure of the cavity (Figure 7); and (8) On July 22, 2020, A one-year follow-up lung CT scan showed no new lesions (Figure 8).
Our patient developed new bilateral pulmonary lesions after 9 months of antituberculosis treatment and the possible causes were as follows: (1) Development of resistance after antituberculosis treatment; (2) Secondary bacterial or fungal infection; and (3) Pulmonary manifestations of connective tissue disease. Subsequent lung biopsy revealed typical OP, but acid fast bacilus (AFB) staining results were negative. The low positive rate of AFB staining and the rapid appearance of new lesions in the right lower lung did not support a diagnosis of tuberculosis, but the persistent cavity lesions in the left lower lung were considered to indicate tuberculosis infection. Since corticosteroid therapy alone carries the risk of tuberculosis dissemination, we administered both antituberculosis and corticosteroid treatments after diagnosing OP. Over time, the newly developed subpleural lesions gradually absorbed and subsided, and cavity lesions in the lower left lung also closed. Therefore, we concluded that the OP was secondary to tuberculosis infection, not related to anti-tuberculosis medication or other factors.
(1) Pulmonary tuberculosis; (2) Tuberculous pleurisy; (3) Drug-induced dermatitis; and (4) Secondary organizing pneumonia.
(1) Treatment began with isoniazid (0.3 g/day), ethambutol (0.75 g/day), pyrazinamide (1.5 g/day), and rifampicin (0.6 g/day). One week after the treatment, the patient developed a rash on the trunk, accompanied by fever, suggesting an allergic reaction to anti-tuberculosis drugs. The anti-tuberculosis treatment was temporarily halted, and anti-allergy treatment was initiated. After the rash subsided, the regimen was adjusted to rifampicin, ethambutol, pyrazinamide, and levofloxacin; and (2) After the organizing pneumonia was confirmed, treatment was initiated with 40 mg methylprednisolone once daily. The steroid was gradually tapered off and discontinued after a total course of six months. Anti-tuberculosis medication was also discontinued at the same time.
Follow-up lung CT scan showed improvement in lesion absorption and gradual closure of the cavity, a one-year follow-up lung CT scan showed no new lesions.
The epidemiology of OP is not well understood currently. An early retrospective study suggests an incidence rate of 1.97 to 7 per 100000 individuals, with the median age of onset between 50 and 60 years[4]. The clinical manifestations of OP are nonspecific, which always delayed diagnosis by 6 to 12 weeks. Common symptoms include dry cough, flu-like symptoms, and respiratory distress, while hemoptysis is rare and acute respiratory distress syndrome is uncommon. Breath crackles are often observed in OP patients, yet around 25% of patients have normal lung auscultation findings. There are no specific laboratory tests for diagnosing OP. About 50% of patients may have leukocytosis, along with elevated c-reactive protein and erythrocyte sedimentation rate. Pulmonary function tests generally show mild-to-moderate diffusion dysfunction, and some smokers may exhibit obstructive ventilation dysfunction[5].
In clinical practice, once OP is diagnosed through tissue biopsy, it should not be readily categorized as COP. It is crucial to differentiate the etiology and actively rule out SOP. In contrast to COP with no determined causes, SOP could be specifically triggered by infection, connective tissue disease, drug damage, inflammatory bowel disease, hematologic malignancies, organ transplantations, and radiation therapy[3]. Histopathological presence of epithelioid cell granulomas or giant multinucleated cells suggests infection-related OP[6], with viruses and fungi as primary causes[7-9]. However, OP secondary to tuberculosis is rarely reported, possibly due to the low incidence of OP itself and the low positive detection rate of both AFB staining and tuberculosis cultures. A previous study analyzed 11 global cases of tuberculosis complicated with OP and found that the positive rate of sputum AFB staining was only 18%, while the positive rate of MTB DNA detection in sputum or bronchoalveolar lavage fliud (BALF) reached 87.5%[10]. Therefore, MTB DNA detection in sputum or BALF should be recommended for early diagnosis of tuberculosis.
Currently, the mechanism of tuberculosis infection leading to OP remains unclear. A previous research suggests that MTB may first enter the alveolar epithelial cells before being phagocytosed. The MTB infection-mediated immune response causes damage to the alveolar epithelial cells. Subsequently, neutrophils and lymphocytes are triggered, becoming activated and aggregated, and release inflammatory mediators and cytokines. Concurrently, fibroblasts are also activated. During alveolar damage, leakage of coagulation proteins occurs, leading to the accumulation of fibrin. The activity of fibrinolysis decreases, eventually resulting in fibrosis and granuloma formation[11]. This process is reversible in its early stages; early diagnosis and glucocorticoid use can prevent irreversible pulmonary fibrosis and impaired gas exchange function.
The imaging features of OP are vary with over 70% of patients showing subpleural ground-glass opacities and/or peribronchovascular consolidation. These lesions are usually bilateral and asymmetric; single or multiple nodules are uncommon, and in some instances, the lesions may exhibit migratory changes. Unlike those in COP and other SOPs, the lesions in infection-associated SOP cases are mostly fixed due to the relatively fixed location of the infection[12]. Previously reported cases of tuberculosis complicated with OP mostly involved localized consolidation or nodular lesions, often presenting as focal OP[10,13,14]. Huang et al[15] reported a patient with multiple migratory ground-glass opacities distributed bilaterally in the subpleural region of the lungs. Uniquely, in the current study, the patient with tuberculosis-induced OP showed scattered bilateral ground-glass opacities, predominantly in the lower lungs, without migratory changes. Furthermore, in previous studies, radiological signs of disseminated tuberculosis, such as cavities and tree-in-bud signs, were seldom observed in patients with pulmonary tuberculosis-related SOP.
The temporal boundary between infection and the occurrence of SOP is unclear. In some patients, infection and OP coexist, whereas in others, the inflammatory response persists even after the infection has been resolved, leading to the development of OP caused by an excessive inflammatory response[16,17]. In all previous domestic and international reports of tuberculosis complicated with OP, the two conditions have coexisted. In this study, our patient developed new bilateral pulmonary lesions after 9 months of antituberculosis treatment and the possible causes were as follows: (1) Development of resistance after antituberculosis treatment; (2) Secondary bacterial or fungal infection; and (3) Pulmonary manifestations of connective tissue disease. To clarify the aetiology, further bronchoscopy and BAL, in addition to tests for drug-resistant tuberculosis and connective tissue disease, were performed, and the results of all tests were negative. Subsequent lung biopsy revealed typical OP, but AFB staining results were negative. The low positive rate of AFB staining and the rapid appearance of new lesions in the right lower lung did not support a diagnosis of tuberculosis, but the persistent cavity lesions in the left lower lung were considered to indicate tuberculosis infection. Since corticosteroid therapy alone carries the risk of tuberculosis dissemination, we administered both antituberculosis and corticosteroid treatments after diagnosing OP. Over time, the newly developed subpleural lesions gradually absorbed and subsided, and cavity lesions in the lower left lung also closed. Therefore, we concluded that the OP was secondary to tuberculosis infection, not related to anti-tuberculosis medication or other factors.
In summary, pulmonary tuberculosis complicated with OP is rare in clinical setting. Its diagnosis requires a comprehensive evaluation of the patient’s medical history, symptoms, radiological findings, and histopathological examination. In treating patients with active pulmonary tuberculosis, if an increase in lesions is observed during anti-tuberculosis treatment, it is necessary to consider the possibility of tuberculosis-related SOP, apart from excluding factors like drug resistance. Specifically, timely lung biopsy is essential for early intervention. In some cases, anti-tuberculosis treatment alone may suffice, but if glucocorticoids are used, enhanced anti-tuberculosis treatment is necessary to prevent tuberculosis dissemination.
We thank Rui P for assisting in preparation of this manuscript.
| 1. | Baque-Juston M, Pellegrin A, Leroy S, Marquette CH, Padovani B. Organizing pneumonia: what is it? A conceptual approach and pictorial review. Diagn Interv Imaging. 2014;95:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Raghu G, Meyer KC. Cryptogenic organising pneumonia: current understanding of an enigmatic lung disease. Eur Respir Rev. 2021;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Krupar R, Kümpers C, Haenel A, Perner S, Stellmacher F. [Cryptogenic organizing pneumonia versus secondary organizing pneumonia]. Pathologe. 2021;42:55-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Gudmundsson G, Sveinsson O, Isaksson HJ, Jonsson S, Frodadottir H, Aspelund T. Epidemiology of organising pneumonia in Iceland. Thorax. 2006;61:805-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Cherian SV, Patel D, Machnicki S, Naidich D, Stover D, Travis WD, Brown KK, Naidich JJ, Mahajan A, Esposito M, Mina B, Lakticova V, Cohen SL, Muller NL, Schulner J, Shah R, Raoof S. Algorithmic Approach to the Diagnosis of Organizing Pneumonia: A Correlation of Clinical, Radiologic, and Pathologic Features. Chest. 2022;162:156-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Sander R, Gómez C, Borderías L. Organizing pneumonia and pulmonary tuberculosis: Coexistent or associated diseases. Arch Bronconeumol. 2016;52:570-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Asai N, Yokoi T, Nishiyama N, Koizumi Y, Sakanashi D, Kato H, Hagihara M, Suematsu H, Yamagishi Y, Mikamo H. Secondary organizing pneumonia following viral pneumonia caused by severe influenza B: a case report and literature reviews. BMC Infect Dis. 2017;17:572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Sakurai A, Yanai H, Ishida T, Kuwata H, Kamei K, Izumi S. Possible relationship between organizing pneumonia and chronic pulmonary aspergillosis: A case report and literature review. Respir Investig. 2017;55:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Ketchersid K. A review of organizing pneumonia. JAAPA. 2023;36:16-19. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Kim EJ, Kim KC. Pulmonary tuberculosis presenting secondary organizing pneumonia with organized polypoid granulation tissue: case series and review of the literature. BMC Pulm Med. 2020;20:252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Kligerman SJ, Franks TJ, Galvin JR. From the radiologic pathology archives: organization and fibrosis as a response to lung injury in diffuse alveolar damage, organizing pneumonia, and acute fibrinous and organizing pneumonia. Radiographics. 2013;33:1951-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Arenas-Jiménez JJ, García-Garrigós E, Ureña Vacas A, Sirera Matilla M, Feliu Rey E. Organizing pneumonia. Radiologia (Engl Ed). 2022;64 Suppl 3:240-249. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Yoon HS, Lee EJ, Lee JY, Chon GR, Lee SH, Kim SJ. Organizing pneumonia associated with M ycobacterium tuberculosis infection. Respirol Case Rep. 2015;3:128-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Hsieh MH, Lin CY. Pulmonary tuberculosis presenting as organizing pneumonia. Am J Respir Crit Care Med. 2014;189:e63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Huang LL, Wang C, Liu Y, Gu XY, Wang WX, Chen W, Hu CM. Resolution of an insidious and migratory Mycobacterium tuberculosis-associated secondary organizing pneumonia: a case report and literature review. BMC Infect Dis. 2023;23:372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Huo Z, Feng R, Tian X, Zhang H, Huo L, Liu H. Clinicopathological findings of focal organizing pneumonia: a retrospective study of 37 cases. Int J Clin Exp Pathol. 2015;8:511-516. [PubMed] |
| 17. | Zhou H, Gu W, Li C. Post-Infectious Organizing Pneumonia: an Indistinguishable and Easily Misdiagnosed Organizing Pneumonia. Clin Lab. 2015;61:1755-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |