Published online Sep 6, 2024. doi: 10.12998/wjcc.v12.i25.5832
Revised: June 21, 2024
Accepted: July 4, 2024
Published online: September 6, 2024
Processing time: 64 Days and 15.7 Hours
Scrub typhus is a naturally occurring acute infectious disease that is primarily transmitted through the bites of chiggers or larval mites infected by Orientia tsutsugamushi (O. tsutsugamushi). Omadacycline, a novel tetracycline, exhibits potent antibacterial efficacy against both typical bacteria and atypical pathogens. However, omadacycline application in the treatment of scrub typhus remains limited.
In the present work, we report several cases of scrub typhus, with the main clini
tNGS is an effective method for diagnosing scrub typhus. Omadacycline can be considered an alternative option for antiinfective therapy in patients with O. tsutsugamushi infections.
Core Tip: Scrub typhus is a naturally occurring acute infectious disease that is primarily transmitted through the bites of chiggers or larval mites infected by Orientia tsutsugamushi (O. tsutsugamushi). In the present work, we report three cases of scrub typhus, with the main clinical symptoms being fever, the formation of eschar or ulcers, local or systemic lymphadenopathy, headache, myalgia and rash. Blood samples were collected before the administration of omadacycline. Omadacycline can be considered an alternative option for anti infective therapy in patients with O. tsutsugamushi infections.
- Citation: Lang XM, Qiu Y, Jia YJ, Sun H, Gao SM, Zhao HM. Omadacycline in the treatment of scrub typhus: Three case reports. World J Clin Cases 2024; 12(25): 5832-5838
- URL: https://www.wjgnet.com/2307-8960/full/v12/i25/5832.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i25.5832
Scrub typhus is a naturally occurring infectious disease that is transmitted primarily through the bites of chiggers or larval mites infected by Orientia tsutsugamushi (O. tsutsugamushi). The clinical manifestations of tsutsugamushi disease include fever, eschars or ulcers at the bite site, lymph node enlargement, and skin rash. Most cases generally resolve on their own, but in a few cases, severe complications or even death may occur[1,2]. The main endemic region for scrub typhus is the "tsutsugamushi triangle", which extends from the northernmost regions of South Korea, Japan, and Russia to northern Australia and westward to Pakistan and parts of Afghanistan[1]. Within this area, China is a significant endemic region[3]. The population most susceptible to tsutsugamushi disease consists primarily of farmers and workers[4].
Omadacycline possesses broad-spectrum antibacterial activity against gram-positive and gram-negative aerobic, anaerobic, and atypical bacteria. In clinical practice, there is only one reported use of oral omadacycline[5], while intravenous omadacycline has not been reported for the treatment of scrub typhus. In this report, we present three cases of scrub typhus treated with intravenous omadacycline, which resulted in favorable outcomes.
Case 1: A 59-year-old female presented with a week-long fever.
Case 2: A 68-year-old elderly woman presented with a fever that had persisted for more than 10 days.
Case 3: A 51-year-old female presented with a fever that had persisted for 13 days.
Case 1: The patient developed a fever one week prior after continuous fieldwork exposure, with a maximum body temperature of 39 °C. The patient visited Huai'an Hospital three days prior. Routine blood work revealed a white blood cell (WBC) count of 4.4 × 109/L and a neutrophil percentage of 53.4%. Despite three days of treatment with moxifloxacin (400 mg by intravenous infusion once daily) for infection, the patient’s fever persisted. The patient was transferred to our hospital for further treatment.
Case 2: The patient was admitted to Shuyang Mercy Hospital on November 3, 2023, due to fever that had persisted for five days. She developed a fever after agricultural work, with a maximum body temperature of 39.5 °C. Other symptoms included chills and pain in the right lower limb. The patient self-administered amoxicillin and ibuprofen but still experienced intermittent fever. Routine blood work revealed a WBC count of 3.7 × 109/L and a neutrophil percentage of 57.5%. Despite receiving a combination of azithromycin (500 mg by intravenous infusion once daily) and moxifloxacin (400 mg by intravenous infusion once daily) for antiinfective therapy for five days, the patient’s fever persisted. The patient was transferred to our hospital for treatment on November 8, 2023.
Case 3: The patient had a fever after outdoor activity in a park, with a maximum body temperature of 39 °C occurring on November 24, 2023. Other symptoms included chills, headache and fatigue. Two days before admission, the patient went to the Huai’an Fifth People’s Hospital for treatment. Piperacillin/tazobactam and Reduning were administered to her in that hospital. However, the effect was not satisfactory.
Case 1: The patient has a history of good health.
Case 2: The patient had a history of hypertension and cerebral infarction.
Case 3: The patient had a history of obsolete pulmonary tuberculosis, uterine leiomyomas and an allergy to quinolones.
No significant family history.
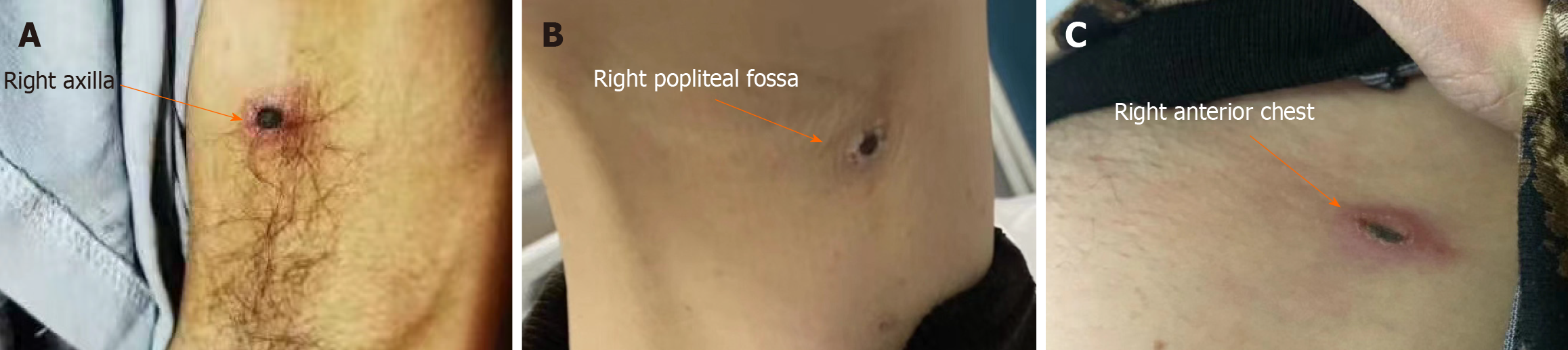
Case 1: The physical examination results were as follows: Body temperature, 36.4 °C; pulse, 106 bpm; respiration rate, 20 breaths/min; and blood pressure, 138/85 mmHg. An eschar with a diameter of approximately 2 cm × 2 cm was found in the right axilla of the patient; the area surrounding the eschar was red (Figure 1A). Enlarged lymph nodes were palpable in both the axillary and inguinal regions. No other significant abnormalities were observed.
Case 2: The physical examination results were as follows: Body temperature, 37 °C; pulse, 78 bpm; respiration rate, 21 breaths/min; and blood pressure, 98/49 mmHg. An eschar with a diameter of approximately 1.5 cm × 1.5 cm was found in the right popliteal fossa (Figure 1B). Enlarged inguinal lymph nodes were palpable.
Case 3: The physical examination results were as follows: Body temperature, 38.1 °C; pulse, 92 bpm; respiration rate, 20 breaths/min; and blood pressure, 115/75 mmHg. An eschar with a diameter of approximately 2.0 cm × 1.5 cm was found on her left anterior chest, with part of the eschar already peeled off (Figure 1C).
Case 1: The main laboratory test findings are shown in Table 1. O. tsutsugamushi was detected through targeted next-generation sequencing (tNGS) examination (Genoxor, Hangzhou).
| Item | Case 1 | Case 2 | Case 3 | Reference range |
| Routine blood work | ||||
| WBC (× 109/L) | 7 | 3.4 | 6.7 | 3.5-9.5 |
| RBC (× 1012/L) | 3.73 | 2.89 | 4.24 | 3.8-5.1 |
| Hb (g/L) | 100 | 87 | 131 | 115-150 |
| PLT (× 109/L) | 155 | 119 | 242 | 125-350 |
| Neutrophil count (× 109/L) | 2.68 | 1.91 | 3.4 | 1.8-6.3 |
| Neutrophil percentage (%) | 38.3 | 56.2 | 50.7 | 40-75 |
| Lymphocyte count (× 109/L) | 3.99 | 1.33 | 3.01 | 1.1-3.2 |
| Eosinophil count (× 109/L) | 0 | 0.01 | 0 | 0.02-0.52 |
| Indices of inflammation | ||||
| CRP (mg/L) | 28.04 | 52.01 | 87.41 | 0-10 |
| IL-6 (pg/mL) | 4 | 0.03 | - | 0-7 |
| PCT (ng/mL) | 0.11 | 0.05 | 0.25 | 0-0.5 |
| Biochemical indexes | ||||
| TBIL (umol/L) | 4.9 | 8.9 | 9.6 | 0-21 |
| DBI (umol/L) | 2 | 4.6 | 0 | 0-8 |
| IBIL (umol/L) | 2.9 | 4.3 | 9.6 | 0-16 |
| ALB (g/L) | 38.4 | 28.9 | - | 40-55 |
| ALT (U/L) | 35.1 | 84.9 | 144 | 7-40 |
| AST (U/L) | 39.5 | 74.5 | 83 | 13-35 |
| LDH (U/L) | 433 | 611 | 387 | 135-214 |
| ADH (U/L) | 55.7 | 51 | - | 0-20 |
| BUN (mmol/L) | 3.97 | 4.2 | 3.18 | 2.6-7.5 |
| Scr (umol/L) | 54.5 | 49.8 | 67.7 | 41-73 |
| Coagulation function | ||||
| PT (seconds) | 13.2 | 13.5 | 13.7 | 12-14 |
| APTT (seconds) | 42.4 | 48.1 | 40.5 | 28-40 |
| FIB (g/L) | 3.57 | 2.47 | 5.23 | 2-4 |
| D-dimer (ug/mL) | 1.14 | 10.8 | 1.46 | 0-0.5 |
| Electrolyte concentrations | ||||
| Na + (mmol/L) | 137.9 | 131.3 | 134.6 | 137-147 |
| K + (mmol/L) | 3.3 | 3.53 | 3.91 | 3.5-5.3 |
| Cl- (mmol/L) | 105.7 | 98 | 101.2 | 99-110 |
| Ca2 + (mmol/L) | 2.15 | 1.96 | 2.13 | 2.11-2.52 |
| Infectious disease | ||||
| HBV | Positive | Negative | Negative | |
| HCV | Negative | Negative | Negative | |
| HIV | Negative | Negative | Negative | |
| Syphilis | Negative | Negative | Negative | |
| Respiratory pathogen spectrum | Negative | Negative | Negative | |
| Antinuclear antibody profile | Negative | Negative | Negative | |
| EBV quantification | Normal | Normal | Normal | |
| T-SPOT | - | - | Negative | |
Case 2: Other laboratory test findings are shown in Table 1. Scrub typhus was diagnosed by tNGS (Genoxor, Hangzhou).
Case 3: The main laboratory test findings are shown in Table 1. We strongly suspected that the patient had scrub typhus and collected a blood sample for tNGS analysis (Genoxor, Hangzhou), which confirmed our diagnosis.
Case 1: Chest and abdominal computed tomography (CT) scans revealed multiple enlarged lymph nodes in the axillary, clavicular, and abdominal regions, accompanied by splenomegaly.
Case 2: A chest CT scan revealed an old lesion in the upper right lung. No abnormalities were detected via cardiac or abdominal color Doppler ultrasound.
Case 3: Chest and abdominal CT scans revealed old lesions in both lungs, slightly enlarged lymph nodes in both axillae, and fatty liver.
The final diagnosis for this patient was scrub typhus.
After admission, the patient was promptly administered omadacycline (HaiZheng Pharmaceutical, China) via int
After hospitalization, the patient received antiinfective treatment with omadacycline (HaiZheng Pharmaceutical, China), following the same administration method as Case 1.
After the diagnosis of scrub typhus was confirmed, omadacycline (HaiZheng Pharmaceutical, China) was administered promptly.
The patient's body temperature normalized on the second day after initiation of omadacycline. Following a five-day course of treatment, the patient was discharged with no recurrence of symptoms.
After two days of treatment, the patient’s axillary temperature returned to normal. The patient recovered and was discharged after five days of hospitalization.
After two days of treatment, the patient’s body temperature returned to normal, and the accompanying symptoms disappeared. Five days later, the patient was discharged with no complaints of discomfort.
O. tsutsugamushi, the pathogen responsible for inducing scrub typhus in humans, is characterized as a gram-negative obligate intracellular bacterium. The main pathological manifestation of scrub typhus is focal or disseminated vasculitis involving small blood vessels and multiple organs[6]. The onset of scrub typhus typically occurs between 6 and 21 days after exposure to O. tsutsugamushi, with clinical symptoms often presenting in an atypical manner. Patients may experience fever, fatigue, muscle aches, headache, and cough, among other symptoms. An eschar, a specific and crucial diagnostic indicator in scrub typhus cases, may manifest in different body regions but is commonly observed in areas with thinner and wetter skin, such as the armpits, groin, neck, and waist[7,8]. All three cases reported in this study presented clinical symptoms within one week after outdoor activities. In addition to some atypical clinical manifestations, the typical eschar appears in various parts of the body. Scrub typhus can lead to multiorgan dysfunction, resulting in damage to the respiratory, digestive, nervous, cardiovascular, and hematological systems, as well as abnormal liver and kidney function[9]. Liver involvement is frequent in scrub typhus, manifesting mainly as increased transaminase levels. A study by Hu et al[10] revealed alanine aminotransferase (ALT) and ALT elevation rates of 89.3% and 91.7%, respectively, suggesting a close association between scrub typhus and elevated liver enzyme levels. All three of our patients had higher than normal levels of aminopherase.
For patients with scrub typhus, early diagnosis and treatment are key factors in ensuring a favorable prognosis. In the past, the Weil-Felix test has been used as an auxiliary diagnostic method for scrub typhus, but its sensitivity and specificity are both low. Currently, indirect immunofluorescence assay (IIFA) is considered the gold standard for diagnosing scrub typhus. However, IIFA is not vigorous enough to detect infection at early stages, and enzyme-linked immunosorbent assay has the same shortcomings[11]. Next-generation sequencing (NGS) is the latest technique for detecting scrub typhus and has superior performance in the diagnosis of scrub typhus in early stages and eschar-negative cases[12,13]. Compared with metagenomic NGS (mNGS), tNGS has greater accuracy and sensitivity in pathogen detection, as well as lower testing costs[14]. Therefore, for patients suspected of having scrub typhus, tNGS may be a better choice than mNGS. The diagnoses of all three cases reported here were confirmed by tNGS. In clinical practice, we also encounter patients who are highly suspected of having scrub typhus but who test negative according to tNGS. We consider that the main reason for this false-negative result may be related to the pathogen not entering the bloodstream or a low presence of the pathogen in the bloodstream.
Early and appropriate antibiotic treatment is crucial to prevent progression to severe scrub typhus. Doxycycline is the most widely used antibiotic, and tetracycline, chloramphenicol, azithromycin, and rifampicin are also effective choices for the treatment of scrub typhus[15]. Owing to its good efficacy and few side effects, azithromycin is the preferred drug for treating scrub typhus in pregnant women and children, and to avoid increasing the resistance of Mycobacterium tuberculosis (M. tuberculosis) to rifampicin, rifampicin is recommended only in areas where M. tuberculosis is resistant to other drugs[16,17]. There is little difference in treatment efficacy between tetracycline and doxycycline[15]. Omadacycline is a novel tetracycline-derived antibiotic. Omadacycline has broad-spectrum antibacterial activity against gram-positive and gram-negative aerobes, anaerobes, and atypical pathogens. Omadacycline overcomes common tetracycline resistance mechanisms, such as bacterial efflux pumps and ribosomal protection proteins; thus, it still has good antibacterial activity against minocycline-resistant, doxycycline-resistant, methicillin-resistant, Staphylococcus aureus, and drug-resistant, gram-negative bacteria[18]. Omadacycline is characterized by a low protein binding rate, large distribution volume, low systemic clearance rate, and long half-life; it is not metabolized by the liver and is excreted mainly through feces (81.1%) and the kidneys (14.4%). Accordingly, for elderly patients or those who have impaired liver or kidney function, the dosage of omadacycline does not need to be adjusted[19]. In our study, before being admitted to our hospital, all three patients received other antibiotic therapies, but all of the patients had unsatisfactory therapeutic outcomes. Furthermore, all of the patients were elderly and had impaired liver function, but after receiving standard intravenous omadacycline treatment, they were discharged from the hospital with no discomfort. The mean time to defervescence with doxycycline or azithromycin for the treatment of scrub typhus ranges from 25 to 224 hours[20]. In our reported cases, all three patients achieved normal body temperature within 24 hours of starting omadacycline therapy, but larger studies are needed to confirm this finding. There are no case reports of the use of intravenous omadacycline to treat scrub typhus, and only one case report of the use of oral omadacycline[5]. Therefore, our study can provide an additional reference for the treatment of scrub typhus with omadacycline. However, our study has a limited number of patients, and further research with more patients is needed to investigate the mechanism of action, safety, and effectiveness of omadacycline in the treatment of scrub typhus.
The presence of an eschar is a crucial diagnostic indicator for scrub typhus, making careful physical examination essential in clinical practice. tNGS represents a novel effective method for diagnosing scrub typhus. Omadacycline can be considered an alternative option for antiinfective therapy in patients with O. tsutsugamushi infections, particularly in patients with a poor response to macrolides or fluoroquinolone antibiotics.
| 1. | Paris DH, Shelite TR, Day NP, Walker DH. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg. 2013;89:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 2. | Park JW, Kim SH, Park DW, Jung SH, Park HJ, Seo MH, Song HJ, Lee JY, Kim DM, Kim CM, Gill BC, Jeong HJ, Lee JM, Ha DR, Kim ES, Chung JK. Molecular Epidemiology of an Orientia tsutsugamushi Gene Encoding a 56-kDa Type-Specific Antigen in Chiggers, Small Mammals, and Patients from the Southwest Region of Korea. Am J Trop Med Hyg. 2018;98:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Qi Y, Yin Q, Shao Y, Cao M, Li S, Chen H, Shen W, Rao J, Li J, Li X, Sun Y, Lin Y, Deng Y, Zeng W, Zheng S, Liu S, Li Y. Development of a rapid and visual nucleotide detection method for a Chinese epidemic strain of Orientia tsutsugamushi based on recombinase polymerase amplification assay and lateral flow test. Int J Infect Dis. 2018;70:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Huang J, Deng K, Chen J, Zhang M. Epidemiological and clinical characteristics of scrub typhus in northern Fujian, China, from 2015 to 2019. BMC Infect Dis. 2023;23:479. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Sun CB, Ma Z, Liu Z. Case Report: Optic neuritis as the initial presentation of Orientia tsutsugamushi infection detected by metagenomic next-generation sequencing. Front Immunol. 2023;14:1129246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Díaz FE, Abarca K, Kalergis AM. An Update on Host-Pathogen Interplay and Modulation of Immune Responses during Orientia tsutsugamushi Infection. Clin Microbiol Rev. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Rajapakse S, Weeratunga P, Sivayoganathan S, Fernando SD. Clinical manifestations of scrub typhus. Trans R Soc Trop Med Hyg. 2017;111:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Akaike T, Ishizuka K, Tominaga N, Motohashi I. Scrub typhus: the clinical significance of the eschar. BMJ Case Rep. 2023;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Peter JV, Sudarsan TI, Prakash JA, Varghese GM. Severe scrub typhus infection: Clinical features, diagnostic challenges and management. World J Crit Care Med. 2015;4:244-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Hu ML, Liu JW, Wu KL, Lu SN, Chiou SS, Kuo CH, Chuah SK, Wang JH, Hu TH, Chiu KW, Lee CM, Changchien CS. Short report: Abnormal liver function in scrub typhus. Am J Trop Med Hyg. 2005;73:667-668. [PubMed] |
| 11. | Kala D, Gupta S, Nagraik R, Verma V, Thakur A, Kaushal A. Diagnosis of scrub typhus: recent advancements and challenges. 3 Biotech. 2020;10:396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Li J, Chen C, Zou F, Liu L, Wang B, Kang H, Liu B, Wang S, Qin T. Diagnosing scrub typhus without eschar: a case report using metagenomic next-generation sequencing (mNGS). Ann Transl Med. 2021;9:1190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 13. | Liu X, Zhang Y, Zhang J, Lou Z, Xia H, Lu Z. The Early Diagnosis of Scrub Typhus by Metagenomic Next-Generation Sequencing. Front Public Health. 2021;9:755228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Yi X, Lu H, Liu X, He J, Li B, Wang Z, Zhao Y, Zhang X, Yu X. Unravelling the enigma of the human microbiome: Evolution and selection of sequencing technologies. Microb Biotechnol. 2024;17:e14364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 15. | El Sayed I, Liu Q, Wee I, Hine P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2018;9:CD002150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Rajapakse S, Rodrigo C, Fernando SD. Drug treatment of scrub typhus. Trop Doct. 2011;41:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Lu D, Wang T, Luo Z, Ye F, Qian J, Zhang J, Wang C. Evaluation of the Therapeutic Effect of Antibiotics on Scrub Typhus: A Systematic Review and Network Meta-Analysis. Front Public Health. 2022;10:883945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Zhanel GG, Esquivel J, Zelenitsky S, Lawrence CK, Adam HJ, Golden A, Hink R, Berry L, Schweizer F, Zhanel MA, Bay D, Lagacé-Wiens PRS, Walkty AJ, Lynch JP 3rd, Karlowsky JA. Omadacycline: A Novel Oral and Intravenous Aminomethylcycline Antibiotic Agent. Drugs. 2020;80:285-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 19. | Rodvold KA, Pai MP. Pharmacokinetics and Pharmacodynamics of Oral and Intravenous Omadacycline. Clin Infect Dis. 2019;69:S16-S22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Gupta N, Boodman C, Jouego CG, Van Den Broucke S. Doxycycline vs azithromycin in patients with scrub typhus: a systematic review of literature and meta-analysis. BMC Infect Dis. 2023;23:884. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |