Published online Sep 6, 2024. doi: 10.12998/wjcc.v12.i25.5775
Revised: May 22, 2024
Accepted: June 24, 2024
Published online: September 6, 2024
Processing time: 100 Days and 16.6 Hours
During the Coronavirus disease 2019 (COVID-19) pandemic, a notable increase in acute macular neuroretinopathy (AMN) cases was observed. This study aimed to investigate the potential association between AMN and COVID-19 by examining 3 cases in China.
The first case involved a 30-year-old man who presented with progressive vision loss following a COVID-19 infection. Optical coherence tomography (OCT) and near-infrared imaging identified hallmark AMN lesions, hyperreflective disru
The findings highlight the diagnostic utility of advanced ocular imaging in detecting AMN in COVID-19 patients and the importance of comprehensive eye examinations.
Core Tip: Advanced ocular imaging has diagnostic value in identifying neuroretinopathy (AMN) in patients with Coronavirus disease 2019 (COVID-19). The clinical variability of AMN is highlighted by individual differences in the occurrence of symptoms, recovery rate, and the degree of visual improvement after COVID-19.
- Citation: Bi C, Huang CM, Shi YQ, Huang C, Yu T. Acute macular neuroretinopathy following COVID-19 infection: Three case reports. World J Clin Cases 2024; 12(25): 5775-5783
- URL: https://www.wjgnet.com/2307-8960/full/v12/i25/5775.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i25.5775
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and emerged as a global pandemic starting in late 2019. The disease spread rapidly worldwide, leading to significant morbidity and mortality[1]. COVID-19 manifests with a broad spectrum of clinical symptoms ranging from mild respiratory issues to severe complications including multi-organ failure and death[2]. Since the easing of epidemic control measures for COVID-19 in China in early December 2022, there has been a rapid resurgence of the virus in the country[3].
Acute macular neuroretinopathy (AMN) is a relatively rare ophthalmic disorder characterized by subtle yet potentially significant visual disturbances, with scotoma being the most prominent symptom[4]. The epidemiological profile of AMN shows a distinct predilection toward women and frequently presents bilaterally[5]. AMN is associated with a diverse range of precipitants, including viral infection, use of oral contraceptives, traumatic events, and systemic conditions such as anemia[4]. Although generally considered a self-limiting condition, the long-term effects of AMN, including persistent visual field defects despite a general trend toward recovery, highlight the need for greater clinical attention.
Onset of the COVID-19 pandemic was associated with an increase in cases exhibiting ocular symptoms, with AMN reported with increased frequency. A retrospective analysis conducted in France revealed a marked rise in the incidence of AMN, from 0.66 per 100000 cases in 2019 to 8.97 per 100000 cases in 2020 following the outbreak of COVID-19[5]. This significant increase underscores the potential role of COVID-19 not only as a systemic health emergency but also as a contributing factor to various ocular conditions, including conjunctivitis, uveitis, and glaucoma in addition to AMN. Despite the apparent correlation, the mechanisms underlying the association between COVID-19 and AMN remain unclear, and the current literature provides limited insight into the potential interplay.
This challenge is further increased by the slow development of clinical management strategies for AMN in the midst of COVID-19 co-infection. This uncertainty underscores the reliance on advanced diagnostic and therapeutic approaches, particularly near-infrared (NIR) imaging and optical coherence tomography (OCT). NIR imaging offers superior detection of AMN lesions beyond the capability of traditional fundus examination[6], and OCT provides detailed visualization of lesion changes[7]. These techniques highlight the critical need for specialized ocular information in managing AMN with COVID-19 co-infection. This case report describes the use of NIR and OCT in a series of three instances of AMN that developed after COVID-19 infection.
Case 1: A 30-year-old man who presented with a 2 d history of progressive vision loss in the left eye.
Case 2: A 24-year-old woman who presented with a complaint of blurred vision in her left eye persisting for 10 d.
Case 3: A 28-year-old woman presenting a sensation of occlusion in her right eye persisting for 1 wk (Table 1).
| Characteristic | Case 1 | Case 2 | Case 3 |
| Chief complaint | Progressive vision loss in left eye for 2 d | Blurring vision in left eye for 10 d | Sensation of occlusion in right eye for 1 wk |
| History of present illness | Fever for 3 d 1 wk ago, confirmed COVID-19 infection via throat swab | Fever 2 wk prior, confirmed COVID-19 infection via throat swab, not pregnant | Confirmed COVID-19 infection via nucleic acid test 2 wk prior, not pregnant |
| Vaccine | Inactivated COVID-19 vaccine (Vero cell, Beijing Institute of Biological Products Co., Ltd.) | ||
| BCVA | 0.6 (left eye), 1.0 (right eye) | 1.0 (right eye), 0.5 (left eye) | 0.3 (right eye), 1.0 (left eye) |
| Fundus exam | Rugby ball-like lesion over macula | Scattered cotton-wool spots in both eyes | |
| NIR | Hyporeflective lesion oriented towards fovea in left eye | Hyporeflective lesions in both eyes, pronounced in left eye | Hyporeflective lesions in right eye |
| OCT/ OCTA | Hyporeflective ELM, hyperreflective MZ, diminished EZ; No initial abnormalities in SCP/DCP | Hyperreflective foci in OPL, hyporeflective foci in EZ/IZ in both eyes, hyperreflective foci in INL in right eye | Hyperreflective lesions in OPL, hyporeflective lesions in EZ/IZ, hyperreflective lesions in INL |
| Treatment | Ibuprofen (0.2 g three times a day) | Acetaminophen (0.3 g three times a day) | Acetaminophen (0.3 g three times a day) |
| Final diagnosis | AMN | AMN and PAMM | AMN and PAMM |
Case 1: The patient had a fever for 3 d in the previous week. COVID-19 infection was subsequently confirmed by a throat swab.
Case 2: The patient experienced a fever approximately 2 wk before her visit. COVID-19 was confirmed by a throat swab.
Case 3: The patient had a COVID-19 infection that was confirmed by nucleic acid testing 2 wk before her visit.
None of the patients had a previous history of myopia or other ophthalmologic disease. Their medical history was otherwise unremarkable.
All 3 patients had received an inactivated COVID-19 vaccine (Vero cell, Beijing Institute of Biological Products Co., Ltd.) 3, 4, and 3 mon before their visit, respectively.
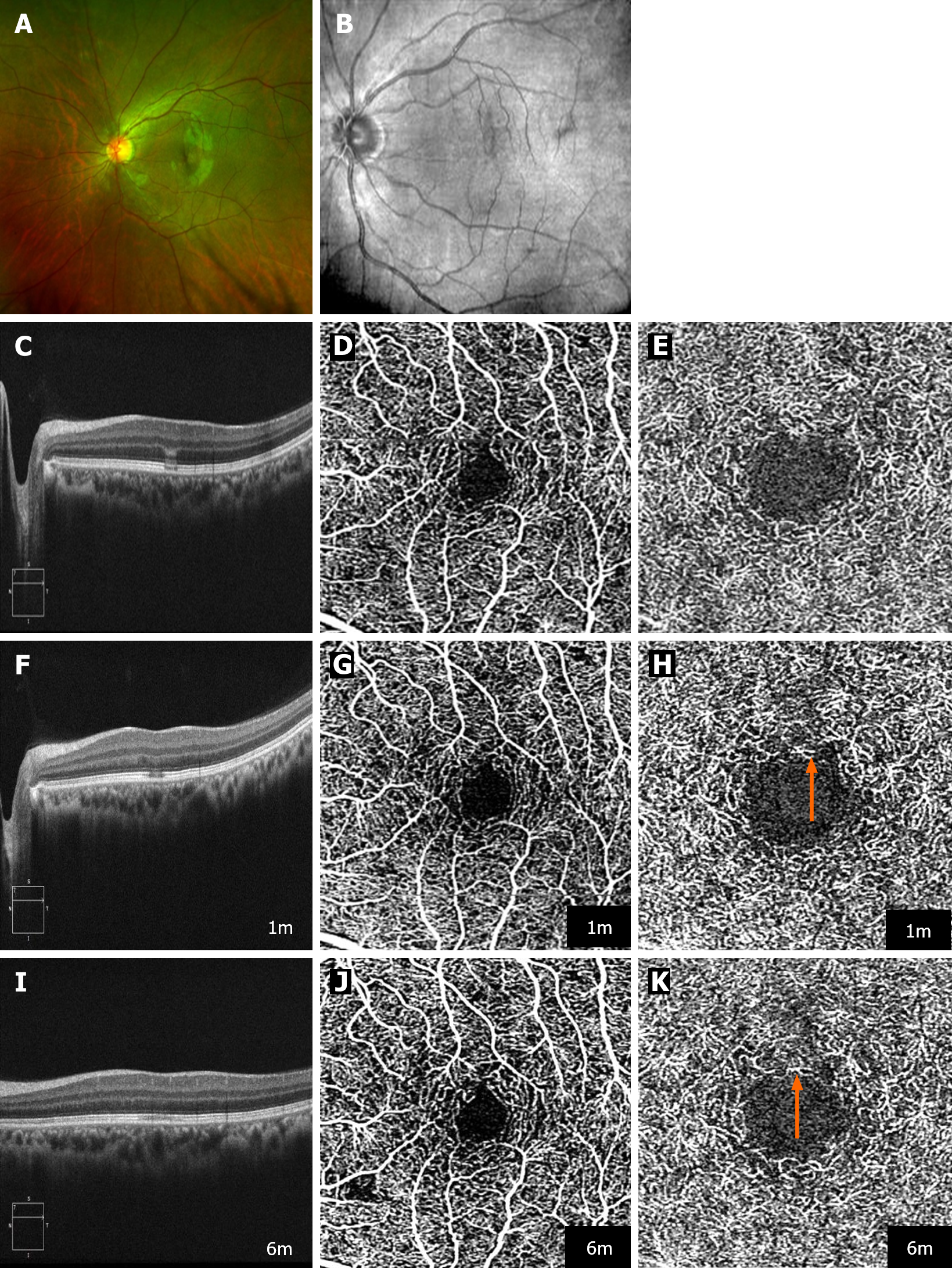
Case 1: Initial ophthalmologic assessment revealed a best-corrected visual acuity (BCVA) of 0.6 in the affected eye and 1.0 in the contralateral eye. Fundus examination identified a distinctive rugby ball-like lesion over the macular region of the left eye (Figure 1A). The blood pressure was 120/80 mmHg.
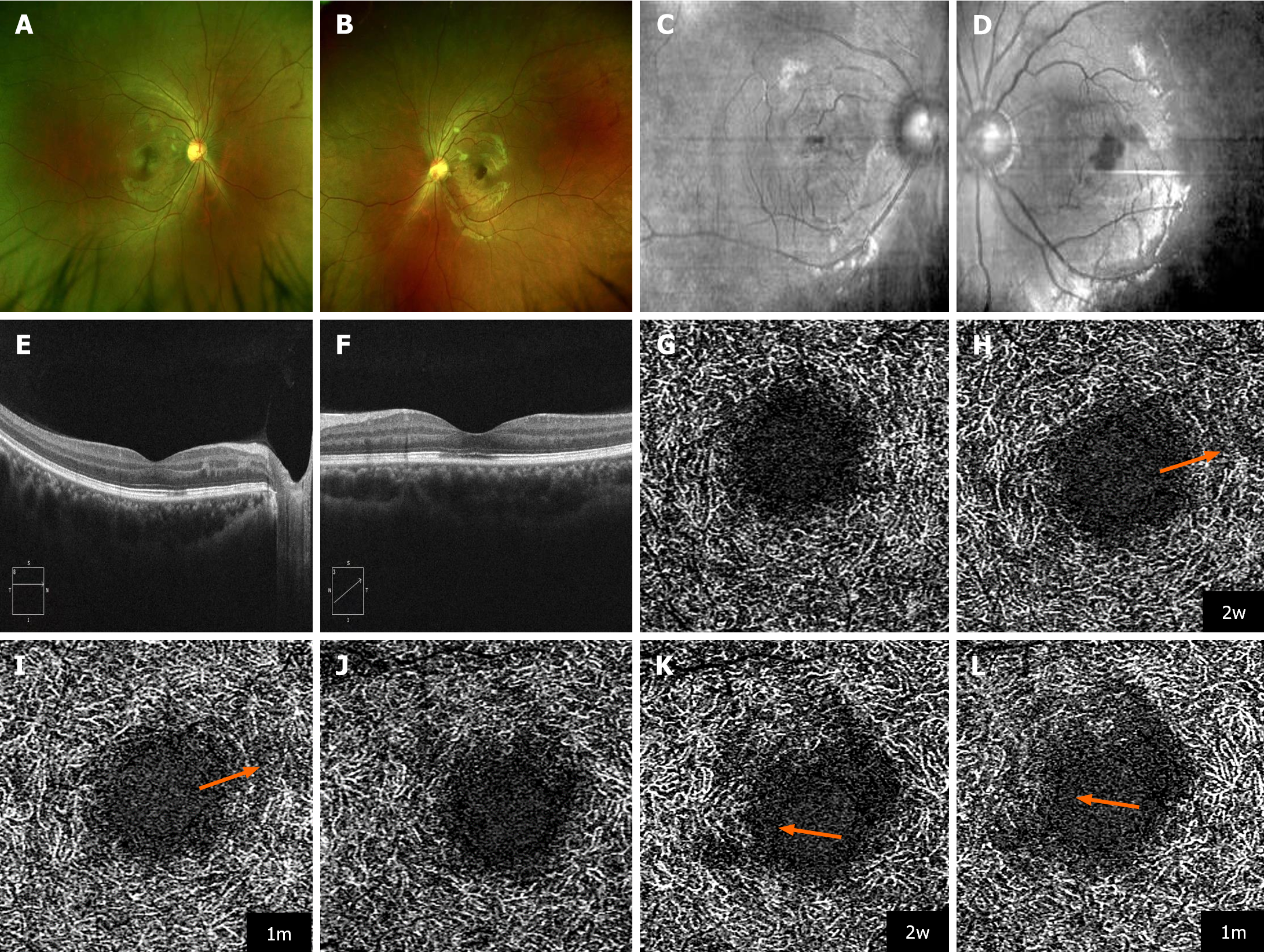
Case 2: Fundus examination disclosed the presence of scattered cotton-wool spots in both eyes (Figure 2A and B). The BCVA was 1.0 in the right eye and 0.5 in the left eye. The blood pressure was 125/80 mmHg.
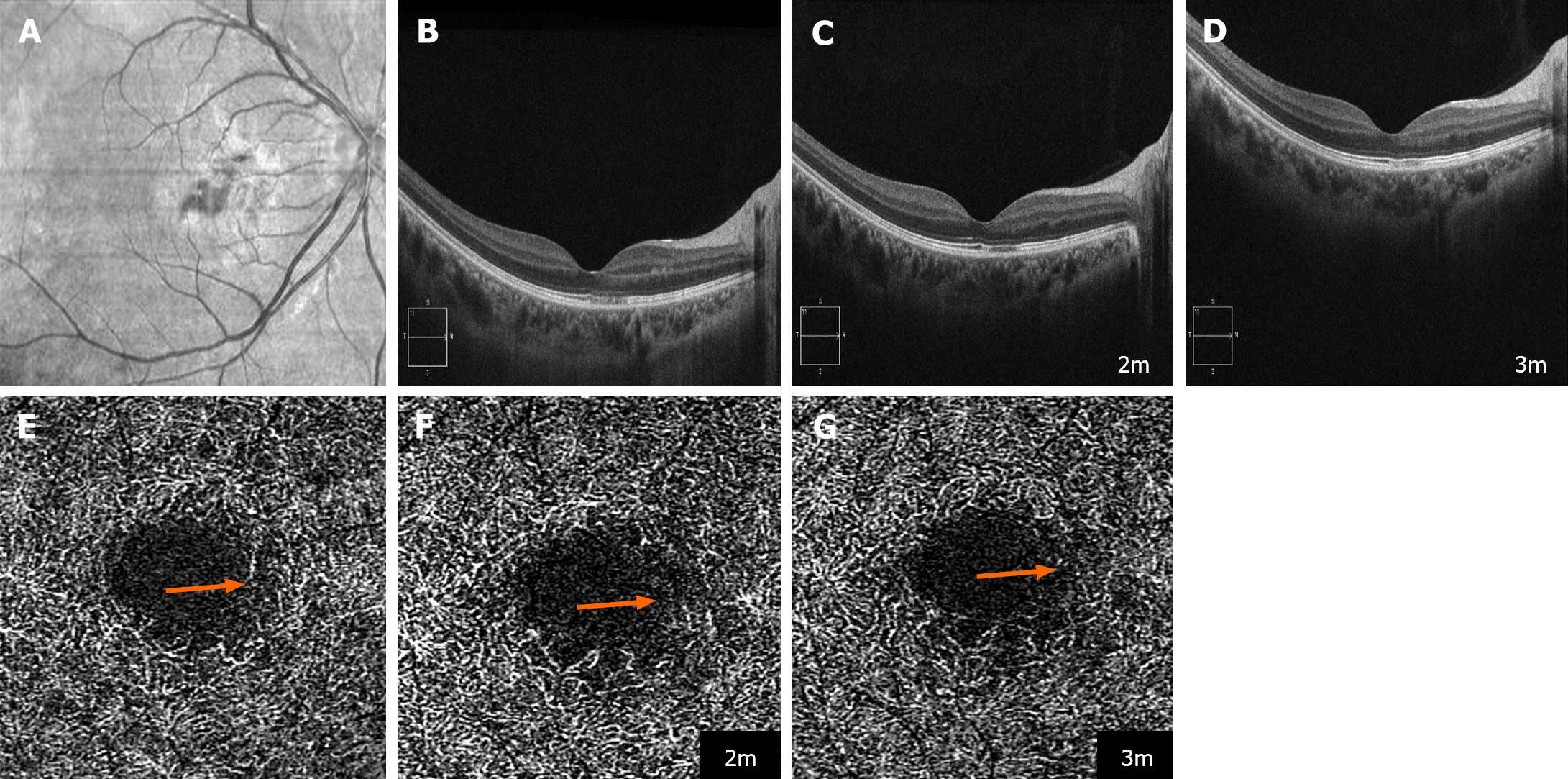
Case 3: The BCVA was 0.3 in the affected eye and 1.0 in the unaffected eye. The blood pressure was 128/78 mmHg.
The patients self-tested with COVID-19 rapid antigen detection kits and a throat swab.
Case 1: NIR imaging delineated a hyporeflective lesion oriented toward the fovea (Figure 1A and B). In addition to the collapse of the outer plexiform layer (OPL), marked by hyper-reflectivity in Henle's fiber layer and the vanishing of the interdigitation zone (IZ), OCT revealed a hyporeflective external limiting membrane, a conspicuously hyperreflective myoid zone, and diminished reflectivity within the ellipsoid zone (EZ), supporting the diagnosis of AMN. OCT angiography (OCTA) initially showed no significant abnormality of the superficial or deep capillary plexus (DCP) (Figure 1C-E).
Case 2: NIR imaging identified hyporeflective lesions within the macular regions of both eyes with a pronounced manifestation in the left eye (Figure 2C and D). OCT imaging provided a more detailed view, revealing hyperreflective foci within the OPL and hyporeflective foci in the EZ/IZ affecting the fovea in both eyes, plus hyperreflective foci in the inner nuclear layer (INL) of the right eye. The observations indicated AMN coexisting with paracentral acute middle maculopathy (PAMM) (Figure 2E and F).
Case 3: Diagnostic imaging revealed hyporeflective lesions within the macular region of the right eye on NIR imaging (Figure 3A), indicative of AMN. OCT findings showed hyperreflective lesions in the OPL, hyporeflective lesions in the EZ/IZ, and hyperreflective lesions in the INL (Figure 3B), suggesting concurrent presentation of PAMM.
AMN.
The 3 patients received ibuprofen (0.2 g three times a day), acetaminophen (0.3 g three times a day), and acetaminophen (0.3 g three times a day) for symptomatic therapy of COVID-19.
Case 1: The patient was COVID-19 negative 2 wk after diagnosis. Follow-up examination 1 mon after diagnosis revealed partial recovery of the BCVA to 1.0/0.8. Despite this improvement, OCTA detected a reduction in vascular density within the DCP together with persistent hyporeflective changes in the EZ and impairment of the IZ (Figure 1F-H). At 6 mon, the BCVA was fully restored at 1.0/1.0, reflecting significant clinical improvement. However, OCT imaging continued to show residual impairment in the IZ, with further decreased vascular density in the DCP observed on OCTA (Figure 1I-K).
Case 2: The patient was COVID-19 negative within 15 d after diagnosis. Subsequent follow-up showed an improvement of the BCVA to 1.0/0.8 within 2 wk, which was maintained 1 mon later. However, OCTA revealed a progressive decrease in vascular density within the DCP of both eyes on follow-up. (Figure 2G-L).
Case 3: The fever resolved within 5 d, and though initially testing positive for COVID-19 after experiencing fever, the patient was found to be negative at the time of her visit. Remarkably, within 2 mon, the patient's BCVA improved to 1.0/1.0, with OCT showing near restoration of the macular structure, albeit with lingering hyporeflective foci in the IZ (Figure 3C). The improvement stabilized over the following month (Figure 3D). Nonetheless, OCTA demonstrated a progressive decline in the vascular density within the DCP at the site of the lesions during the follow-up period (Figure 3E-G).
In our case series, the common characteristics observed across all three cases of AMN following COVID-19 infection included visual symptoms and specific imaging signatures on OCT and NIR imaging. Each case exhibited hyporeflective lesions in the macular region and alterations in the DCP, indicative of ischemic changes. Individual differences in symptom onset post-COVID-19, variable recovery rates, and degree of visual acuity improvement were noted, underscoring the clinical variability of AMN.
The etiology of AMN remains under investigation, as current theories cannot fully account for its diverse clinical manifestations. It is hypothesized that the development of AMN may be linked to ischemic disturbance of the choroidal circulation, particularly within the DCP[7-10]. A review of the literature indicates that AMN may have pathological features similar to conditions that show angular signs of Henle’s fiber layer hyperreflectivity on OCT, such as trauma, central retinal vein occlusion, acute posterior multifocal placoid pigment epitheliopathy, cancer-associated retinopathy, and laser-related retinal damage[11]. Similar to AMN, these conditions are believed to damage photoreceptors and Müller cells through ischemic impact on the choroid or DCP. Taken together, the evidence underscores the complex pathogenesis of AMN. And highlights the need for further study of its ischemic mechanism and the potential influence of systemic conditions such as COVID-19.
Pathological studies have detected the presence of SARS-CoV-2 in the optic nerve, choroid, and retinal tissues, with particular impact on ganglion cells, the IPL, and the OPL[12]. These findings suggest specific tissue affinities, or tropisms of the virus, that may influence the pathogenesis of acute macular AMN commonly associated with viral infection. These tropisms could lead to distinct pathological pathways that result in the retinal impairment observed in AMN. Furthermore, COVID-19 has been associated with microcirculatory disturbance within the retina, characterized by a reduction in microvessel density near the optic nerve and veins, venous occlusion, and preceding hemorrhage linked to venous constriction[12]. The incidence of AMN during the COVID-19 pandemic highlights the adverse effects of the virus on retinal vasculature, and the need for further investigation of the vascular effects of COVID-19 within the ocular sphere to increase our understanding of its role in AMN pathogenesis.
In this study, we observed cases of AMN co-occurring with PAMM, a combination rarely reported in previous literature[13,14]. During the course of the disease, OCT imaging demonstrated recovery of both PAMM and AMN lesions, yet persistent defects in the IZ were noted. This lingering abnormality suggests that the lesions may represent an immune-mediated inflammatory response. The retinal vasculature, with its distinctive hammock-like arrangement and the direct flow of arterial vessels returning to the main veins, plays a crucial role[15]. Specifically, the DCP, which is known to have a low oxygen partial pressure and high hydrostatic pressure, appears especially vulnerable to the initial effects of venous constriction or obstruction, potentially aggravating intermediate capillary plexus. These observations, highlighted by the varied ocular manifestations seen in COVID-19 patients, indicate a diverse immune response to infection. Notably, in some patients who reported monocular visual impairment, comprehensive examination disclosed bilateral lesions, consistent with previous reports of occult infections[16]. This emphasizes the vital importance of performing careful ophthalmological evaluations of patients with COVID-19, even in the absence of ocular symptoms. Such assessments are indispensable for a full understanding of the impact of the virus on ocular health and for the early detection and management of potential complications.
In our cases, initial OCT imaging identified AMN lesions on presentation, but OCTA did not show any concurrent alterations of blood flow in the affected regions. This discrepancy suggests that AMN pathogenesis in COVID-19 infection may involve not only changes in vascular density, but also a direct viral impact on photoreceptors as well as induction of inflammatory vasoreaction. Following the resolution of AMN, we noted reductions in vessel density within the lesion areas that coincided with improvement of vision. These changes persisted in cases of mild infection or for several months after the infection onset. Such findings underscore the enduring impact of COVID-19, consistent with studies that have reported microvascular occlusion without significant dysfunction of major organs like the lungs and kidneys in COVID-19 patients[17]. Microvascular angiopathy, presumed to be triggered by acute endothelial dysfunction and proinflammatory cytokines, might increase the risk of microvascular thrombosis that could lead to multiorgan dysfunction[18]. In addition, postmortem analysis has revealed direct viral damage in organs such as the brain, liver, and kidneys[19-21]. Intriguingly, neurological changes persist for months after recovery from COVID-19 in some patients despite the absence of initial neurological symptoms[22]. This suggests that SARS-CoV-2 infection may impact the metabolism of the outer retina, with reduced energy demands potentially leading to vascular atrophy and similar to changes seen in inherited metabolic disorders like Leber’s hereditary optic neuropathy. The possibility of recurrent infection raises questions about the long-term clinical implications of these findings and emphasizes the need for ongoing monitoring of retinal microvascular changes.
Recent studies have reported an association between COVID-19 vaccination and the onset of AMN, particularly in young women shortly after vaccination[23,24]. The three patients in this report developed AMN symptoms after receiving a COVID-19 vaccine, which supporting an association of AMN with vaccination. The pathogenesis of vaccine-associated AMN is elusive, but it is thought to be related to inflammatory responses that precipitate vascular or thromboembolic events that lead to ischemia in the deep retinal capillary plexus[25]. The proposed mechanism includes molecular mimicry and hypersensitivity reactions that induce microvascular pathology akin to that observed in AMN[26]. Increasing evidence underscores the importance of further investigation of the ocular ramifications of COVID-19 vaccination.
In our case series, the time from COVID-19 infection to the onset of AMN varied, with symptoms appearing from a few days to 1 wk after infection. This variability is in line with the findings of other studies. Masjedi et al[27] reported a 29-year-old woman with AMN symptoms that appeared 2 wk after the initial COVID-19 symptoms and 5 d after a positive SARS-CoV-2 test[27]. Similarly, Macé and Pipelart[28] described a case of AMN that appeared in a 39-year-old woman 2 d after COVID-19 infection was diagnosed[28]. Kovalchuk et al[29] reported a case involving 16-year-old girl who noticed paracentral scotomas about 10 d after experiencing mild COVID-19 symptoms. Giacuzzo et al[30] reported a case involving a 23-year-old woman with AMN following a positive PCR test and systemic symptoms of 2 wk duration[30]. Our cases reported vision loss 1 wk after confirmed COVID-19 in case 1, blurred vision 2 wk after fever and COVID-19 diagnosis in case 2, and ocular symptoms 2 wk after a positive COVID-19 test in case 3. These observations show that AMN can occur at different times during COVID-19 infection, from the acute phase to post-symptomatic recovery, and can involve direct viral effects or an immune-mediated response.
In summary, this case series highlights the clinical importance of diagnosing and understanding AMN in the context of COVID-19 infection and vaccination. The observed correlation between COVID-19 and increased incidence of AMN, particularly following vaccination, underscores the need for increased vigilance and diagnostic precision, using imaging modalities such as OCT and NIR. These techniques are invaluable for detecting characteristic lesions and vascular alterations associated with AMN. They are essential for adding to our insight into the pathophysiology and the overarching impact of COVID-19.
| 1. | Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3736] [Cited by in RCA: 3191] [Article Influence: 638.2] [Reference Citation Analysis (0)] |
| 2. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30069] [Article Influence: 6013.8] [Reference Citation Analysis (3)] |
| 3. | Goldberg EE, Lin Q, Romero-Severson EO, Ke R. Swift and extensive Omicron outbreak in China after sudden exit from 'zero-COVID' policy. Nat Commun. 2023;14:3888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 4. | Bhavsar KV, Lin S, Rahimy E, Joseph A, Freund KB, Sarraf D, Cunningham ET Jr. Acute macular neuroretinopathy: A comprehensive review of the literature. Surv Ophthalmol. 2016;61:538-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 218] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 5. | Azar G, Bonnin S, Vasseur V, Faure C, Salviat F, Clermont CV, Titah C, Farès S, Boulanger E, Derrien S, Couturier A, Duvilliers A, Manassero A, Hage R, Tadayoni R, Behar-Cohen F, Mauget-Faÿsse M. Did the COVID-19 Pandemic Increase the Incidence of Acute Macular Neuroretinopathy? J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Abdolrahimzadeh S, Ciancimino C, Grassi F, Sordi E, Fragiotta S, Scuderi G. Near-Infrared Reflectance Imaging in Retinal Diseases Affecting Young Patients. J Ophthalmol. 2021;2021:5581851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Thanos A, Faia LJ, Yonekawa Y, Randhawa S. Optical Coherence Tomographic Angiography in Acute Macular Neuroretinopathy. JAMA Ophthalmol. 2016;134:1310-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Sanjari N, Moein HR, Soheilian R, Soheilian M, Peyman GA. Enhanced depth imaging OCT and indocyanine green angiography changes in acute macular neuroretinopathy. Ophthalmic Surg Lasers Imaging Retina. 2013;44:S36-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Fawzi AA, Pappuru RR, Sarraf D, Le PP, McCannel CA, Sobrin L, Goldstein DA, Honowitz S, Walsh AC, Sadda SR, Jampol LM, Eliott D. Acute macular neuroretinopathy: long-term insights revealed by multimodal imaging. Retina. 2012;32:1500-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Sarraf D, Rahimy E, Fawzi AA, Sohn E, Barbazetto I, Zacks DN, Mittra RA, Klancnik JM Jr, Mrejen S, Goldberg NR, Beardsley R, Sorenson JA, Freund KB. Paracentral acute middle maculopathy: a new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol. 2013;131:1275-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 346] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 11. | Ramtohul P, Cabral D, Sadda S, Freund KB, Sarraf D. The OCT angular sign of Henle fiber layer (HFL) hyperreflectivity (ASHH) and the pathoanatomy of the HFL in macular disease. Prog Retin Eye Res. 2023;95:101135. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Jidigam VK, Singh R, Batoki JC, Milliner C, Sawant OB, Bonilha VL, Rao S. Histopathological assessments reveal retinal vascular changes, inflammation, and gliosis in patients with lethal COVID-19. Graefes Arch Clin Exp Ophthalmol. 2022;260:1275-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Vinzamuri S, Pradeep TG, Kotian R. Bilateral paracentral acute middle maculopathy and acute macular neuroretinopathy following COVID-19 vaccination. Indian J Ophthalmol. 2021;69:2862-2864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Virgo J, Mohamed M. Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Eye (Lond). 2020;34:2352-2353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 15. | Cabral D, Fradinho AC, Pereira T, Ramakrishnan MS, Bacci T, An D, Tenreiro S, Seabra MC, Balaratnasingam C, Freund KB. Macular Vascular Imaging and Connectivity Analysis Using High-Resolution Optical Coherence Tomography. Transl Vis Sci Technol. 2022;11:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Abbinante G, Plaitano C, Gallo FG, Magli A. A case of retinal vascular involvement in a 6-year-old patient with Covid-19. Eur J Ophthalmol. 2022;32:NP1-NP5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Idilman IS, Telli Dizman G, Ardali Duzgun S, Irmak I, Karcaaltincaba M, Inkaya AC, Demirkazik F, Durhan G, Gulsun Akpinar M, Ariyurek OM, Akpinar E, Rello J, Akova M, Akata D. Lung and kidney perfusion deficits diagnosed by dual-energy computed tomography in patients with COVID-19-related systemic microangiopathy. Eur Radiol. 2021;31:1090-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res. 2020;69:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 19. | Younger DS. Postmortem neuropathology in COVID-19. Brain Pathol. 2021;31:385-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Zhao CL, Rapkiewicz A, Maghsoodi-Deerwester M, Gupta M, Cao W, Palaia T, Zhou J, Ram B, Vo D, Rafiee B, Hossein-Zadeh Z, Dabiri B, Hanna I. Pathological findings in the postmortem liver of patients with coronavirus disease 2019 (COVID-19). Hum Pathol. 2021;109:59-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 21. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4567] [Article Influence: 913.4] [Reference Citation Analysis (0)] |
| 22. | Qin Y, Wu J, Chen T, Li J, Zhang G, Wu D, Zhou Y, Zheng N, Cai A, Ning Q, Manyande A, Xu F, Wang J, Zhu W. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 167] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 23. | Fekri S, Khorshidifar M, Dehghani MS, Nouri H, Abtahi SH. Acute macular neuroretinopathy and COVID-19 vaccination: Case report and literature review. J Fr Ophtalmol. 2023;46:72-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 24. | Dutta Majumder P, Agarwal A. Acute Macular Neuroretinopathy and Paracentral Acute Middle Maculopathy during SARS-CoV-2 Infection and Vaccination. Vaccines (Basel). 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | Ahmed W, Suri A, Ahmed A. COVID-19 and Acute Macular Neuroretinopathy - An underlying association? Ann Med Surg (Lond). 2022;78:103847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 26. | Jalink MB, Bronkhorst IHG. A Sudden Rise of Patients with Acute Macular Neuroretinopathy during the COVID-19 Pandemic. Case Rep Ophthalmol. 2022;13:96-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Masjedi M, Pourazizi M, Hosseini NS. Acute macular neuroretinopathy as a manifestation of coronavirus disease 2019: A case report. Clin Case Rep. 2021;9:e04976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Macé T, Pipelart V. Acute macular neuroretinopathy and SARS-CoV-2 infection: Case report. J Fr Ophtalmol. 2021;44:e519-e521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Kovalchuk B, Kessler LJ, Auffarth GU, Mayer CS. [Paracentral scotomas associated with COVID-19 infection]. Ophthalmologie. 2023;120:323-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Giacuzzo C, Eandi CM, Kawasaki A. Bilateral acute macular neuroretinopathy following COVID-19 infection. Acta Ophthalmol. 2022;100:e611-e612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |