Published online Aug 26, 2024. doi: 10.12998/wjcc.v12.i24.5589
Revised: May 5, 2024
Accepted: June 4, 2024
Published online: August 26, 2024
Processing time: 172 Days and 0.3 Hours
The complexity of immunoglobulin G4 (IgG4)-related diseases and their potential connection to hematologic malignancies remains unclear. This article provided a review of the diagnosis and treatment of a patient with IgG4-related sclerosing cholangitis (SC) and essential thrombocythemia (ET), along with an analysis of relevant literature to enhance comprehension of this disease.
A 56-year-old male was admitted to two hospitals with deteriorating jaundice and pruritus prior to hospitalization. Beyond our expectations, the patient was first diagnosed with IgG4-SC and ET with the Janus kinase 2 V617F mutation. Inte
When IgG4-SC is suspected without histopathological evidence, diagnostic the
Core Tip: The case diagnosed with immunoglobulin G4 (IgG4)-related sclerosing cholangitis (SC) and essential thrombocythemia was first reported. In this article, we described the clinical features of this case and reported the diagnosis and treatment process and prognosis. The relationship between IgG4-SC and Janus kinase 2 V617F mutation diseases was analyzed and summarized by retrieving literature.
- Citation: Wu ZN, JI R, Xiao Y, Wang YD, Zhao CY. IgG4-related sclerosing cholangitis associated with essential thrombocythemia: A case report. World J Clin Cases 2024; 12(24): 5589-5595
- URL: https://www.wjgnet.com/2307-8960/full/v12/i24/5589.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i24.5589
Prior research identified a connection between autoimmune disorders and the development of myeloproliferative neoplasms (MPNs)[1]. Immunoglobulin G4 (IgG4)-related sclerosing cholangitis (SC) was first reported in Japan, and it refers to SC of unknown cause. It is characterized by increased serum IgG4 levels and fibrosis associated with marked infiltration of local lesions by lymphocytes and IgG4-positive plasma cells[2]. Precise epidemiological data are currently lacking, and the available data were mainly obtained in Japanese studies. The overall incidence and annual prevalence of IgG4-SC in Japan are 2.1 and 0.63 per 100000 people, respectively[2]. It is challenging to distinguish IgG4-SC from pri
Meanwhile, essential thrombocythemia (ET) is an MPN with an annual incidence of approximately 1.2-3.0 per 100000 people[3]. It is rare for patients to experience both ET and IgG4-SC simultaneously. We experienced a patient exhibiting jaundice, pruritus, a high platelet (PLT) count, and bleeding as clinical features. This was the first reported case both do
A 56-year-old male was admitted to our hospital on September 29, 2022 with progressive jaundice and intractable pruritus persisting for 1 mo.
The patient experienced poor appetite, abdominal distension, and intractable pruritus but did not report abdominal pain, diarrhea, rash, hemorrhagic spots, or ecchymosis prior to hospitalization. Prior to coming to our hospital, the patient had visited Municipal hospitals on September 15, 2022 and a tertiary hospital on September 23, 2022. The results of blood tests, as shown in Table 1, revealed significantly elevated serum biomarkers including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT), and bilirubin. Addi
| Variable | September 15, 2022 | September 23, 2022 | September 30, 2022 | October 04, 2022 | October 09, 2022 | October 10, 2022 | October 14, 2022 | October 18, 2022 | October 28, 2022 |
| TBIL in μmol/L | 151.79 | 186.78 | 156.36 | 135.41 | 123.79 | 99.15 | 83.87 | 134.00 | 53.00 |
| ALP in U/L | 895.2 | 856.2 | 722.0 | 837.0 | 1077.0 | 856.0 | 715.0 | 827.0 | 745.0 |
| GGT in U/L | NA | 393.3 | 407.0 | 475.0 | 709.0 | 580.0 | 469.0 | 889.0 | 1021.0 |
| PLT as × 109/L | 998 | 760 | 771 | 813 | 910 | NA | 804 | 1035 | 813 |
| D-dimer in g/L | NA | NA | 0.39 | 0.39 | 0.55 | NA | 1.45 | 2.33 | NA |
| PT in s | 11.4 | NA | 11.0 | 11.1 | 11.0 | NA | 10.9 | 9.9 | NA |
| APTT in s | 27.9 | NA | 42.5 | 35.6 | 42.5 | NA | 31.6 | 30.6 | NA |
| INR | 1.00 | NA | 0.99 | 1.00 | 0.99 | NA | 0.98 | 0.89 | NA |
| FIB in g/L | 3.68 | NA | 3.99 | 3.57 | 3.99 | NA | 3.72 | 3.48 | NA |
| COL/EPI-CT in s | NA | NA | NA | NA | NA | NA | > 292 | NA | NA |
| COL/ADP-CT in s | NA | NA | NA | NA | NA | NA | 244 | NA | NA |
| P2Y12-CT in s | NA | NA | NA | NA | NA | NA | 136 | NA | NA |
The patient had no history of liver disease or hemopathy. There was also no record of taking drugs that could potentially cause liver injury in the prior 6 mo.
The patient had no history of alcohol consumption, smoking, or genetic disease in the family.
The patient’s temperature was 36.4 °C, heart rate was 72 beats/minute, and blood pressure was 134/71 mmHg. Severe jaundice was observed in the skin, mucous membrane, and sclera along with widespread scratches due to pruritus. No other regions showed any signs.
On September 30, 2022, blood examinations were performed. The results were as follows: Total bilirubin 156.36 μmo1/L; direct bilirubin 126.66 μmo1/L; ALP 722 U/L; GGT 407 U/L; total bile acid 617 μmo1/L; and PLT counts 771 × 109/L. These levels were significantly elevated. Blood AST and ALT levels were mildly increased. The levels of serum pancreatic enzymes were in the normal range. There was no infectious evidence of hepatitis A-E virus, HIV, Epstein-Barr virus, and cytomegalovirus. The levels of serum tumor markers, including ferritin 354.74 ng/mL (normal range: 23.9-33.6 ng/mL), carbohydrate antigen 199 367.4 U/mL (normal range: 0-25 U/mL), protein induced by vitamin K absence or antagonist-II 80.54 mAU/mL (normal range: 0-40 mAU/mL), and carbohydrate antigen 125 36.5 U/mL (normal range: 0-35 U/mL) were elevated, while alpha-fetoprotein and carcinoembryonic antigen levels were normal. The outcomes of the ex
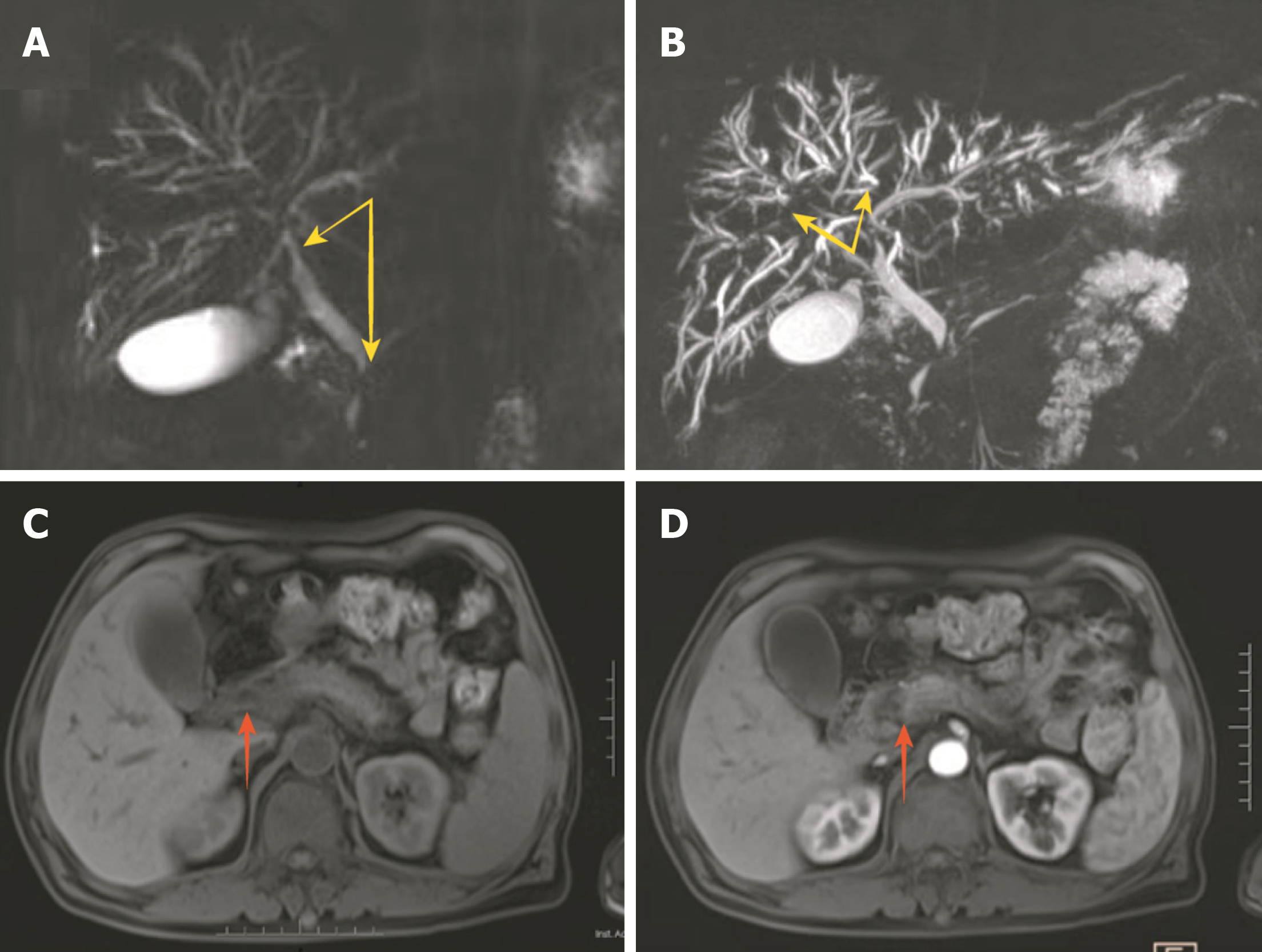
Magnetic resonance imaging revealed dilation of the intrahepatic bile duct, local narrowing, and uneven thickness of the bilateral hepatic duct, common hepatic duct, and lower segment of the common bile duct. The head of the pancreas was enlarged, and the imaging physician considered possible localized pancreatitis (Figure 1).
Unfortunately, jaundice did not vanish gradually. At the same time, PLT levels progressively increased (813 × 109/L) starting on October 4, 2022. To prevent thrombosis development and embolism because of significantly high PLT counts, aspirin (0.1 g per day) and low-molecular-weight heparin (4250 IU q12h ih) were administered. After 4-d anticoagulation and anti-PLT therapy, ecchymosis and a lump with tenderness were found at the needle sites and the back of the left lower leg, respectively. Color doppler ultrasound showed a liquid mass in the muscle layer of the left lower leg, and there were no signs of clot or deep vein thrombosis in the legs. We considered the lump a hematoma. Therefore, aspirin and low-molecular-weight heparin were discontinued immediately.
According to the above clues, the cause of jaundice in patients may be any of the following: PSC; IgG4-SC; and tumor. Due to the patient’s high risk of bleeding, a liver biopsy was not conducted. This decision complicated the process of distinguishing between PSC and a bile duct tumor, but quantitative analysis of immunoglobulin subclasses was carried out. The coagulation function examination suggested a normal or hypercoagulable state and decreased PLT function (Table 1). To determine the cause of hyperthrombocytosis and bleeding, we conducted bone marrow and genetic testing.
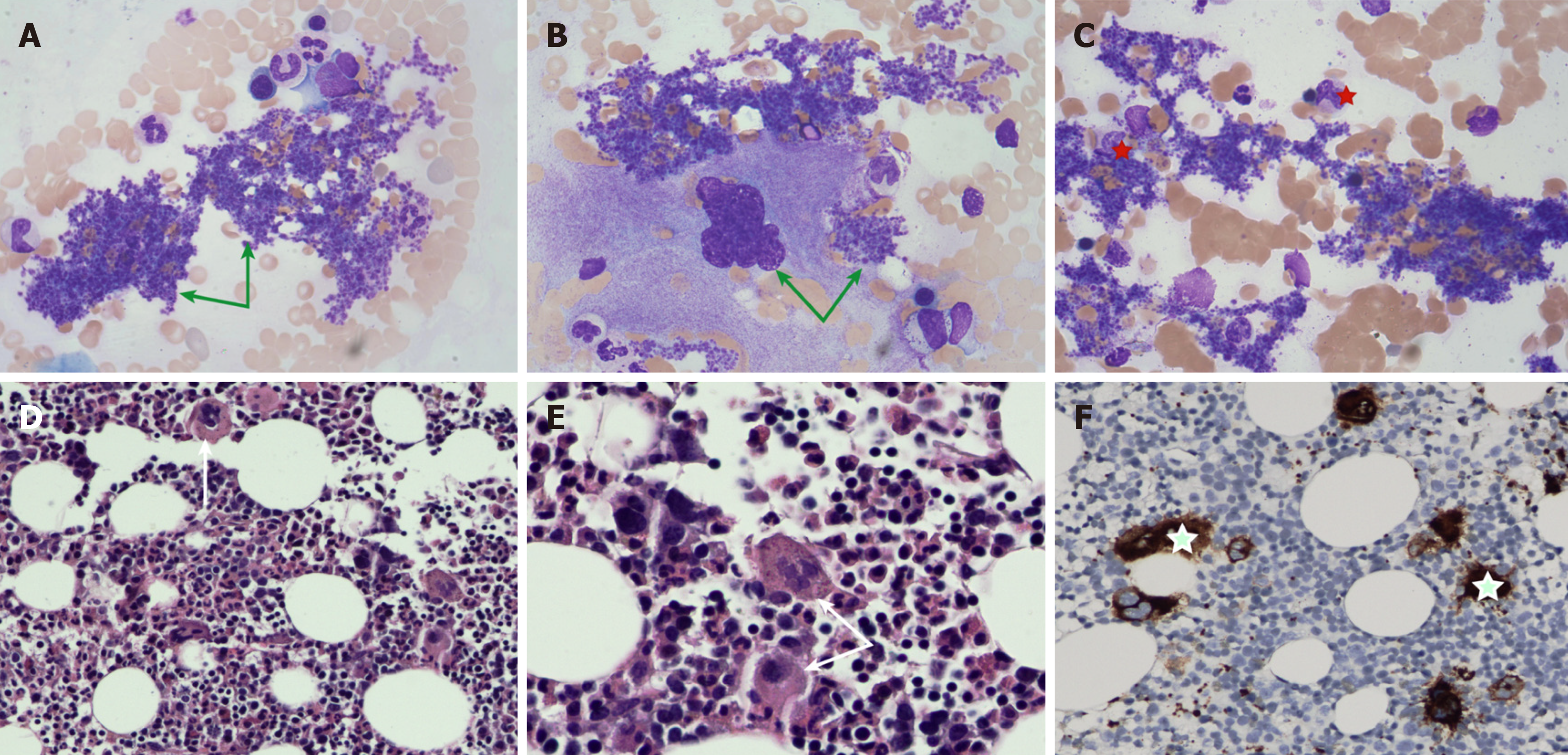
The outcomes of the examinations were gradually reported. Quantitative analysis of immunoglobulin subclasses: Serum IgG4 (19 g/L, normal range: 0.03-2.01 g/L) level was significantly elevated. Bone marrow cytology examination and bone marrow histopathology are depicted in Figure 2. Gene testing found that the Janus kinase 2 (JAK2) V617F mutation was positive.
Although pancreatic enzymes did not show significant abnormalities, localized pancreatitis was still be considered in conjunction with imaging studies conducted before and after hospital admission. Based on the significantly elevated IgG4 levels, we concluded that the patient’s diagnosis was autoimmune pancreatitis. Additionally, the clinical presentation of this patient was mainly jaundice, and imaging findings showed significant bile duct abnormalities, therefore the final diagnosis included IgG4-SC[4]. ET was diagnosed in the patient in accordance with relevant guidelines (Table 2)[5].
| Major criteria | Minor criteria |
| Platelet count ≥ 450000 per mm3 | Presence of clonal marker or of evidence of reactive thrombocytosis |
| Bone marrow biopsy showing proliferation mainly of the megakaryocytic lineage, with increased numbers of enlarged, mature megakaryocytes with hyperlobated nuclei; no substantial increase or left shift in neutrophil granulopoiesis or erythropoiesis; in rare instances, minor (grade 1) increase in reticulin fibers | |
| Criteria for BCR-ABL1-positive chronic myeloid leukemia, polycythemia vera, primary myelofibrosis, or other myeloid neoplasm not met; JAK2 V617F, CALR, or MPL mutation. |
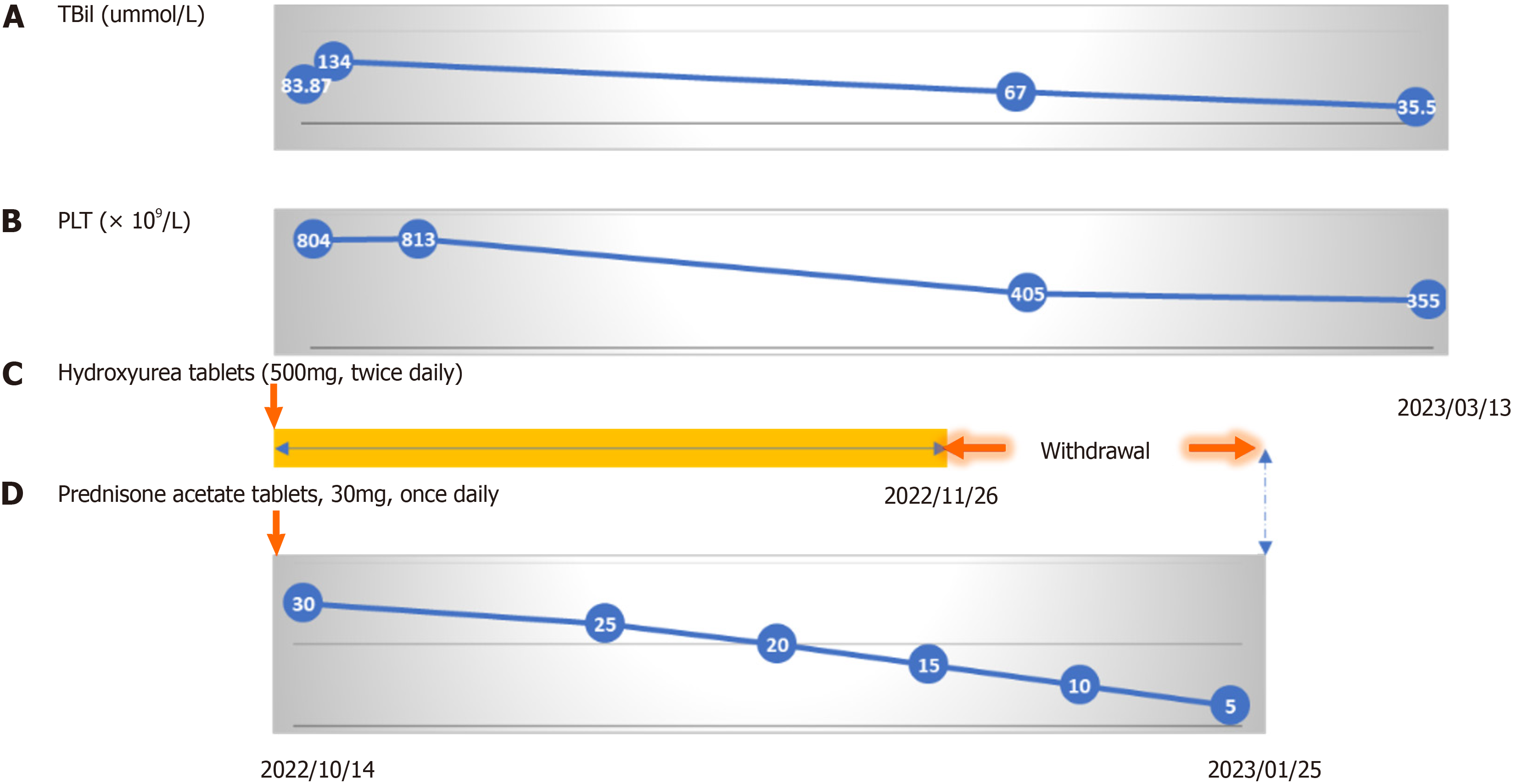
At admission, the patient was given symptomatic treatments, including liver protection and choleretic treatment. The patient (body weight: 65 kg) was treated with oral glucocorticoids (acetate prednisone tablets, 30 mg, once daily) com
The patient’s jaundice resolved, and he was discharged on October 20, 2022. One month after discharge, the patient stopped taking hydroxyurea tablets by himself. The dosage of corticosteroids was gradually reduced. Follow-up was conducted 7 mo later by phone. Jaundice and hematoma had subsided, and the bleeding tendency had disappeared. Bi
IgG4-SC is an autoimmune disease closely associated with IgG4 and falls under the umbrella of IgG4-related diseases. These conditions are known for causing ongoing chronic inflammation in individuals who have not received treatment. Glucocorticoids are recommended as the first-line treatment therapy for IgG4-SC[4].
ET is a kind of MPN characterized by the proliferation of bone marrow megakaryocytes and increased PLT counts in peripheral blood[5]. The diagnosis of ET is mainly based on the diagnostic criteria proposed by Tefferi and Pardanani[5] in 2019, and this case fulfills all the diagnostic criteria. Low-dose aspirin (81-100 mg, once daily), hydroxyurea (500 mg, twice daily), or interferon alpha-2α (45 µg once a week, the maximum dose of 180 µg once a week) should be given for patients diagnosed with ET until normal PLT counts[5,6]. It is well-known that glucocorticoids promote PLT production, which will not be conducive to the treatment of ET. Therefore, whether glucocorticoids should be used in this patient be
To enhance our understanding of the pathogenesis of ET, we conducted an extensive literature review. Chronic inflammation is considered a prerequisite for defending against clonal evolution and cancer development due to its effective DNA repair mechanism in response to sustained oxidative stress caused by chronic inflammation[7]. It is important to note that mutations resulting from DNA repair mechanisms may also increase the risk of clonal evolution. Early studies have shown that sustained inflammation can activate JAK2 (including the V617F mutation) and lead to genome insta
IgG4-SC is an autoimmune disease closely related to IgG4 and is classified as one of the IgG4-related diseases. These diseases are known for causing persistent chronic inflammation in untreated individuals. Emerging evidence indicates that the pathophysiological mechanisms linked to immune inflammation could potentially affect PLT production. Kristin
During follow-up of this case, the patient came to our clinic again due to weakness, and examination showed ALT 637 U/L, AST 394 U/L, total bilirubin 38.8 μmo1/L, direct bilirubin 27.6 μmo1/L, GGT 1141 U/L, ALP 1285 U/L, WBC 14.46 × 109/L, N% 75.4%, eosinophil 0.88 × 109/L, and PLT 1461 × 109/L (because of poverty, the patient did not receive inpatient treatment). Multiple simultaneous increases in biliary enzymes and PLT counts further supported a potential link between IgG4-SC and ET.
Mechanistically, aberrant B cell activation is present in both diseases[11,12]. This suggests that the presence of IgG4-SC and ET in this case is not merely a combination of the two diseases but rather distinct manifestations triggered by inflammatory and immune-related factors. Based on these theories, JAK inhibitors hold promise as alternative treatment options for IgG4-related diseases and MPNs[13,14]. Importantly, this case study can aid clinicians in expanding their diagnostic and treatment approaches.
IgG4-SC can be challenging to diagnose as it lacks specific clinical manifestations and can be mistaken for biliary malignancies, particularly when obtaining pathological examination results is difficult. Long-term follow-up and glu
| 1. | Kristinsson SY, Landgren O, Samuelsson J, Björkholm M, Goldin LR. Autoimmunity and the risk of myeloproliferative neoplasms. Haematologica. 2010;95:1216-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Tanaka A. Immunoglobulin G4-related sclerosing cholangitis. J Dig Dis. 2019;20:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Szuber N, Mudireddy M, Nicolosi M, Penna D, Vallapureddy RR, Lasho TL, Finke C, Begna KH, Elliott MA, Hook CC, Wolanskyj AP, Patnaik MM, Hanson CA, Ketterling RP, Sirhan S, Pardanani A, Gangat N, Busque L, Tefferi A. 3023 Mayo Clinic Patients With Myeloproliferative Neoplasms: Risk-Stratified Comparison of Survival and Outcomes Data Among Disease Subgroups. Mayo Clin Proc. 2019;94:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 4. | Nakazawa T, Kamisawa T, Okazaki K, Kawa S, Tazuma S, Nishino T, Inoue D, Naitoh I, Watanabe T, Notohara K, Kubota K, Ohara H, Tanaka A, Takikawa H, Masamune A, Unno M. Clinical diagnostic criteria for IgG4-related sclerosing cholangitis 2020: (Revision of the clinical diagnostic criteria for IgG4-related sclerosing cholangitis 2012). J Hepatobiliary Pancreat Sci. 2021;28:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Tefferi A, Pardanani A. Essential Thrombocythemia. N Engl J Med. 2019;381:2135-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 6. | Mascarenhas J, Kosiorek HE, Prchal JT, Rambaldi A, Berenzon D, Yacoub A, Harrison CN, McMullin MF, Vannucchi AM, Ewing J, O'Connell CL, Kiladjian JJ, Mead AJ, Winton EF, Leibowitz DS, De Stefano V, Arcasoy MO, Kessler CM, Catchatourian R, Rondelli D, Silver RT, Bacigalupo A, Nagler A, Kremyanskaya M, Levine MF, Arango Ossa JE, McGovern E, Sandy L, Salama ME, Najfeld V, Tripodi J, Farnoud N, Penson AV, Weinberg RS, Price L, Goldberg JD, Barbui T, Marchioli R, Tognoni G, Rampal RK, Mesa RA, Dueck AC, Hoffman R. A randomized phase 3 trial of interferon-α vs hydroxyurea in polycythemia vera and essential thrombocythemia. Blood. 2022;139:2931-2941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 7. | Hasselbalch HC. Chronic inflammation as a promotor of mutagenesis in essential thrombocythemia, polycythemia vera and myelofibrosis. A human inflammation model for cancer development? Leuk Res. 2013;37:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 203] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 8. | Hermouet S, Vilaine M. The JAK2 46/1 haplotype: a marker of inappropriate myelomonocytic response to cytokine stimulation, leading to increased risk of inflammation, myeloid neoplasm, and impaired defense against infection? Haematologica. 2011;96:1575-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Fisher DAC, Fowles JS, Zhou A, Oh ST. Inflammatory Pathophysiology as a Contributor to Myeloproliferative Neoplasms. Front Immunol. 2021;12:683401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 10. | Sobas M, Podolak-Dawidziak M, Lewandowski K, Bator M, Wróbel T. Primary Immune Thrombocytopenia and Essential Thrombocythemia: So Different and yet Somehow Similar-Cases Series and a Review of the Literature. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 11. | Liu CC, Wang SC, Kao CW, Hsieh RK, Chang MC, Chang YF, Lim KH, Chen CG. B cells facilitate platelet production mediated by cytokines in patients with essential thrombocythaemia. Thromb Haemost. 2014;112:537-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Perugino CA, Stone JH. IgG4-related disease: an update on pathophysiology and implications for clinical care. Nat Rev Rheumatol. 2020;16:702-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 13. | How J, Garcia JS, Mullally A. Biology and therapeutic targeting of molecular mechanisms in MPNs. Blood. 2023;141:1922-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 14. | Khan S, Gordins P, Durairaj S. JAK Inhibition as a Therapeutic Strategy for IgG4-RD. J Investig Allergol Clin Immunol. 2021;31:280-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |