Published online Sep 6, 2024. doi: 10.12998/wjcc.v12.i25.5729
Revised: May 27, 2024
Accepted: June 19, 2024
Published online: September 6, 2024
Processing time: 85 Days and 16.4 Hours
Previous epidemiologic investigations have consistently demonstrated a strong association between the ratio of cholesterol to total lipids in medium very-low-density lipoprotein (VLDL) and the occurrence of peptic ulcers (PU). However, the precise causal relationship between these factors remains ambiguous. Consequently, this study aims to elucidate the potential correlation between the ratio of cholesterol to total lipids in medium VLDL and the incidence of peptic ulcer.
To investigate the ratio of cholesterol to total lipids in medium very-low-density lipoprotein (VLDL) association with PU via genetic methods, guiding future clinical research.
Genome-wide association study (GWAS) datasets for the ratio of cholesterol to total lipids in intermediate VLDL and peptic ulcer were retrieved from the IEU OpenGWAS project (https://gwas.mrcieu.ac.uk). For the forward Mendelian randomization (MR) analysis, 72 single nucleotide polymorphisms (SNPs) were identified as instrumental variables. These SNPs were selected based on their association with the ratio of cholesterol to total lipids in intermediate VLDL, with peptic ulcer as the outcome variable. Conversely, for the inverse MR analysis, no SNPs were identified with peptic ulcer as the exposure variable and the ratio of cholesterol to total lipids in intermediate VLDL as the outcome. All MR analyses utilized inverse variance weighted (IVW) as the primary analytical method. Additionally, weighted median and MR-Egger methods were employed as supplementary analytical approaches to assess causal effects. Egger regression was used as a supplementary method to evaluate potential directional pleiotropy. Heterogeneity and multiplicity tests were conducted using the leave-one-out method to evaluate result stability and mitigate biases associated with multiple testing.
The genetically predicted ratio of cholesterol to total lipids in medium VLDL was significantly associated with an elevated risk of peptic ulcer (IVW: OR = 2.557, 95%CI = 1.274-5.132, P = 0.008). However, no causal association of peptic ulcer with the ratio of cholesterol to total lipids in medium VLDL was observed in the inverse Mendelian randomization analysis.
In conclusion, our study reveals a significant association between the ratio of cholesterol to total lipids in medium VLDL and an elevated risk of peptic ulcers. However, further validation through laboratory investigations and larger-scale studies is warranted to strengthen the evidence and confirm the causal relationship between these factors.
Core Tip: Higher ratios of cholesterol to total lipids in medium very-low-density lipoprotein (VLDL) levels were causally related to a reduced risk of peptic ulcer (PU) or the main pathways implicated in its development, as suggested by our Mendelian randomization investigation. Further laboratory studies and exploration of molecular mechanisms are required to clarify the role of the ratio of cholesterol to total lipids in medium VLDL in the onset of PU and its main pathways.
- Citation: Lin CM, Meng Q, Li YJ, Zhang SX, Luo QX, Dai ZY. Causal associations between intermediate very-low-density lipoprotein cholesterol-to-total lipids ratio and peptic ulcer: A bidirectional Mendelian randomization study. World J Clin Cases 2024; 12(25): 5729-5738
- URL: https://www.wjgnet.com/2307-8960/full/v12/i25/5729.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i25.5729
Peptic ulcers (PUs) are characterized by lesions formed through chronic inflammatory reactions, necrosis, and detachment of the gastrointestinal mucosa, potentially penetrating deeper layers of the gastrointestinal tract[1,2]. Studies indicate a lifetime prevalence of PU ranging between 5% and 10% in the general population, with a notably higher prevalence among men compared to women (with a ratio of 1:0.94 in 2019). A survey conducted in Shanghai in 2021 revealed a gastroscopically confirmed diagnosis rate of 9.1%[2], with duodenal ulcers, gastric ulcers, and compound ulcers comprising the majority of cases. The global incidence of PU has significantly increased, with 8.09 million reported cases worldwide in 2019, marking a 25.82% increase from 1990[3].
PU arises from a multifactorial etiology, including factors such as inflammation, ischemia, medication use, metabolic disorders, viral infections, and cellular infiltration. Recent cross-sectional studies have implicated the ratio of cholesterol to total lipids in medium very-low-density lipoproteins (VLDL) as a potential risk factor for the development of peptic ulcers[4]. However, establishing a clear correlation between the ratio of cholesterol to total lipids in medium VLDL and peptic ulcer has been challenging due to issues of reverse causality and residual confounding.
Mendelian randomization (MR) is a new research idea that has emerged in recent years and has been widely used in infectious disease research. MR is an assessment of causality between exposure-endpoints using genetic variation as an instrumental variable (IV)[5,6]. It better simulates randomized controlled experiments because genetic variants are randomly assigned among offspring and are not confounded by confounders and reverse causality[7]. The effectiveness of MR has been demonstrated in the literature. Liu et al[8] used MR to demonstrate that osteoporosis and sarcopenia may be causally related to each other, and Wang et al[9] used MR to provide genetic evidence for the relationship between inflammatory factors and multiple myeloma. Carrasquilla et al[10] used MR to show a bidirectional causal relationship between physical inactivity and obesity. In this study, we aim to investigate the relationship between the ratio of cholesterol to total lipids in medium VLDL and the development of peptic ulcer using a two-sample, two-way MR approach with large-scale genome-wide association studies (GWAS) data. Our goal is to contribute new insights into the prevention and treatment of PU through elucidating potential causal pathways involving lipid metabolism.
Data on the ratio of cholesterol to total lipids in medium VLDL were obtained from the IEU OpenGWAS project (https://gwas.mrcieu.ac.uk). This dataset comprises pooled GWAS data with a total of 115082 cases. Similarly, GWAS summary data for peptic ulcers were sourced from the IEU OpenGWAS project (https://gwas.mrcieu.ac.uk). These data provided information on genetic associations with peptic ulcers for further analysis.
For MR studies, IVs must satisfy three key assumptions: (1) The IV is significantly associated with the exposure; (2) the IV is not associated with confounding factors; and (3) the IV affects the outcome solely through the exposure[7]. In the forward MR analysis, where the ratio of cholesterol to total lipids in medium VLDL was considered as the exposure and PU as the outcome, the following steps were taken to fulfill these assumptions:
Selection of IVs associated with exposure: In this study, single nucleotide polymorphisms (SNPs) that were significantly associated with the ratio of cholesterol to total lipids in intermediate VLDL on a genome-wide basis (P < 5 × 10-8) and were not associated with linkage disequilibrium (LD) (r2 < 0.001, kb = 10000) were selected.
Exclusion of confounding factors: Peptic ulcers develop mainly due to aggressive factors such as gastric Helicobacter pylori (H. pylori) infection, excessive stress, smoking, alcohol consumption, and excessive caffeine intake. To exclude potential confounders, the PhenoScannerV2 website (http://www.PhenoScanner.medschl.cam.ac.uk/) was searched (setup: P < 5 × 10-8, r2 > 0.8), and SNPs directly associated with the above risk factors were excluded.
Exclusion of SNPs associated with outcome: SNPs significantly associated with peptic ulcer on a genome-wide basis (P < 5 × 10-8) were also excluded from the analysis.
For the reverse MR analysis, where peptic ulcer was considered as the exposure and the ratio of cholesterol to total lipids in medium VLDL as the outcome, similar steps were followed:
Selection of IVs associated with exposure: SNPs significantly associated with peptic ulcer on a genome-wide basis and without LD were selected.
Exclusion of confounding factors: SNPs directly associated with risk factors related to dyslipidemia were excluded using the PhenoScannerV2 website (settings: P < 5 × 10-8, r2 > 0.8).
Exclusion of SNPs associated with dyslipidemia: SNPs significantly associated with dyslipidemia on a genome-wide basis were excluded. Additionally, SNPs with inconsistent exposure and outcome alleles and palindromic SNPs with medium allele frequencies were excluded from the analysis. SNPs that passed these rigorous screening criteria were utilized in the final causal analysis.
In this study, the following methods were employed for MR analysis:
IVW: IVW was utilized as the primary analysis method. This method provides accurate estimates of causal association effects when all selected SNPs are valid IVs[11].
Weighted median: The weighted median (WM) method was used as a supplementary analysis. It provides valid causal estimates even when more than half of the SNPs are valid IVs[12].
MR Egger: MR Egger method was also employed as a supplementary analysis. It offers reliable causal estimates when all SNPs are considered invalid IVs[13]. These methods were chosen to ensure robustness in estimating causal effects and to account for potential biases or violations of assumptions in the MR analysis.
Several sensitivity analyses were conducted to assess the robustness of the MR results:
Heterogeneity detection: Heterogeneity was assessed using the IVW and MR Egger regression methods. Cochran's Q statistic was calculated, and heterogeneity was considered present if the P value was less than 0.05. In such cases, the random effects model was employed for MR analysis[14].
Horizontal pleiotropy detection: Horizontal pleiotropy was evaluated by examining the intercept of the MR Egger regression. A P value less than 0.05 for the intercept indicates the presence of horizontal pleiotropy, suggesting that MR results may be unreliable.
Outlier identification: Outliers were identified using the MR-PRESSO global test. A P value less than 0.05 for this test suggests the presence of outliers, which should be excluded from the analysis. Subsequently, the analysis should be re-conducted[15].
Leave-one-out analysis: Leave-one-out analysis was performed to assess whether individual SNPs unduly influenced the MR results. This analysis helps identify SNPs that may have a disproportionate impact on the results.
Assessment of instrument strength: The strength of the genetic instrument was evaluated using the F-statistic. An F-statistic greater than 10 indicates that MR results are unlikely to be affected by a weak instrument. The F-statistic was calculated using the formula: f = β2/SE2.
All MR correlation analyses were conducted in R (version 4.2.2) using the TwoSampleMR and MRPRESSO software packages. These sensitivity analyses help ensure the reliability and validity of the MR findings by addressing potential sources of bias or confounding.
In the forward MR analysis, 72 SNPs were selected as IVs based on their significant association with the ratio of cholesterol to total lipids in medium VLDL. These SNPs were obtained from the GWAS pooled data for the ratio of cholesterol to total lipids in medium VLDL. They were chosen because they met the criteria of being significantly associated with the exposure (ratio of cholesterol to total lipids in medium VLDL) at a genome-wide significance level (P < 5 × 10-8) and were mutually independent, ensuring that they did not exhibit linkage disequilibrium.
Out of these 72 SNPs, eight were not present in the GWAS pooled data for peptic ulcer. Additionally, palindromic SNPs with intermediate allele frequencies were excluded from the analysis, as they were not significantly associated with peptic ulcer.
Ultimately, the remaining 72 SNPs were considered valid instrumental variables for the causal analysis of the ratio of cholesterol to total lipids in medium VLDL with respect to peptic ulcer. These SNPs were used to assess the causal relationship between the exposure (ratio of cholesterol to total lipids in medium VLDL) and the outcome (peptic ulcer) in the MR analysis.
As shown in Table 1, The two-sample MR analysis, using the IVW and MR Egger methods, revealed a significant association between the genetically predicted ratio of cholesterol to total lipids in medium VLDL and an increased risk of peptic ulcer.
| Exposure | Outcome | Method | Nsnp | OR (95%CI) | P value |
| Ratio of cholesterol to total lipids in medium VLDL | Peptic ulcer | IVW | 72 | 2.557 (1.274-5.132) | 0.008 |
| WM | 72 | 2.106 (0.709-6.252) | 0.179 | ||
| MR Egger | 72 | 6.394 (1.958-20.878) | 0.003 |
Specifically, the IVW method estimated an odds ratio (OR) of 2.557, with a 95% confidence interval (CI) ranging from 1.274 to 5.132, indicating a statistically significant positive association. Similarly, the MR Egger method estimated an OR of 6.394, with a wider 95%CI ranging from 1.958 to 20.878, also suggesting a significant positive association.
In addition, the WM method also indicated a positive direction of causal estimation, albeit not statistically significant. The WM method estimated an OR of 2.106, with a 95%CI ranging from 0.709 to 6.252.
Overall, these results consistently suggest that the genetically predicted ratio of cholesterol to total lipids in medium VLDL is associated with an increased risk of peptic ulcer, with the IVW and MR Egger methods providing statistically significant estimates.
The sensitivity analysis conducted in this study aimed to assess the robustness of the MR results and detect potential sources of bias.
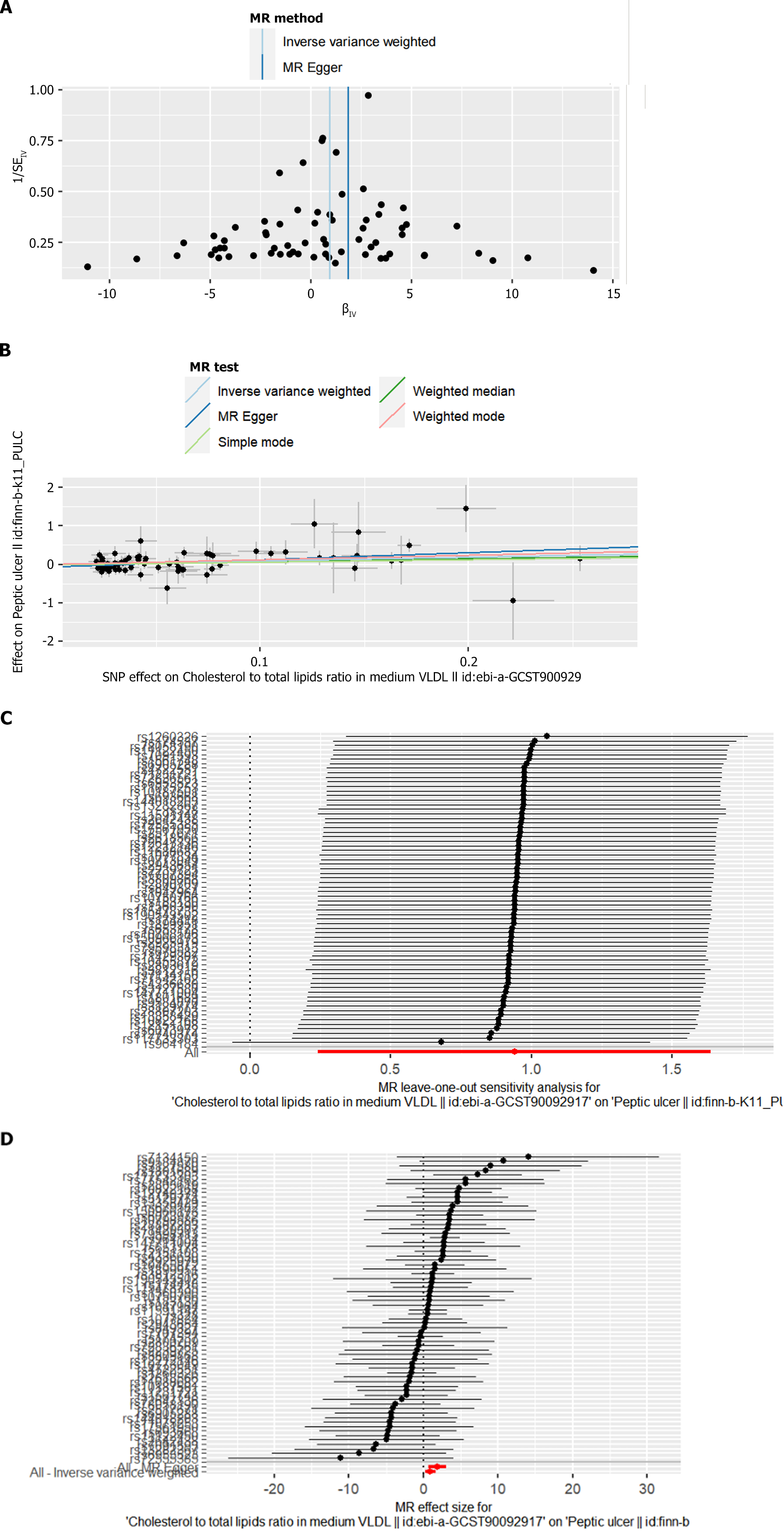
Cochran 's Q test was used in IVW and MR Egger regressions to detect heterogeneity, and the MR Egger intercept was used to detect horizontal pleiotropy. As shown in Table 2, no heterogeneity was detected in all analyses (P > 0.05). A funnel plot of the MR analyses was drawn to visualize the study heterogeneity (Figure 1A). The MR Egger intercept test did not detect horizontal pleiotropy (P > 0.05) (Table 2, Figure 1B), and the MR-PRESSO global test did not detect any outliers. The F-statistics of all SNPs were large 10, indicating that the results of the MR analysis were not affected by weak IV bias. In addition, the results of leave-one-out analysis indicated that the MR analysis results were not driven by a single SNP (Figure 1C). A series of sensitivity analyses indicated that the MR results of this study were robust. In addition, the present study provides a forest plot of the causal effect of each SNP as an IV on the risk of the outcome (ratio of cholesterol to total lipids in moderate VLDL/peptic ulcer) (Figure 1D).
| Exposure/Outcome | Heterogeneity test | Multi-efficiency test | ||||
| MR Egger, IV | MR Egger | |||||
| Q | P value | Q | P value | Intercept | P value | |
| Ratio of cholesterol to total lipids in medium VLDL/peptic ulcer | 59.919 | 0.687 | 63.448 | 0.6 | -0.061 | 0.064 |
In the reverse two-sample MR analysis conducted in this study, peptic ulcer was considered as the exposure, and the ratio of cholesterol to total lipids in medium VLDL was treated as the outcome. The same stringent SNP screening criteria applied in the forward MR analysis were employed.
However, despite the rigorous screening process, no SNPs met the criteria to serve as IVs in the reverse MR analysis. Consequently, the analysis could not establish a causal relationship between genetic susceptibility to peptic ulcer and the ratio of cholesterol to total lipids in medium VLDL.
The absence of significant SNPs underscores the complexity of the relationship between peptic ulcer and lipid metabolism, suggesting that genetic predisposition to peptic ulcer may not directly influence the ratio of cholesterol to total lipids in medium VLDL. This finding highlights the need for further research to elucidate the underlying mechanisms linking these two traits and explore other potential factors contributing to the development of peptic ulcers.
In this study, we used the TSMR method to analyze published GWAS datasets to determine whether there is a causal relationship between the ratio of cholesterol to total lipids in medium VLDL and peptic ulcers in a European population. Our results support a causal relationship between genetic susceptibility to the ratio of cholesterol to total lipids in intermediate VLDL and an increased risk of peptic ulcers (IVW: OR = 2.557, 95%CI = 1.274-5.132, P = 0.008). The MR results were robust and reliable in sensitivity analysis.
The inherent connection between the ratio of cholesterol to total lipids in intermediate VLDL and the onset of peptic ulcers remains ambiguous. Recent research indicates a potential close link between the ratio of cholesterol to total lipids in intermediate VLDL and various factors including body inflammation, insulin resistance, H. pylori infection, and intestinal microecology. Initially, previous studies have proposed that alterations in the secretion of adipokines occur following the accumulation of body fat in individuals, correlating with various pathophysiologic conditions such as insulin resistance and inflammatory responses. This inflammatory cascade can contribute to damage in the gastrointestinal mucosa, potentially leading to PU[16]. However, the precise relationship between PU and the ratio of cholesterol to total lipids in medium VLDL remains contentious. Several potential explanations exist for this discrepancy. Adipose tissue, functioning as a crucial endocrine organ, secretes adipokines that play pivotal roles in the inflammatory response[17,18]. Research indicates that adipose and inflammatory cells can induce inflammatory reactions and insulin resistance by releasing adhesion molecules and pro-inflammatory cytokines[19]. The convergence of these factors may foster a chronic low-grade inflammatory state, ultimately culminating in the development of PU. Furthermore, systemic low-grade inflammation in visceral fat, which serves as a major source of inflammatory cytokines, is likely associated with additional inflammatory responses such as esophagitis[20]. Numerous products of adipose tissue, termed adipocytokines, have been identified[21]. Studies by Tilg et al[20,21] propose that visceral adipocytes and infiltrating macrophages secrete various inflammatory mediators, including adipocytokines, which play crucial roles in the development of diseases such as obesity and multiple sclerosis. Previous research from our group revealed that various mediators released by visceral fat, including lipocalin, leptin, tumor necrosis factor-a (TNF-α), and interleukin-6 (IL-6), can exert distal effects in the gastric and/or esophagogastric junction region. Additionally, it has been demonstrated that pro-inflammatory cytokines such as IL-1 and TNF-α promote gastrin secretion in the antrum of the human gastric lobe. Thus, pro-inflammatory mediators such as adipokines may contribute to the initiation and progression of localized inflammation, ultimately leading to the development of PU.
Pro-inflammatory cytokines can affect various tissues, contributing to the characteristic features of type 2 diabetes mellitus (T2DM)[22]. The inflammatory response in adipose tissue plays a pivotal role in insulin resistance. Specifically, the autoinflammatory response mediated by interleukin-1β (IL-1β) is a significant factor leading to impaired insulin secretion in individuals with T2DM. Our previous research demonstrated a notable inflammatory reaction in the adipose tissue of obese mice, characterized by an increased infiltration of bone marrow-derived macrophages. These macrophages promote the secretion of chemokines and cytokines, including IL-1β[23], C-reactive protein, IL-6, and TNF[24]. Subsequently, these factors, along with others secreted by macrophages, activate serine kinases through a paracrine pathway. These serine kinases, such as c-junk-terminal kinase and inhibitor of nuclear factor kappa B kinase β, phosphorylate insulin receptor substrate proteins, thereby inducing insulin resistance in adipose tissue[25].
Furthermore, several other studies have indicated an association between the ratio of cholesterol to total lipids in medium VLDL and peptic ulcers. A retrospective cross-sectional study[26] revealed a strong correlation between elevated triglyceride levels and gastric ulcers in 5102 patients, with hypertriglyceridemic individuals being more prone to developing gastric ulcers. Additionally, a case-control study involving 35194 cases investigated the use of statins and the development of gastrointestinal ulcer disease (PU). The study found that statins significantly decreased the risk of PU, with the reduction being attributed to the high cumulative daily dose of statins. Hence, the aggressive administration of statins may effectively lower the incidence of PU.
H. pylori infects cells by targeting lipid rafts, which are specialized domains rich in cholesterol distributed across the cell membrane[27]. The bacterium's cholesterol-α-glucosyltransferase (CGT) enzyme converts cholesterol into cholesterol-based glucosides, which bind to the bacterial cell wall[28]. This process helps H. pylori evade the host's immune defenses[29] and establish colonization. While the exact molecular mechanism remains unknown, previous studies have shown that CGT enables H. pylori to adhere to gastric epithelial cells in a cholesterol-dependent manner. Another key aspect is the externalization of cell membrane phosphatidylserine, facilitated by CGT, which enhances the interaction between H. pylori and host cells, leading to cell death.
Among the pathogenic mechanisms, the role of the cytotoxin-associated gene A (CagA) is particularly prominent[30]. Additionally, exogenous cholesterol can modulate the activity of H. pylori CGT on intracellular cholesterol levels, suggesting that the initial binding of H. pylori to host cells post-infection is closely linked to the host's cholesterol levels. Excessive cholesterol encapsulated by CGT-containing H. pylori affects inflammatory signaling pathways[31]. It has been investigated whether exogenous cholesterol influences CGT-mediated H. pylori adhesion to cells. The initial attachment of H. pylori to cells relies on host cholesterol, while exogenous cholesterol interacts with CGT-mediated cellular cholesterol, inhibiting its binding to cells.
Previous research has shown that bacterial virulence factors gain entry into host cells through cholesterol-rich microdomains in their membranes[32]. Cholesterol depletion or consumption is an effective strategy to block the invasion of pathogenic bacteria[33]. For instance, statins have been demonstrated to significantly reduce the incidence of H. pylori-related diseases[34,35]. Moreover, cholesterol sequestration by antagonists competes with the effects of virulence factors like VacA and CagA via lipid rafts, thereby mitigating H. pylori-induced pathogenesis[36].
Likewise, a cross-sectional study involving 462 patients revealed[37] that H. pylori infection correlated with elevated LDL cholesterol levels in an elderly Korean population. Additionally, a large cross-sectional study observed a significant increase in LDL cholesterol and a decrease in HDL cholesterol among male patients with H. pylori infection[38]. Furthermore, researchers such as Jia and others have proposed[39] that reduced high-density lipoprotein cholesterol levels may be linked to H. pylori infection.
Thirdly, research has demonstrated the significant role of the interaction between the diversity or composition of intestinal flora and intestinal cells in human health[40]. This interaction is crucial for maintaining intestinal and immune homeostasis[41]. Ivanov et al[42] discovered that a single segmented filamentous bacterium could induce T-lymphocyte differentiation in the intestinal lamina propria in a study on mice. Furthermore, some commensal bacteria have been shown to regulate inflammatory responses in the intestinal mucosa by modulating regulatory T cells and their downstream signaling pathways such as transforming growth factor-β and IL-10[43]. Additionally, studies have indicated that the intestinal flora regulates Th17 cells, which modulate the innate immune response and lipid uptake by intestinal epithelial cells[44,45]. Moreover, gut flora breaks down indigestible polysaccharides to produce short-chain fatty acids (SCFAs), which are then translocated to other sites, thereby impacting normal cellular physiological activities[46]. Insufficient levels of SCFAs lead to the weakening of tight junctions between intestinal epithelial cells, resulting in disruption of the intestinal epithelial barrier[47,48]. This disruption promotes the progression of H. pylori infection and inflammatory bowel disease.
In addition, this study did not find a causal association of PU on the ratio of cholesterol to total lipids in moderate VLDL. The possible explanation for this is that this bidirectional effect makes PUs less effective on lipids and thus less influential on the ratio of cholesterol to total lipids in intermediate VLDL. Luo et al[49] also used bidirectional two-sample Mendelian randomization to study the causal association between depression and inflammatory bowel disease and confirmed a causal association between depression and inflammatory bowel disease but did not support a causal association of inflammatory bowel disease on depression. Similarly, Chen et al[50] found a protective effect of several gut flora against depression, but a two-way Mendelian randomization study showed no effect of depression on gut microbiota composition.
Finally, the reverse MR analysis of this study did not find an effect of peptic ulcer on the ratio of cholesterol to total lipids in moderate VLDL. This finding further affirms the causal relationship from the ratio of cholesterol to total lipids in moderate VLDL to peptic ulcer.
The findings of this study carry significant clinical implications as they could aid in identifying specific populations at risk for peptic ulcers, allowing for early intervention to mitigate the risk. Moreover, additional research is warranted to investigate whether lipid-lowering therapies can effectively reduce the risk of peptic ulcers by targeting the ratio of cholesterol to total lipids in medium VLDL.
This study possesses several strengths. Randomized controlled trials, while considered the gold standard in clinical research, are often expensive, challenging to follow up, and difficult to implement. In contrast, the two-sample MR study design utilized in this research can mitigate confounding factors and reverse causality, simulating the effect of a randomized controlled trial. Moreover, SNPs that highly correlated with exposure (F > 10) were carefully selected as IVs, and multiple sensitivity analysis methods detected no heterogeneity, pleiotropy, or outliers, enhancing the reliability of the study results.
However, there are several limitations to consider. Despite the MR design and efforts to exclude known confounders, the study results may still be influenced by unaccounted potential confounders. In addition, prevalence rates vary between races, and generalizability of the findings to other racial groups may be limited due to the fact that only GWAS data from European populations were utilized. Furthermore, due to the lack of statistical power, definitive causal effect analysis awaits future GWAS studies with larger sample sizes. Lastly, although a number of possible biological mechanisms have been proposed in the discussion to explain the relationship between the ratio of cholesterol to total lipids in moderate VLDL and PUs, these mechanisms have not yet been explored in depth, and further laboratory studies (e.g., simulated PUs after intervention of the ratio of cholesterol to total lipids in VLDL in mice in animal experiments, to further explore the relationship with inflammatory factors, insulin resistance, and gut flora) and larger studies (e.g., further database mining of large-scale cohort and cross-sectional studies to increase the level of evidence) are needed to reveal the relationship between cholesterol-to-total lipid ratio in VLDL and peptic ulcer.
In conclusion, this study suggests a causal association between the ratio of cholesterol to total lipids in medium VLDL and an increased risk of PU.
In conclusion, bidirectional TSMR analyses supported a causal relationship between genetic susceptibility to the ratio of cholesterol to total lipids in medium VLDL and increased risk of PU, and no causal association of PU on the ratio of cholesterol to total lipids in medium VLDL was found. However, due to the limitations of the study, further research is necessary.
We thank all those who participated in our study.
| 1. | Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 558] [Article Influence: 69.8] [Reference Citation Analysis (37)] |
| 2. | Huang Y, Li H, Long X, Liang X, Lu H. Lessons learned from upper gastrointestinal endoscopy in asymptomatic Chinese. Helicobacter. 2021;26:e12803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 3. | Xie X, Ren K, Zhou Z, Dang C, Zhang H. The global, regional and national burden of peptic ulcer disease from 1990 to 2019: a population-based study. BMC Gastroenterol. 2022;22:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 4. | Lin CJ, Liao WC, Chen YA, Lin HJ, Feng CL, Lin CL, Lin YJ, Kao MC, Huang MZ, Lai CH, Kao CH. Statin Therapy Is Associated with Reduced Risk of Peptic Ulcer Disease in the Taiwanese Population. Front Pharmacol. 2017;8:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. 2017;26:2333-2355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 1071] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 6. | Swanson SA, Tiemeier H, Ikram MA, Hernán MA. Nature as a Trialist?: Deconstructing the Analogy Between Mendelian Randomization and Randomized Trials. Epidemiology. 2017;28:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA. 2017;318:1925-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 2139] [Article Influence: 267.4] [Reference Citation Analysis (0)] |
| 8. | Liu C, Liu N, Xia Y, Zhao Z, Xiao T, Li H. Osteoporosis and sarcopenia-related traits: A bi-directional Mendelian randomization study. Front Endocrinol (Lausanne). 2022;13:975647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Wang Q, Shi Q, Lu J, Wang Z, Hou J. Causal relationships between inflammatory factors and multiple myeloma: A bidirectional Mendelian randomization study. Int J Cancer. 2022;151:1750-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Carrasquilla GD, García-Ureña M, Fall T, Sørensen TIA, Kilpeläinen TO. Mendelian randomization suggests a bidirectional, causal relationship between physical inactivity and adiposity. Elife. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology. 2017;28:30-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 1263] [Article Influence: 180.4] [Reference Citation Analysis (0)] |
| 12. | Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40:304-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4015] [Cited by in RCA: 5664] [Article Influence: 629.3] [Reference Citation Analysis (0)] |
| 13. | Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2275] [Cited by in RCA: 6180] [Article Influence: 618.0] [Reference Citation Analysis (0)] |
| 14. | Greco M FD, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34:2926-2940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 971] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 15. | Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 5310] [Article Influence: 758.6] [Reference Citation Analysis (0)] |
| 16. | Sogabe M, Okahisa T, Kimura T, Okamoto K, Miyamoto H, Muguruma N, Takayama T. Influence of metabolic syndrome on upper gastrointestinal disease. Clin J Gastroenterol. 2016;9:191-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Gonçalves P, Magro F, Martel F. Metabolic inflammation in inflammatory bowel disease: crosstalk between adipose tissue and bowel. Inflamm Bowel Dis. 2015;21:453-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 18. | Kahn CR, Wang G, Lee KY. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J Clin Invest. 2019;129:3990-4000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 424] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 19. | Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Kouroumalis EA. The effect of infliximab on circulating levels of leptin, adiponectin and resistin in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2007;19:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Tilg H, Moschen AR. Visceral adipose tissue attacks beyond the liver: esophagogastric junction as a new target. Gastroenterology. 2010;139:1823-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2065] [Cited by in RCA: 2245] [Article Influence: 118.2] [Reference Citation Analysis (0)] |
| 22. | Fernández-Real JM, Pickup JC. Innate immunity, insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2008;19:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 261] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 24. | Vaarala O, Yki-Järvinen H. Diabetes: Should we treat infection or inflammation to prevent T2DM? Nat Rev Endocrinol. 2012;8:323-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2726] [Cited by in RCA: 3078] [Article Influence: 162.0] [Reference Citation Analysis (0)] |
| 26. | Loke SS, Li WC. Peptic Ulcer Disease Associated with Central Obesity. J Pers Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Chatterjee R, Chowdhury AR, Mukherjee D, Chakravortty D. Lipid larceny: channelizing host lipids for establishing successful pathogenesis by bacteria. Virulence. 2021;12:195-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Lebrun AH, Wunder C, Hildebrand J, Churin Y, Zähringer U, Lindner B, Meyer TF, Heinz E, Warnecke D. Cloning of a cholesterol-alpha-glucosyltransferase from Helicobacter pylori. J Biol Chem. 2006;281:27765-27772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Wunder C, Churin Y, Winau F, Warnecke D, Vieth M, Lindner B, Zähringer U, Mollenkopf HJ, Heinz E, Meyer TF. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat Med. 2006;12:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 218] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 30. | Murata-Kamiya N, Kikuchi K, Hayashi T, Higashi H, Hatakeyama M. Helicobacter pylori exploits host membrane phosphatidylserine for delivery, localization, and pathophysiological action of the CagA oncoprotein. Cell Host Microbe. 2010;7:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Morey P, Pfannkuch L, Pang E, Boccellato F, Sigal M, Imai-Matsushima A, Dyer V, Koch M, Mollenkopf HJ, Schlaermann P, Meyer TF. Helicobacter pylori Depletes Cholesterol in Gastric Glands to Prevent Interferon Gamma Signaling and Escape the Inflammatory Response. Gastroenterology. 2018;154:1391-1404.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 32. | Chen Y, Huang K, Chen LK, Wu HY, Hsu CY, Tsai YS, Ko WC, Tsai PJ. Membrane Cholesterol Is Crucial for Clostridium difficile Surface Layer Protein Binding and Triggering Inflammasome Activation. Front Immunol. 2020;11:1675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Gagliardi MC, Iwabuchi K, Lai CH. Editorial: Role of Lipid Rafts in Anti-microbial Immune Response. Front Immunol. 2021;12:654776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Liao WC, Huang MZ, Wang ML, Lin CJ, Lu TL, Lo HR, Pan YJ, Sun YC, Kao MC, Lim HJ, Lai CH. Statin Decreases Helicobacter pylori Burden in Macrophages by Promoting Autophagy. Front Cell Infect Microbiol. 2016;6:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Lin CJ, Liao WC, Lin HJ, Hsu YM, Lin CL, Chen YA, Feng CL, Chen CJ, Kao MC, Lai CH, Kao CH. Statins Attenuate Helicobacter pylori CagA Translocation and Reduce Incidence of Gastric Cancer: In Vitro and Population-Based Case-Control Studies. PLoS One. 2016;11:e0146432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Yeh JY, Lin HJ, Kuo CJ, Feng CL, Chou CH, Lin CD, Wu HY, Li CY, Chiu CH, Lai CH. Campylobacter jejuni Cytolethal Distending Toxin C Exploits Lipid Rafts to Mitigate Helicobacter pylori-Induced Pathogenesis. Front Cell Dev Biol. 2020;8:617419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Kim HL, Jeon HH, Park IY, Choi JM, Kang JS, Min KW. Helicobacter pylori infection is associated with elevated low density lipoprotein cholesterol levels in elderly Koreans. J Korean Med Sci. 2011;26:654-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Satoh H, Saijo Y, Yoshioka E, Tsutsui H. Helicobacter Pylori infection is a significant risk for modified lipid profile in Japanese male subjects. J Atheroscler Thromb. 2010;17:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Jia EZ, Zhao FJ, Hao B, Zhu TB, Wang LS, Chen B, Cao KJ, Huang J, Ma WZ, Yang ZJ, Zhang G. Helicobacter pylori infection is associated with decreased serum levels of high density lipoprotein, but not with the severity of coronary atherosclerosis. Lipids Health Dis. 2009;8:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Reinhardt C, Reigstad CS, Bäckhed F. Intestinal microbiota during infancy and its implications for obesity. J Pediatr Gastroenterol Nutr. 2009;48:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 41. | Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 657] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 42. | Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3529] [Cited by in RCA: 3514] [Article Influence: 219.6] [Reference Citation Analysis (0)] |
| 43. | Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1311] [Cited by in RCA: 1205] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 44. | Kawano Y, Edwards M, Huang Y, Bilate AM, Araujo LP, Tanoue T, Atarashi K, Ladinsky MS, Reiner SL, Wang HH, Mucida D, Honda K, Ivanov II. Microbiota imbalance induced by dietary sugar disrupts immune-mediated protection from metabolic syndrome. Cell. 2022;185:3501-3519.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 178] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 45. | Adolph TE, Meyer M, Schwärzler J, Mayr L, Grabherr F, Tilg H. The metabolic nature of inflammatory bowel diseases. Nat Rev Gastroenterol Hepatol. 2022;19:753-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 146] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 46. | Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020;11:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 780] [Cited by in RCA: 1575] [Article Influence: 315.0] [Reference Citation Analysis (0)] |
| 47. | Pérez-Reytor D, Puebla C, Karahanian E, García K. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front Physiol. 2021;12:650313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 99] [Reference Citation Analysis (0)] |
| 48. | Singh R, Zogg H, Wei L, Bartlett A, Ghoshal UC, Rajender S, Ro S. Gut Microbial Dysbiosis in the Pathogenesis of Gastrointestinal Dysmotility and Metabolic Disorders. J Neurogastroenterol Motil. 2021;27:19-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 49. | Luo J, Xu Z, Noordam R, van Heemst D, Li-Gao R. Depression and Inflammatory Bowel Disease: A Bidirectional Two-sample Mendelian Randomization Study. J Crohns Colitis. 2022;16:633-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 50. | Chen M, Xie CR, Shi YZ, Tang TC, Zheng H. Gut microbiota and major depressive disorder: A bidirectional Mendelian randomization. J Affect Disord. 2022;316:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |