Published online Sep 16, 2024. doi: 10.12998/wjcc.v12.i26.5960
Revised: June 19, 2024
Accepted: July 2, 2024
Published online: September 16, 2024
Processing time: 118 Days and 17.8 Hours
Gastrointestinal tract metastasis from lung cancer is rare and compared to small cell lung cancer (SCLC), non-SCLC (NSCLC) is even less likely to metastasize in this manner. Additionally, small intestinal tumors can also present with diverse complications, some of which require urgent intervention.
In this report, we detail a unique case of stage IV lung cancer, where the presence of small intestine tumors led to intussusception. Subsequent to a small intestine resection, pathology confirmed that all three tumors within the small intestine were metastases from adenocarcinoma of the lung. The postoperative follow-up period extended beyond 14 mo.
In patients with stage IV NSCLC, local tumor control can be achieved with va
Core Tip: Small bowel metastases may occur in advanced lung cancer, leading to gastrointestinal obstruction and requiring aggressive surgical intervention. In such patients, survival may be improved if the gastrointestinal obstruction is relieved and the primary lung lesion can be controlled.
- Citation: Niu QG, Huang MH, Kong WQ, Yu Y. Stage IV non-small cell lung cancer with multiple metastases to the small intestine leading to intussusception: A case report. World J Clin Cases 2024; 12(26): 5960-5967
- URL: https://www.wjgnet.com/2307-8960/full/v12/i26/5960.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i26.5960
Lung cancer is one of the leading causes of mortality among malignant diseases in humans and is characterized by high incidence and mortality rates, with the majority of deaths resulting from metastasis. Based on histopathological characteristics, lung cancer is primarily classified into non-small cell lung cancer (NSCLC) and SCLC. NSCLC accounts for approximately 80%-85% of all lung cancer cases[1]. Metastasis in lung cancer involves the spread of primary malignant tumors of the lung from their origin to distant sites through various mechanisms. The most common sites of metastasis include the brain, bones, lymph nodes, and liver[1,2], among which metastasis to the bone is the most frequent site, occurring in about 20%–40% of lung cancer patients[2,3]. NSCLC with brain metastases has the worst prognosis, with a 2-year overall survival rate of no more than 10%-20%[3,4]. Compared to SCLC, the occurrence of multiple metastases in NSCLC is significantly rarer. In NSCLC, cases of metastasis to soft tissue and organs such as the kidneys, pancreas, spleen, peritoneal cavity, intestines, bone marrow, eyes, ovaries, thyroid, heart, breasts, tonsils, and nasal cavity have been reported and found to be associated with a poor prognosis[5,6]. An analysis of 17431 deceased lung cancer patients diagnosed from 2002 to 2010 from the nationwide Swedish Cancer Registry revealed that the most frequent metastatic sites were the nervous system, bone, liver, respiratory system, and adrenal gland. Liver (35%) and nervous system (47%) metastases were common in patients with metastases from SCLC, and bone (39%) and respiratory system (22%) me
The incidence of gastrointestinal metastases from lung cancer is extremely low, and among sites, metastasis to the gastrointestinal tract is exceedingly rare, accounting for only 0.19% to 0.26% of all cases[13]. Among these, small intestinal metastasis is even rarer, reported in 11.9% of gastrointestinal cases, but has been found to substantially shorten the survival of lung cancer patients[14]. The occurrence of multiple metastases in NSCLC is also rarer compared to SCLC. In this case report, we present a rare case of NSCLC with multiple metastases to the small intestine. And we describe all treatment procedures and postoperative follow-ups.
Nausea and vomiting for one day with fever.
A 62-year-old female patient presented with sudden onset diarrhea, which began one day prior without an identifiable trigger. Her symptoms included watery stools with frequent episodes, accompanied by abdominal distension and an absence of flatus. The patient also reported nausea and vomiting, with vomitus comprising gastric contents. She developed a fever, peaking at 38 °C (100.4 °F). Due to persistent symptoms, she was admitted to the general surgery department on September 7, 2022.
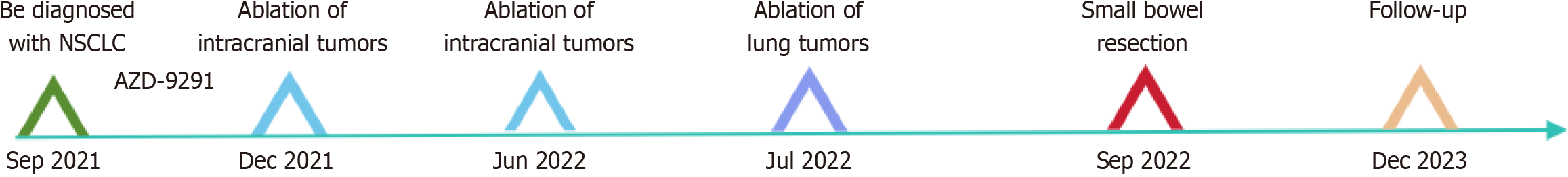
The patient was diagnosed with NSCLC in September 2021 at Zhongshan Hospital affiliated with Fudan University (Shanghai, China). An enhanced chest computed tomography (CT) scan at that time revealed a lobulated soft tissue mass adjacent to the right pulmonary hilum, measuring 3.9 cm in diameter, with irregular margins and uneven internal density, accompanied by enhancement. Biopsy pathology indicated poorly differentiated NSCLC, with the specific pathological type not further clarified. A cranial CT scan suggested possible intracranial metastasis. The patient did not undergo surgery but began oral targeted therapy with osimertinib (AZD-9291). Subsequently, the patient developed neurological symptoms, including headaches and seizures. Due to brain metastasis from lung cancer, the patient underwent two intracranial tumor ablation surgeries in December 2021 and June 2022 at another hospital, followed by a lung tumor ablation surgery in July 2022.
The patient reported no remarkable personal and family history.
Upon admission, physical examination revealed a flat and soft abdomen with scattered epigastric tenderness, without rebound pain or muscle tensing. The patient’s vital signs were: Body temperature, 37.3 °C (90.14 °F); heart rate, 100 beats/min; respiration rate: 18 breaths/min; blood pressure, 12.13/7.33 kPa.
The patient’s laboratory testing results were: White blood cell count, 10.65 × 109/L; C-reactive protein, 61.87 mg/L; neutrophil percentage, 87.4%; haemoglobin, 87 g/L; platelet count: 210 × 109/L.
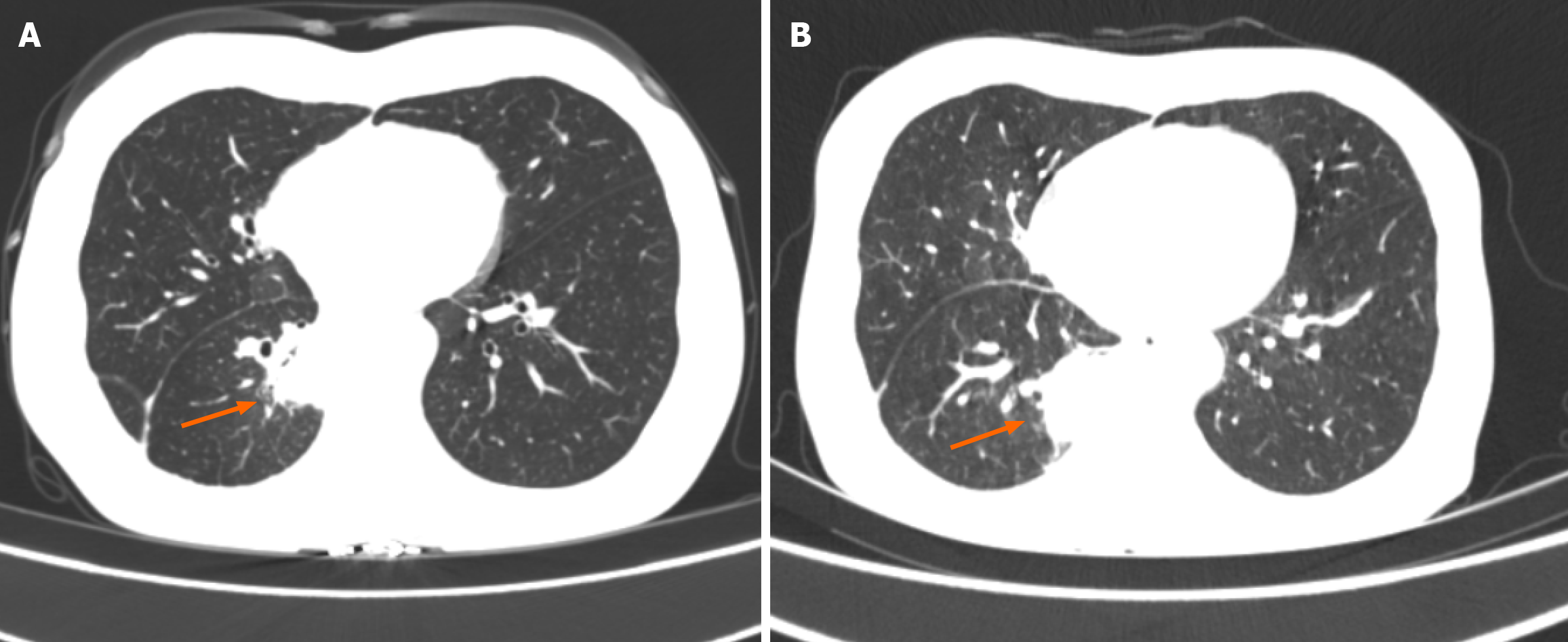
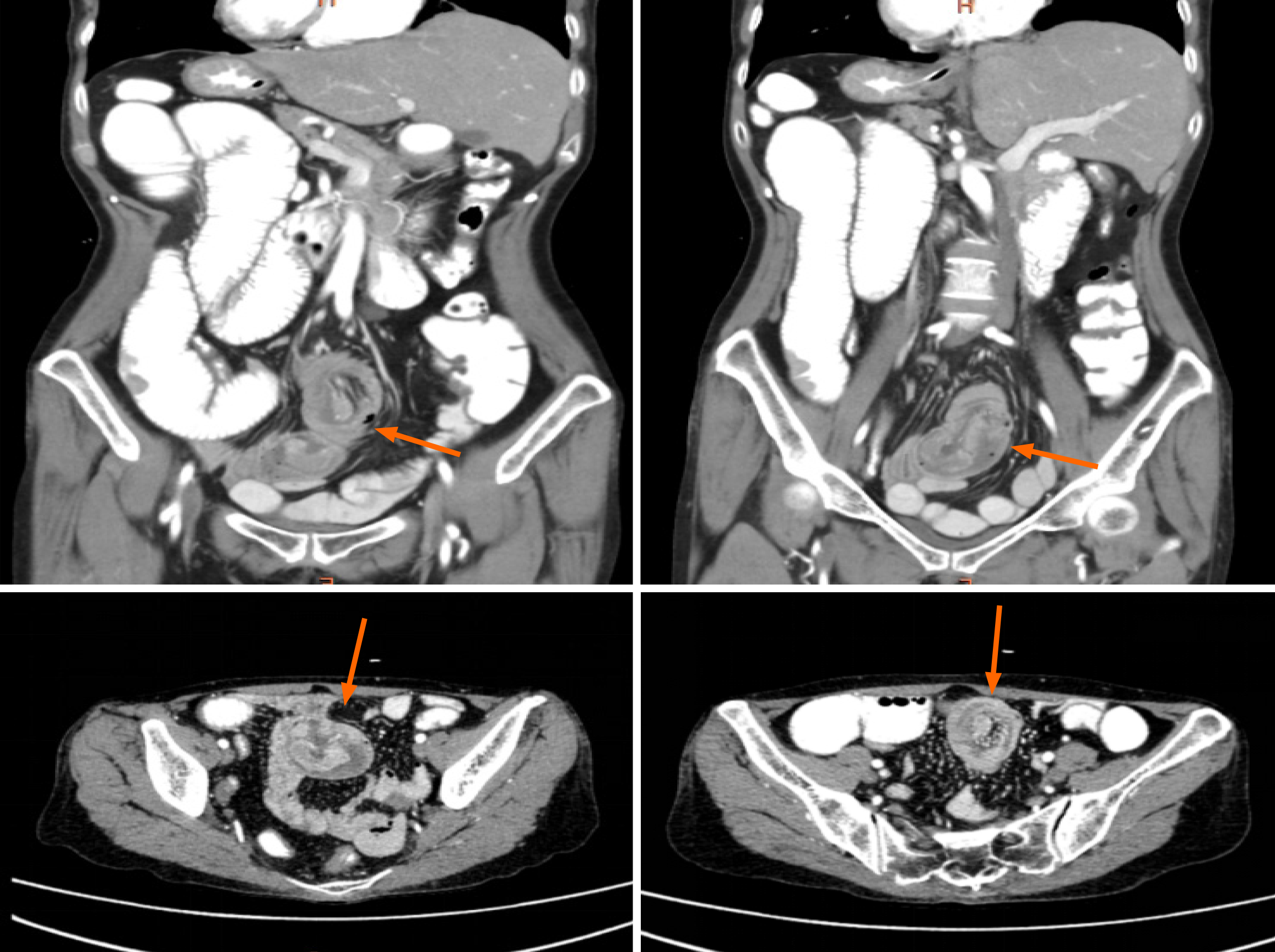
A pre-admission plain CT scan indicated partial small intestinal obstruction and a possible intra-abdominal hernia. A subsequent chest CT scan was also conducted upon admission (Figure 1). After oral contrast administration, an enhanced CT scan (Figure 2) suggested nodular thickening of the small intestinal wall in the left abdomen. A segment of the small intestine in the pelvic region was found to exhibit a circular intussusception structure, with localized wall thickening and uneven enhancement post-contrast, and was accompanied by significant dilation of the upstream small intestine, accumulation of gas and fluid with fluid levels, and the presence of gas within the wall, indicating a high likelihood of strangulation.
Based on the patient's history, the clinical diagnosis was as follows: Small intestine tumor with intussusception, small intestinal obstruction, post-lung cancer ablation status, post-brain tumor ablation status, and anemia.
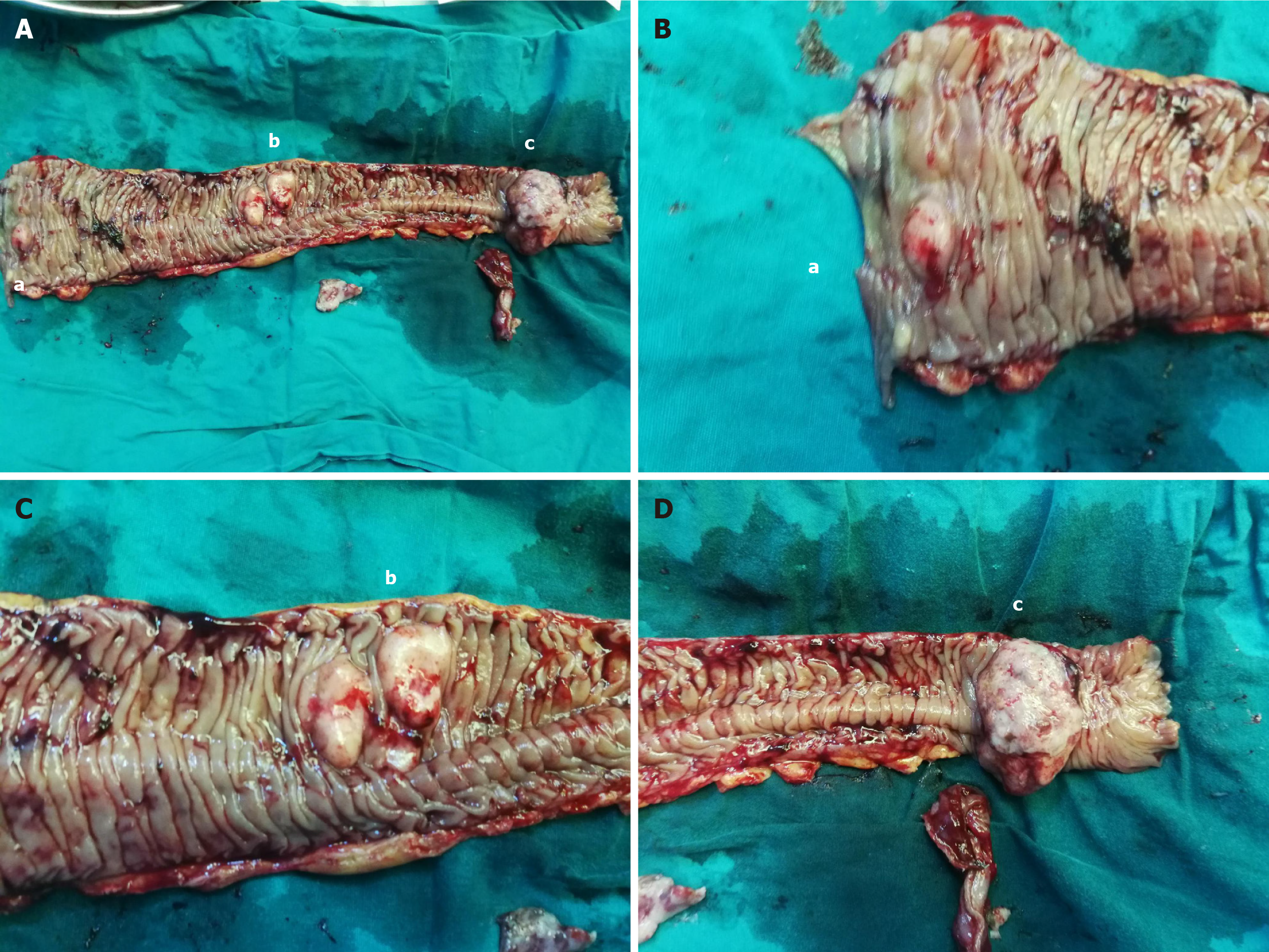
After consultations with the patient and her family, an exploratory laparotomy was performed. During the surgery, approximately 200 mL of brownish-yellow ascites was found in her abdominal cavity. An intussusception of the small intestine was identified about 100 cm from the Treitz ligament, with the intussuscepted segment measuring approximately 40 cm in length and showing significant local edema. The proximal small intestine was slightly dilated, and at the site of intussusception, three small intestinal tumors were identified. They were palpable and firm in consistency, with the largest being 6 cm and the smallest 1 cm in diameter. A segment of the small intestine, approximately 50 cm in length, including the tumors and the intussuscepted portion, was resected 5 cm away from the tumors, followed by a side-to-side anastomosis using a linear cutting stapler. No significant lymph node enlargement was observed at the root of the mesentery. The resected specimen was visually examined, as shown in Figure 3.
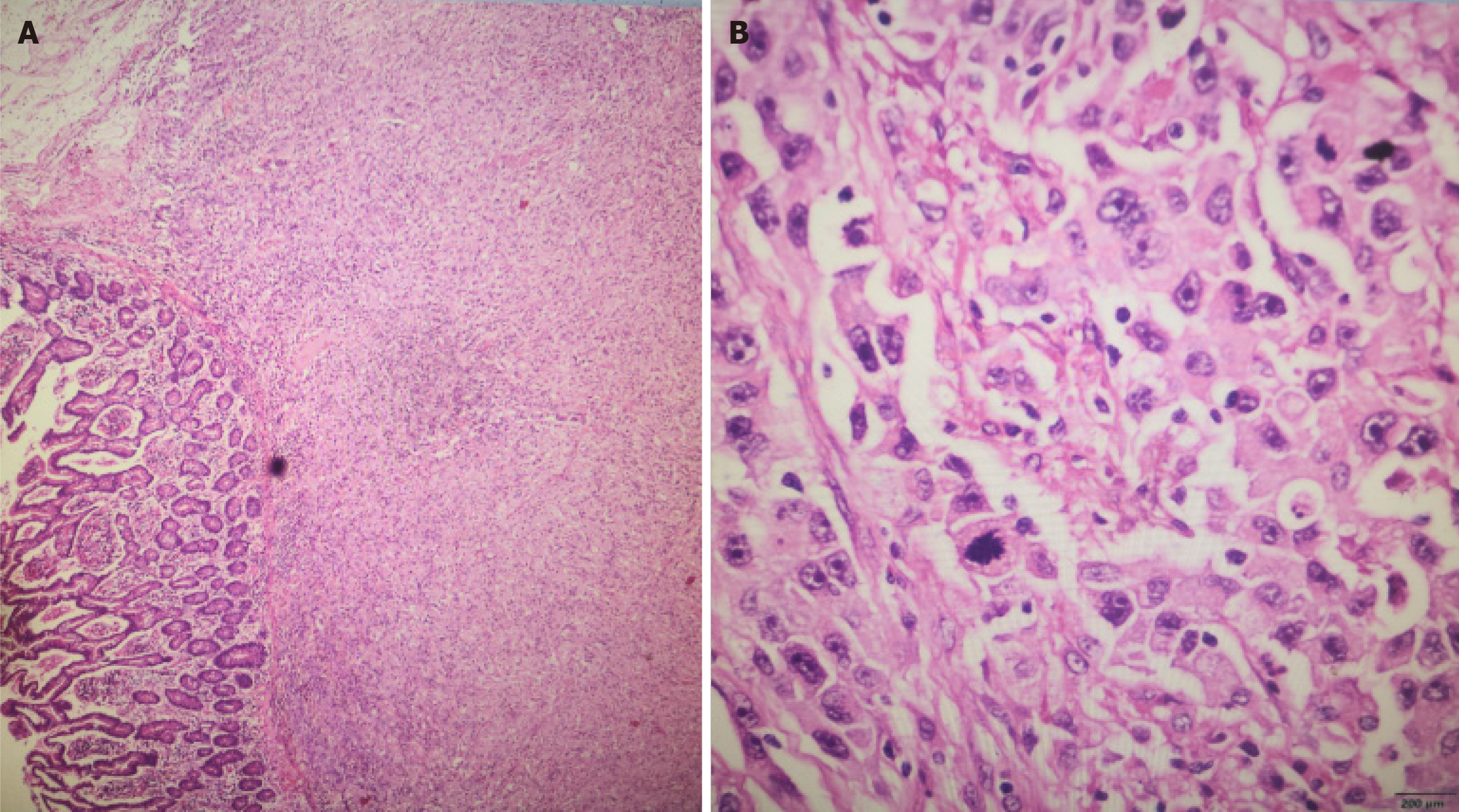
On the fifth postoperative day, the patient's surgical wound showed satisfactory healing. She tolerated a semi-liquid diet without discomfort and was discharged after the normal resumption of gas and bowel movements. The postoperative pathology report indicated a malignant tumor in the small intestine, consistent with metastatic poorly differentiated adenocarcinoma from the lung (Figure 4). The tumor infiltrated up to the serosal layer, exhibited vascular invasion, and had clear resection margins. There was no evidence of nerve invasion. Immunohistochemistry results were as follows: CK7 (+), Napsin A (+), Ki67 (80%+), ALK (-), AE1/AE3 (+), TTF-1 (+), P40 (-), CK20 (-), Syn (-), Villin (-), CD34 (-), CD117 (-), Dog-1 (-), and D2-40 (+). Special staining for AB-PAS was also performed.
The patient was followed for over 14 mo after discharge. The timeline of the patient's disease diagnosis and treatment is shown in Figure 5. At the most recent successful follow-up conducted by telephone in April 2024, she was continuing her medication regimen, but her quality of life was declining. Regrettably, the patient is currently lost to follow-up, suggesting that she might have either passed away or sought treatment at another hospital.
This case report describes a patient who developed multiple small bowel metastases from advanced lung cancer with multiple metastases. The patient had undergone various non-radical therapies, including targeted agents and ablative therapy, and was receiving oral osimertinib. Osimertinib is a third-generation, irreversible, oral epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor that potently and selectively inhibits both EGFR-sensitizing mutations and the T790M mutation. It has shown a 71% objective response rate with a median progression-free survival of 8.5 mo[15]. After the discovery of brain metastases, the patient underwent intracranial tumor ablation. Brain metastases are the most common intracranial tumors, occurring ten times more frequently than primary brain tumors[16]. To address inoperable cases, minimally invasive techniques like magnetic resonance imaging-guided laser interstitial thermotherapy (LITT) have been explored. In this regard, LITT delivers precise heat in a controlled manner, aiming to minimize collateral damage[17-19]. The patient's lung tumor was also ablated. Microwave ablation (MWA) is an important alternative therapy to conventional surgery for the treatment of lung cancer. In addition to eliminating local tumors, MWA may promote antitumor immunological responses, such as abscopal effects in distant lesions[20], and for inoperable early-stage and metastatic lung cancer, MWA is usually performed[21]. It causes water molecules within tumor tissue to vibrate and collide in microwave electromagnetic fields, resulting in high temperatures and a coagulative necrotic zone in which local tumor cells are killed[22]. For local treatment, MWA is precise, with minimal invasiveness and few complications. In our case, the patient also required a small bowel resection for intussusception, and follow-up after surgery showed that the patient was still undergoing treatment. We report this case not only due to its clinical rarity but also to illustrate a potential treatment strategy for similar cases. Comprehensive evaluations, targeted surgical interventions, and systemic therapies tailored to individual patients - conducted through multidisciplinary team discussions - can enhance survival rates and quality of life[23,24]. The primary goal of surgery is to alleviate symptoms while minimizing the tumor burden. Early detection, accurate diagnosis, and prompt treatment are essential for improving patient outcomes.
Gastrointestinal metastasis from lung cancer is caused by haematogenous spread and usually occurs in the end stage of lung cancer. Symptoms always present with clinical complications, including gastrointestinal perforation, haemorrhage, and obstruction. Our case suggested that aggressive management of complications has the potential to improve patient survival. Xie et al[25] reported on two male patients in their 60s with primary poorly differentiated NSCLC with initially undetectable distant metastases. Both cases presented with abdominal pain and distension, and immunohistochemistry of small bowel biopsy samples obtained by endoscopy confirmed lung cancer metastasis. The biopsies were then subjected to second-generation sequencing, which identified mutations in the genes TP53, LRP1B, and FGFR2, which are associated with the onset and progression of poorly differentiated NSCLC and its small bowel metastasis. Because digestive tract metastasis of lung cancer is often insidious, studying the genetic profile of NSCLC small bowel metastasis by second-generation sequencing is crucial for early detection and prevention[25]. Recent studies have observed that estrogen promotes (NSCLC) metastasis by facilitating the formation of invasive pseudopods in NSCLC cells via estrogen receptor beta[26].
Therefore, if occult metastases from advanced lung cancer can be recognized as early as possible so that early intervention can be carried out, it may improve the patient's prognosis considerably. This will also broaden new ideas on treatment strategies for other patients with advanced NSCLC.
In conclusion, patients diagnosed with stage IV NSCLC and small bowel metastases could be recommended to undergo prompt surgical intervention, provided that their condition allows, to alleviate obstruction, and may continue pharmacological therapy postoperatively. This approach outlines a potential treatment strategy for metastatic lesions, emphasizing the reduction of tumor burden through feasible surgical or ablative methods, with ongoing pharmacological management of the primary lung lesion aiming to enhance patient survival.
| 1. | Tamura T, Kurishima K, Nakazawa K, Kagohashi K, Ishikawa H, Satoh H, Hizawa N. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol. 2015;3:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 242] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 2. | Zhou Q, Zu L, Li L, Chen X, Chen X, Li Y, Liu H, Sun Z. [Screening and establishment of human lung cancer cell lines with organ-specific metastasis potential]. Zhongguo Fei Ai Za Zhi. 2014;17:175-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | D'Antonio C, Passaro A, Gori B, Del Signore E, Migliorino MR, Ricciardi S, Fulvi A, de Marinis F. Bone and brain metastasis in lung cancer: recent advances in therapeutic strategies. Ther Adv Med Oncol. 2014;6:101-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Dodson C, Richards TJ, Smith DA, Ramaiya NH. Tyrosine Kinase Inhibitor Therapy for Brain Metastases in Non-Small-Cell Lung Cancer: A Primer for Radiologists. AJNR Am J Neuroradiol. 2020;41:738-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Lin L, Wang X, Tang C, Liang J. Clinical Characteristics and Prognosis of Gastrointestinal Metastases in Solid Tumor Patients: A Retrospective Study and Review of Literatures. Anal Cell Pathol (Amst). 2019;2019:4508756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Niu FY, Zhou Q, Yang JJ, Zhong WZ, Chen ZH, Deng W, He YY, Chen HJ, Zeng Z, Ke EE, Zhao N, Zhang N, Sun HW, Zhang QY, Xie Z, Zhang XC, Wu YL. Distribution and prognosis of uncommon metastases from non-small cell lung cancer. BMC Cancer. 2016;16:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Gunev V. [Prenatal diagnosis of hereditary diseases]. Akush Ginekol (Sofiia). 1975;14:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 601] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 8. | Song Z, Lin B, Shao L, Zhang Y. Cutaneous metastasis as a initial presentation in advanced non-small cell lung cancer and its poor survival prognosis. J Cancer Res Clin Oncol. 2012;138:1613-1617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Li Y, Wong M, Zhan L, Corke L, Brown MC, Cheng S, Khan K, Balatnaram K, Chowdhury M, Sabouhanian A, Herman J, Walia P, Strom E, Patel D, García-Pardo M, Schmid S, Eng L, Sacher AG, Leighl N, Bradbury PA, Shepherd FA, Shultz D, Liu G. Single organ metastatic sites in non-small cell lung cancer: Patient characteristics, treatment patterns and outcomes from a large retrospective Canadian cohort. Lung Cancer. 2024;192:107823. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Smith S, Kao S, Boyer M, Franco M, Moore M. Treatment selection and real-world analysis of immunotherapy with or without chemotherapy in PD-L1-high metastatic non-small cell lung cancer. Intern Med J. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 11. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15320] [Article Influence: 3064.0] [Reference Citation Analysis (4)] |
| 12. | Hu Y, Feit N, Huang Y, Xu W, Zheng S, Li X. Gastrointestinal metastasis of primary lung cancer: An analysis of 366 cases. Oncol Lett. 2018;15:9766-9776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Wang M, Chen G, Luo J, Fan Z, Liu Y, Xie C, Gong Y. Case Report: Genetic profiling of small intestine metastasis from poorly differentiated non-small cell lung cancer: report of 2 cases and literature review of the past 5 years. Front Oncol. 2023;13:1265749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Yoshimoto A, Kasahara K, Kawashima A. Gastrointestinal metastases from primary lung cancer. Eur J Cancer. 2006;42:3157-3160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS; FLAURA Investigators. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:113-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2556] [Cited by in RCA: 3682] [Article Influence: 526.0] [Reference Citation Analysis (0)] |
| 16. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 9954] [Article Influence: 4977.0] [Reference Citation Analysis (2)] |
| 17. | Jethwa PR, Barrese JC, Gowda A, Shetty A, Danish SF. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: initial experience. Neurosurgery. 2012;71:133-44; 144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Salem U, Kumar VA, Madewell JE, Schomer DF, de Almeida Bastos DC, Zinn PO, Weinberg JS, Rao G, Prabhu SS, Colen RR. Neurosurgical applications of MRI guided laser interstitial thermal therapy (LITT). Cancer Imaging. 2019;19:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 19. | Shah AH, Semonche A, Eichberg DG, Borowy V, Luther E, Sarkiss CA, Morell A, Mahavadi AK, Ivan ME, Komotar RJ. The Role of Laser Interstitial Thermal Therapy in Surgical Neuro-Oncology: Series of 100 Consecutive Patients. Neurosurgery. 2020;87:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 20. | Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol. 2018;11:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 322] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 21. | Ma F, Lin Y, Ni Z, Wang S, Zhang M, Wang X, Zhang Z, Luo X, Miao X. Microwave ablation enhances the systemic immune response in patients with lung cancer. Oncol Lett. 2024;27:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 22. | Ni Y, Xu H, Ye X. Image-guided percutaneous microwave ablation of early-stage non-small cell lung cancer. Asia Pac J Clin Oncol. 2020;16:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Guo Z, Han B, Wang Y. [The status of diagnosis and therapy for gastrointestinal metastasis from primary lung cancer]. Zhongguo Fei Ai Za Zhi. 2011;14:69-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Kini S, Kapadia RM, Amarapurkar A. Intussusception due to intestinal metastasis from lung cancer. Indian J Pathol Microbiol. 2010;53:141-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Xie S, Wu Z, Qi Y, Wu B, Zhu X. The metastasizing mechanisms of lung cancer: Recent advances and therapeutic challenges. Biomed Pharmacother. 2021;138:111450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Qiu W, Chen J, Meng W, Zhao R, Lin W, Mei P, Diao M, Xiao H, Liao Y. ERβ promoted invadopodia formation-mediated non-small cell lung cancer metastasis via the ICAM1/p-Src/p-Cortactin signaling pathway. Int J Cancer. 2023;153:1287-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |