Published online Aug 26, 2024. doi: 10.12998/wjcc.v12.i24.5523
Revised: May 29, 2024
Accepted: June 12, 2024
Published online: August 26, 2024
Processing time: 83 Days and 19.5 Hours
The results of existing lower extremity robotics studies are conflicting, and few relevant clinical trials have examined short-term efficacy. In addition, most of the outcome indicators in existing studies are scales, which are not objective enough. We used the combination of objective instrument measurement and scale to explore the short-term efficacy of the lower limb A3 robot, to provide a clinical reference.
To investigate the improvement of lower limb walking ability and balance in stroke treated by A3 lower limb robot.
Sixty stroke patients were recruited prospectively in a hospital and randomized into the A3 group and the control group. They received 30 min of A3 robotics training and 30 min of floor walking training in addition to 30 min of regular rehabilitation training. The training was performed five times a week, once a day, for 2 wk. The t-test or non-parametric test was used to compare the three-dimensional gait parameters and balance between the two groups before and after treatment.
The scores of basic activities of daily living, Stroke-Specific Quality of Life Scale, FM balance meter, Fugl-Meyer Assessment scores, Rivermead Mobility Index, Stride speed, Stride length, and Time Up and Go test in the two groups were significantly better than before treatment (19.29 ± 12.15 vs 3.52 ± 4.34; 22.57 ± 17.99 vs 4.07 ± 2.51; 1.21 ± 0.83 vs 0.18 ± 0.40; 3.50 ± 3.80 vs 0.96 ± 2.08; 2.07 ± 1.21 vs 0.41 ± 0.57; 0.89 ± 0.63 vs 0.11 ± 0.32; 12.38 ± 9.00 vs 2.80 ± 3.43; 18.84 ± 11.24 vs 3.80 ± 10.83; 45.12 ± 69.41 vs 8.41 ± 10.20; 29.45 ± 16.62 vs 8.68 ± 10.74; P < 0.05). All outcome indicators were significantly better in the A3 group than in the control group, except the area of the balance parameter.
For the short-term treatment of patients with subacute stroke, the addition of A3 robotic walking training to conventional physiotherapy appears to be more effective than the addition of ground-based walking training.
Core Tip: In this study, two groups of stroke patients underwent 2 wk of A3 lower extremity robotics and ground walking training, respectively, and gait spatiotemporal and balance parameters were recorded before and after the 2-wk intervention, which were compared by statistical analysis. It was finally concluded that for the short-term treatment of patients with subacute stroke, the addition of A3 robotic walking training to conventional physiotherapy appears to be more effective than the addition of ground-based walking training.
- Citation: Zhang LJ, Wen X, Peng Y, Hu W, Liao H, Liu ZC, Liu HY. Effectiveness of the A3 robot on lower extremity motor function in stroke patients: A prospective, randomized controlled trial. World J Clin Cases 2024; 12(24): 5523-5533
- URL: https://www.wjgnet.com/2307-8960/full/v12/i24/5523.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i24.5523
Stroke can lead to permanent disability and even death[1]. In many cases following a stroke, mobility, balance, and walking are affected[2], The majority of stroke survivors have initial mobility deficits, and 6 mo after stroke, more than 30% of survivors still cannot walk independently[3] and impaired gait still severely limits daily life. Gait pattern abnormalities associated with stroke are often characterized by altered hip, knee, and ankle kinematics[4], and stroke patients walk asymmetrically and with reduced gait speed[5]. One of the main goals of stroke rehabilitation is to regain gait function[6], and therapists spend a considerable amount of time and effort on it[7].
Lower limb robot is a new treatment method to promote the recovery of stroke patients. It has the advantages of repetition, specificity, and quantitative evaluation[8,9]. Studies have shown that patients with subacute stroke who received lower limb robots in combination with conventional treatment showed greater improvement in functional gait than those who received conventional treatment alone[10-12]. But other studies have found no difference in outcomes between robotic therapy and traditional therapy[13-15] and the issue remains controversial and unresolved. Therefore, the application of gait robots to the field of stroke needs more research to expand some obscure and controversial areas. In addition, most of the previous clinical trials of lower limb robots used scales such as the Fugl-Meyer Assessment scale, Berg Balance Scale scores, and River mead Mobility Index (RMI) to evaluate the efficacy[12], which is subjective to a certain extent. Three-dimensional gait analysis has the advantages of objective data, quantitative data, and high credibility[16].
Based on the above background, a clinical trial was conducted to verify the effectiveness of 2-wk A3 lower limb robot-assisted gait training on patients with subacute stroke, and three-dimensional gait spatiotemporal parameters were used as evaluation indexes. We propose a basic hypothesis: in the short term, patients with subacute stroke who added A3 robotic walking training to conventional physical therapy showed significant gait and balance improvements in spatial-temporal parameters and balance function that may be superior to conventional ground-based walking training.
This study was a single-center, single-blind, and prospective randomized controlled trial. The clinical trial was approved by the Ethics Committee of Yuebei People's Hospital (KY-2021-327; Shaoguan, China). Written informed consent was provided by all participants and the procedures were carried out following the Helsinki Declaration. The program was registered online in the Chinese Clinical Trial Registry (Registration No. ChiCTR2100052767).
Before the trial, eligible participants were randomly assigned to two groups according to a random number generated by Excel software. The random numbers were written on a small card and placed in an opaque envelope. An investigator determined the eligibility of participants for inclusion, but he was not involved in the allocation and was not aware of subsequent grouping (hidden assignment). After the patient grouping was confirmed by two therapist interveners, the grouping sheet was kept in a sealed opaque envelope until the end of the trial. At the end of the trial, no errors were found after we uncovered and checked the allocation.
Subjects were selected according to the following inclusion criteria: (1) Enrolled patients had a first stroke diagnosed by computerized tomography or magnetic resonance imaging (MRI); (2) All patients have unilateral limb motor dysfunction, and the patient can complete a 10 m walk alone or with the assistance of an assistive device; (3) The patient's condition, including vital signs, remained stable; (4) Aged 25 years to 80 years; (5) Lower extremity Brunnstrom stage III (inclusive) or higher, lower extremity modified Ashworth grading muscle tone below grade II; (6) Patients can cooperate with researchers in various examinations and rehabilitation training; and (7) The patient himself and his family members provided written informed consent.
Exclusion criteria are as follows: (1) Patients with other nervous system or bone and joint diseases that may affect the function of the lower extremities, such as arthritis, lower extremity joint contractures, deformities or other peripheral nervous system lesions, pain conditions; (2) Pregnant and lactating patients; (3) Patients with severe cardiopulmonary insufficiencies, such as heart failure, unstable angina, etc, or implanted pacemakers; (4) Patients with severe osteoporosis, malignant tumor of bone and joint; and (5) Patients with severe sensory impairment.
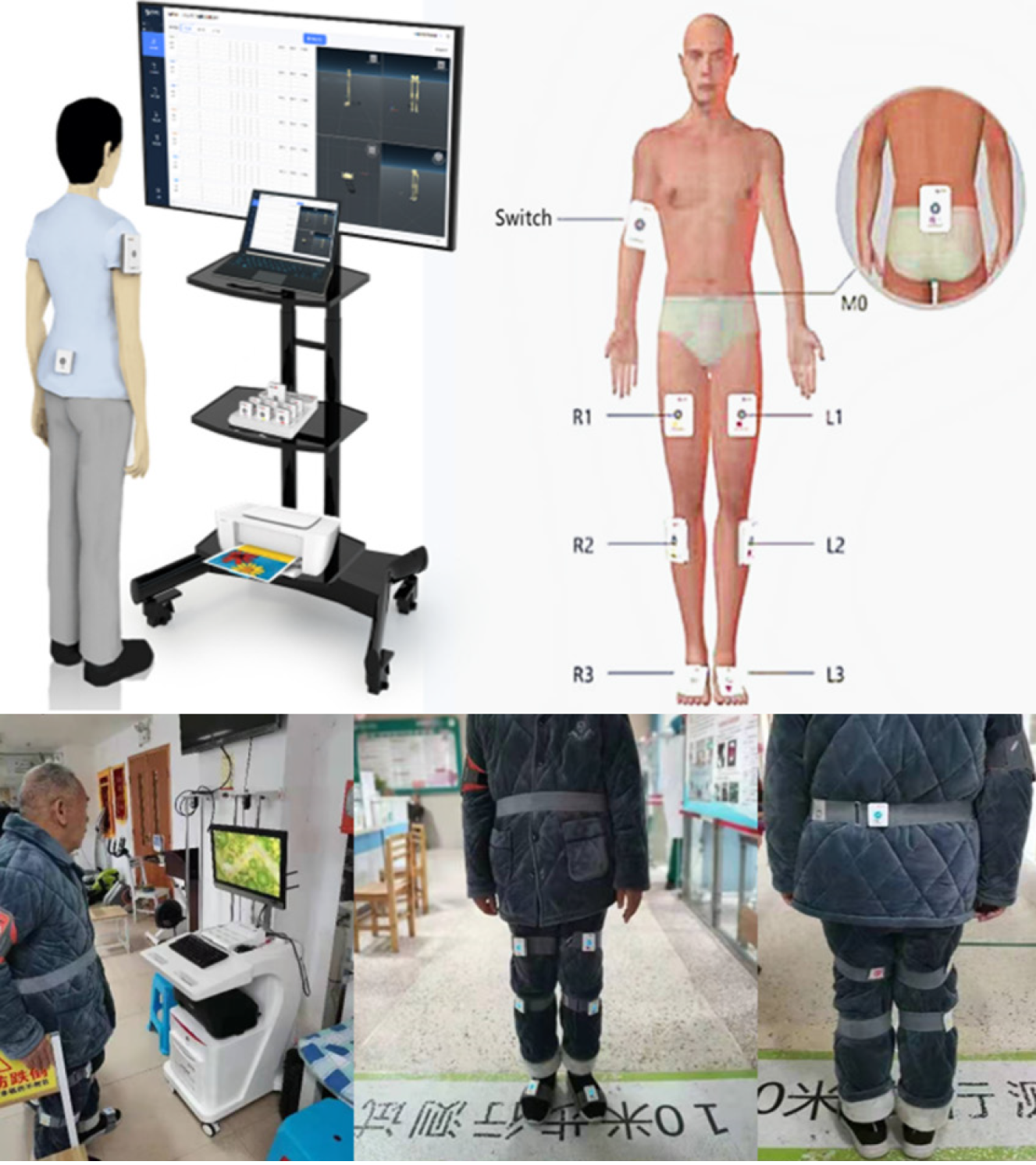
Intervention group (A3 group). The intervention group received 30 min of routine lower extremity training and robot-assisted gait assessment and training system A3 (RAGATS-A3, NX, Shanghai, China) supplemented with conventional rehabilitation training. RAGATS-A3 is a rehabilitation robot device, as presented in Figure 1, which assists patients with lower limb dysfunction to conduct gait correction and motor relearning training. It includes exoskeleton mechanical legs, dynamic and static weight reduction system, buffer runway, and situational feedback display screen, etc. Perhaps due to its high price, few clinical trials have investigated the robot's short-term effectiveness on lower limb gait in patients with subacute stroke. Based on the principle of neural plasticity, walking function can be restored through continuous training. The robot consists of an exoskeleton mechanical leg, running table, weight loss system, situational feedback game, and gait analysis system. The operator can monitor the patient's movement in real-time through sensors mounted on the hip, knee, and ankle, thus accurately controlling the range and walking speed of the hip, knee, and ankle joints. Combined with virtual reality technology and dynamic or static weight loss systems, the robot can provide high-intensity, repea
Control group. Patients in the control group received ground walking training under the supervision or with little assistance from the therapist. The primary role of the therapist is to prevent the patient from falling and to give verbal instructions when necessary. Each treatment lasted for 30 min, once a day, five times/wk.
In addition, prior to each intervention in the clinical trial, all patients will receive 30 min of conventional physical training based on traditional neurodevelopmental techniques including sitting and standing balance training, functional transfer training, affected limb weight training, foot following training, stride training, and dynamic balance training. This training aims to improve the patient's gait, posture, and stability during walking, correct the muscular lines of the body during walking, and assist the patient to re-establish a normal walking pattern. Conventional physical training for all patients was performed by the same experienced senior rehabilitation therapist who did not participate in the entire assessment process. Each treatment lasted for 30 min, once a day, 5 times/wk. Briefly, patients in the A3 group received 30 min of conventional physical training and 30 min of A3 training, whereas the control group received 30 min of conventional physical training and 30 min of conventional ground walking. All participants received a 60-min training intervention per day for 2 wk (5 times/wk, a total of 10 times). In addition, other treatments, including medication, were the same for two groups of patients. We chose a 2-wk treatment period because Chinese and local health insurance policies stipulate that stroke patients can be hospitalized for up to 2 wk when using health insurance to reimburse hospitalization costs. Without health insurance coverage, the burden of healthcare for Chinese residents would be very heavy. This also poses a huge challenge for us as healthcare professionals, and we hope to find ways to maximize the benefits for our patients in the short term.
The primary results were the three-dimensional gait spatiotemporal parameters monitored by the machine (Gait Watch; Zhang He Zhi Neng, Guangzhou, China) (Figure 2) in real time. Specific operation (additional information regarding the manufacturer's website: http://www.ezhanghe.com/) was as follows. The motion sensors were fixed on each joint of the patient's lower limbs (Figure 2), telling the patient to walk 12 m, in the presence of medical staff and family members (no contact with the patient, let the patient walk independently) to and from two times. The machine sensors evaluate and record detailed temporal and spatial parameters of the gait, dynamically observe the patient's gait, and provide a comprehensive picture of the progress of each joint movement during walking. Spatial parameters of the gait include stride frequency, stride length, stride speed, and other basic parameters.
Secondary assessment results were balance parameters (balancing apparatus: Union Rehab, Balance test training system: PC708; Beijing, China) (Figure 3), The balance parameters we selected included gravity center moving track length (Lng), gravity center moving track area (Area), and track length per unit area (TL index)[20], which were automatically generated by the balancing instrument. First, subjects were required to stand on the gravity-sensitive platform of the machine in a standard posture, look directly at the screen in front of them, and relax their hands. Two tests lasting 30 s were carried out: one with eyes open and one with eyes closed. During this process, the machine automatically measured and calculated the value of the balance parameter. Each patient was performed twice and the average score was recorded (Figure 3)[21]. TL index is the value obtained by dividing the total trajectory length of the center of gravity movement by the area of the trajectory of the center of gravity movement within a given time. This value is inversely proportional to the area and directly proportional to the Lng, which reflects the balance stability of the body and the adjustment ability of posture balance. The larger the value, the stronger the adjustment ability[20,22].
In addition, Fugl-Meyer-Assessment (FMA) scores (including aspects of balance and lower extremity) and RMI[23], Time Up and Go test (TUG)[24], and basic activities of daily living (BADL)[25], FM balance meter, Stroke-Specific Quality of Life Scale (SSQOL)[26], and Holden Walking Ability Scale(HWAS)[21] values were collected for recording. Adverse events are also recorded. The patients were evaluated at baseline and after 2 wk of treatment by a specialist evaluator who did not know the exact allocation, and each assessment was carried out more than twice and then averaged.
The data were analyzed using SPSS Statistics version 26.0. The assumption of normality of data was assessed using the Kolmogorov-Smirnov test. The χ2 test was used to test the baseline data of the two groups, such as sex, stroke type, and other categorical data. Data are expressed as the mean ± standard deviation; otherwise, the median and interquartile range was used. The independent t-test was used for normally distributed data, and the Mann-Whitney U test was used for non-normally distributed data. Paired t-test was used for statistical analysis of the changes before and after treatment in the same group. P < 0.05 was considered statistically significant.
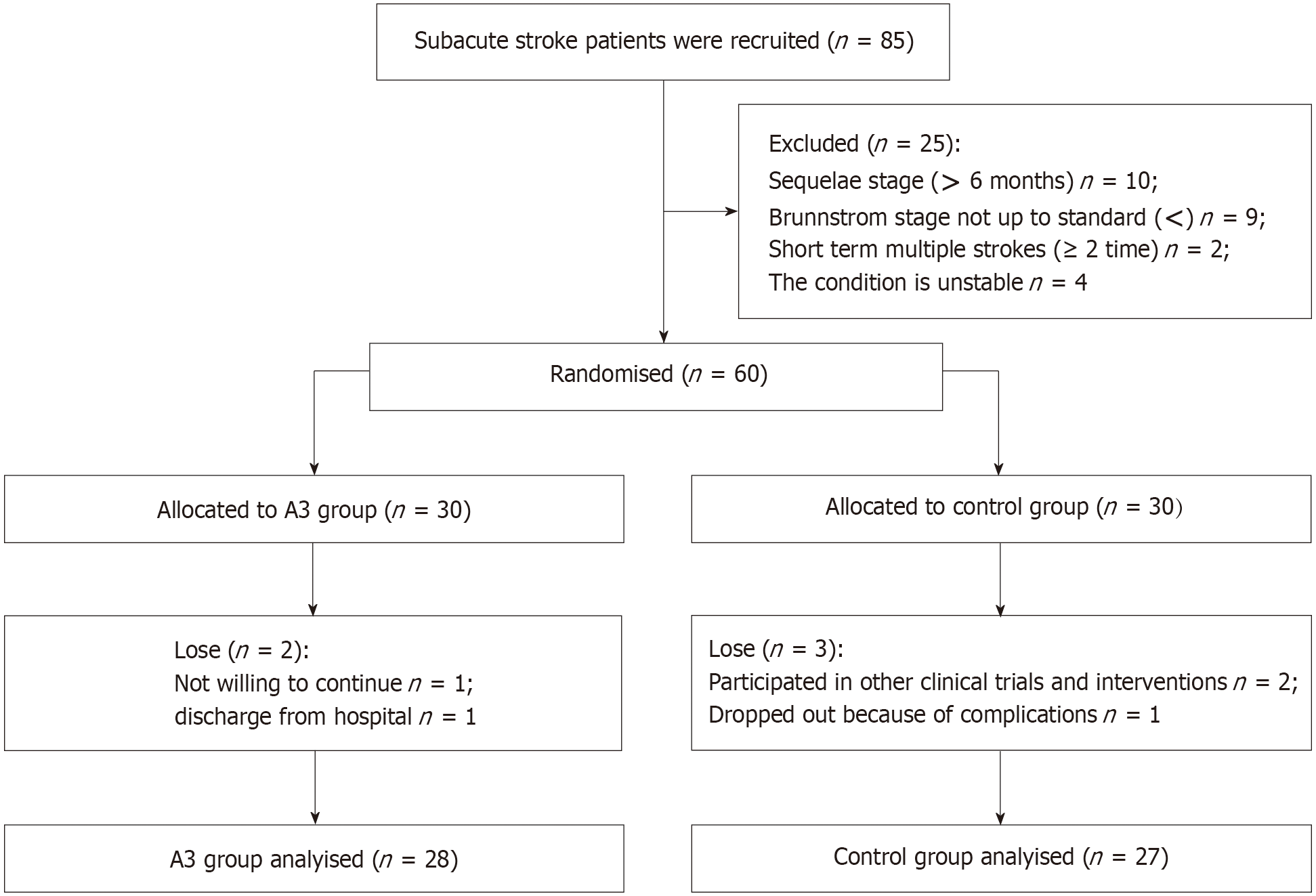
A total of 60 stroke patients who met the inclusion criteria volunteered to participate in the study between November 2021 and November 2022. They were randomly assigned to either the control group or the intervention group (A3 group). The Control group (n = 30) received conventional rehabilitation training while the A3 group (n = 30) received a combination of robot-assisted training and conventional rehabilitation therapy. Five fell off, and fifty-five were eventually included in the analysis. The screening and allocation process is shown in Figure 4.
The A3 group included 28 patients and the control group included 27 patients. There were no significant differences in sex ratio, stroke type, the proportion of hypertension and diabetes mellitus, average age, and course of disease between the two groups (P > 0.05), indicating that the two groups were comparable. The baseline data of patients are shown in Table 1.
| Parameter | A3 group | Control group | P value | |
| Sex | Male | 22 | 22 | 0.787 |
| Female | 6 | 5 | ||
| Age in yr | 52.14 ± 15.352 | 58.48 ± 17.194 | 0.155 | |
| Height in cm | 163.21 ± 4.756 | 165.52 ± 7.361 | 0.177 | |
| Weight in kg | 63.21 ± 7.733 | 67.48 ± 9.928 | 0.080 | |
| Type of stroke | Infarction | 17 | 17 | 0.864 |
| Hemorrhage | 11 | 10 | ||
| Paretic side | Right | 12 | 14 | 0.504 |
| Left | 16 | 13 | ||
| Hypertension | Yes | 20 | 21 | 0.589 |
| No | 8 | 6 | ||
| Diabetes | Yes | 5 | 6 | 0.686 |
| No | 23 | 21 | ||
| Mean time since stroke in d | 49.71 ± 36.054 | 43.63 ± 42.164 | 0.567 | |
| MMSE | 27.50 ± 4.308 | 27.19 ± 2.354 | 0.739 | |
| BADL | 67.14 ± 15.836 | 71.67 ± 10.561 | 0.220 | |
| Brunnstrom stage | 3.68 ± 0.863 | 3.89 ± 0.801 | 0.353 | |
| SSQOL | 156.36 ± 23.429 | 154.67 ± 17.236 | 0.762 | |
| FM balance meter | 8.54 ± 0.999 | 8.52 ± 0.700 | 0.942 | |
| FMA of lower limbs | 20.07 ± 5.643 | 21.22 ± 5.820 | 0.460 | |
| RMI | 8.75 ± 1.936 | 8.44 ± 1.281 | 0.495 | |
| HWAS | 2.86 ± 0.756 | 2.81 ± 0.736 | 0.834 | |
| TUG in s | 32.404 ± 14.726 | 29.643 ± 12.348 | 0.455 | |
| Stride frequency as step/min | 76.018 ± 19.978 | 86.093 ± 17.100 | 0.050 | |
| Stride speed in cm/s | 45.643 ± 19.187 | 53.389 ± 23.009 | 0.180 | |
| Stride length in cm | 70.054 ± 18.921 | 72.426 ± 24.722 | 0.690 | |
| Lng | 1360.61 ± 774.611 | 1463.70 ± 1061.382 | 0.682 | |
| Area | 1889.64 ± 1334.954 | 2165.63 ± 1474.666 | 0.470 | |
| TL index | 0.901 ± 0.361 | 0.941 ± 0883 | 0.828 |
The situation of patients in both groups after 2 wk of intervention can be seen in Table 2. The scores of BADL, SSQOL, FM balance meter, FMA, RMI, Stride speed, Stride length, and TUG in the two groups were significantly better than before treatment (P < 0.05). Although the HWAS, Stride frequency, Lng, Area, and TL index scores of the control group after 2 wk were better than that of the 2 wk before, there was no statistically significant difference (P > 0.05). By contrast, the above indexes of A3 group were significantly better after 2 wk than before 2 wk (P < 0.05).
| Outcome | A3 group | Control group | ||
| Baseline | 2 wk | Baseline | 2 wk | |
| BADL | 67.14 ± 15.836 | 86.43 ± 10.874a | 71.67 ± 10.561 | 75.19 ± 9.556a |
| SSQOL | 156.36 ± 23.429 | 178.93 ± 23.650a | 154.67 ± 17.236 | 158.74 ± 17.295a |
| FM balance meter | 8.54 ± 0.999 | 9.75 ± 1.456a | 8.52 ± 0.700 | 8.70 ± 0.542a |
| FMA (lower limbs) | 20.07 ± 5.643 | 23.57 ± 5.473a | 21.22 ± 5.820 | 22.19 ± 6.032a |
| RMI | 8.75 ± 1.936 | 10.82 ± 1.679a | 8.44 ± 1.281 | 8.85 ± 1.460a |
| HWAS | 2.86 ± 0.756 | 3.75 ± 0.585a | 2.81 ± 0.736 | 2.93 ± 0.675b |
| TUG(s) | 32.404 ± 14.726 | 20.029 ± 7.228a | 29.643 ± 12.348 | 26.842 ± 9.600a |
| Stride frequency as step/min | 76.018 ± 19.978 | 94.857 ± 17.678a | 86.093 ± 17.100 | 89.889 ± 13.480b |
| Stride speed in cm/s | 45.643 ± 19.187 | 90.768 ± 77.937a | 53.389 ± 23.009 | 61.796 ± 21.825a |
| Stride length in cm | 70.054 ± 18.921 | 99.500 ± 26.606a | 72.426 ± 24.722 | 81.111 ± 22.263a |
| Lng | 1360.61 ± 774.611 | 925.36 ± 408.597a | 1463.70 ± 1061.382 | 1397.04 ± 948.131b |
| Area | 1889.64 ± 1334.954 | 980.96 ± 728.462a | 2165.63 ± 1474.666 | 1755.59 ± 987.181b |
| TL index | 0.901 ± 0.361 | 1.352 ± 0.665a | 0.941 ± 0883 | 0.952 ± 0.499b |
We used the change from baseline to 2 wk later as the effect size to objectively compare the efficacy of the two groups, which could overcome the inconsistencies in the baseline and be more objective and accurate than the direct comparison of efficacy results after treatment. The results of the efficacy comparison between the A3 and control groups are shown in Table 3.
| Change | A3 group | Control group | P value |
| BADL | 19.286 ± 12.150 | 3.518 ± 4.344 | < 0.01 |
| SSQOL | 22.571 ± 7.988 | 4.074 ± 2.510 | < 0.01 |
| FM balance meter | 1.214 ± 0.832 | 0.185 ± 0.396 | < 0.01 |
| FMA | 3.500 ± 3.805 | 0.963 ± 2.084 | < 0.01 |
| RMI | 2.071 ± 1.215 | 0.407 ± 0.572 | < 0.01 |
| HWAS | 0.893 ± 0.629 | 0.111 ± 0.320 | < 0.01 |
| TUG | 12.376 ± 8.997 | 2.801 ± 3.430 | < 0.01 |
| Stride frequency | 18.839 ± 11.236 | 3.796 ± 10.829 | < 0.01 |
| Stride speed | 45.125 ± 69.409 | 8.407 ± 10.202 | 0.009 |
| Stride length | 29.446 ± 16.624 | 8.685 ± 10.736 | < 0.01 |
| Lng | 435.250 ± 570.537 | 66.666 ± 517.694 | 0.015 |
| Area | 908.678 ± 972.848 | 410.037 ± 1186.491 | 0.094 |
| TL index | 0.451 ± 0.543 | 0.012 ± 0.732 | 0.014 |
We could find that adding the A3 robot intervention to the control group significantly improved patients' BADL, SSQOL, FM balance meter, FMA, RMI, HWAS, and TUG scores (P < 0.01), and the A3 rehabilitation robot can significantly improve the temporal and spatial parameters of patients in terms of gait frequency, stride length, and gait speed (P < 0.01). In terms of balance, the A3 rehabilitation robot group showed significantly higher improvement in Lng and TL indices than the control group (P < 0.05); however, there was no significant difference in improvement in Area (P = 0.094).
In this study, there were no serious adverse events in either group and no adverse effects in the control group. In the A3 group, 2 patients reported crotch-pulling pain during weight loss, which was reduced or disappeared after appropriate adjustment of the weight loss index and adjustment of wear, and all discomfort disappeared completely within 1 h after completion of the A3 robot.
The purpose of this randomized controlled trial was to compare the short-term therapeutic effects of the A3 gait robot combined with conventional walking training on the improvement of motor function and balance coordination in stroke patients; thus, providing medical evidence for the clinical value of this robot. Our results showed that the combined treatment improved walking function more significantly than traditional gait training in the short term. Notably, the control group showed a numerical but not statistically significant improvement in balance parameters, HWAS, and Stride frequency after 2 wk. After team discussion and analysis, we agreed that this may be related to the small sample size and the short duration of the intervention, after all, 2 wk of regular walking training showed limited improvement in patients in Brunnstrom stage III, because we included patients who were able to stand and had a walking base. At least for the indicator of stride frequency, our results are consistent with those of Yu et al[19] in that 2 wk of training did not change the patients' stride frequency, which could reasonably explain the clinical benefit but no statistical difference. When we used the amount of change before and after the intervention as an effect size to compare the efficacy of the two groups, all results (including quality of life, walking ability, gait parameters, motor balance function, etc., except Area, showed greater improvement in the A3 group than in the control group. This also suggests that a 2-wk robotic intervention can accelerate the improvement of gait and balance coordination function in the lower extremities of patients with subacute stroke. Understood from another perspective, we believe that perhaps the duration of the robotic intervention is recommended to be 2 wk or longer to see a significant improvement, because in the present trial, even with 2 wk of robotic intervention, there was still no significant difference in one of our 13 outcome indicators.
We chose to use this A3 robot from China Yikang because the product has its own characteristics. Compared to the Walkbot and Lokomat robots that are widely used today, the A3 system optimizes the training program and gait data by incorporating the normal gait curves of the Chinese population. Compared to the two foreign systems, the system analyzes results that are more suitable for the exercise patterns of the experimental population, reduces the error of the experimental results, and ensures that the patients establish the correct exercise patterns. In addition, the lower limb exoskeleton size of the A3 robot, which has been applied and improved for more than 10 years, conforms to the physical characteristics of Chinese people, and the changes in the results before and after the experiment can effectively reflect the treatment efficacy. The adjustable hip and knee offset range in the A3 system is -10°-10 degrees, with 20 adjustable steps, which can gradually correct abnormal movement patterns during training. Not only that, the A3 robot also has a spasm detection and stopping protection function, which can satisfy all stages of walking rehabilitation in the early stage of stroke patients' rehabilitation treatment with the dynamic and static weight reduction function. Adjustable active and passive training guiding force adapts to different training progress of patients, providing challenging training and stimulating neural remodeling. And the rich gaming experience of A3 with no less than six game scenes and visual input feedback help activate new neural circuits and realize neural remodeling. Considering these reasons, we believe it is necessary to conduct this study to validate the efficacy of the A3 robot.
Our innovation is to observe whether a short-term A3 robotic intervention is effective in improving lower extremity walking and balance in patients with subacute stroke, in addition to using a combination of objective instrumental measurements and subjective scales, which is more objective and reliable. Several previous studies[27,28] have reported the effects of lower extremity robotics compared to conventional walking training in patients with subacute stroke, but more attention has been paid to the long-term effects between 1 and 2 mo or 6 mo later[29]. Considering the limited resources and time cost, it is difficult for many patients to complete the entire course of treatment, thus affecting the judgment of the therapeutic effect of this product. Therefore, our study focused on its short-term benefits. Compared with previous similar studies we developed more stringent screening inclusion criteria, focusing on patients with certain walking potential within 6 mo after the onset of disease, to most intuitively measure and compare patients' function before and after the intervention. Furthermore, the total length and total area of the walking trajectory of patients were also included in the comparison and analysis of outcome indicators, reflecting patients' dynamic balance and walking stability. Barthel Index and SSQOL scores were also analyzed and compared to more fully assess patients' functional walking ability in daily life rather than just the immediate improvement after training.
There is some basis for the hypothesis that lower extremity robots promote mechanisms of walking and balance. By altering mitochondrial dynamics, exercise training improves skeletal muscle oxidative capacity[30]. The exoskeleton part of the A3 robot wraps around the patient's lower limbs to drive walking, which enhances the patient's proprioception and leads to an easier somatosensory sensation, which is also one of the reasons for the improved balance function[27]. The A3 robot's weight reduction system allows for lateral weight shifting to reduce the weight on the affected side to help the patient develop a symmetrical gait pattern[31,32]. Some studies suggest robot improves stability by altering muscle coordination patterns, partial weight-bearing gait training results in changes in the average burst amplitude of the gastrocnemius and tibialis anterior muscles[33], with changes in the amount of body weight support and control of stride frequency, there is greater activation of the gastrocnemius and less activation of the tibialis anterior, and this change in muscle coordination patterns will provide better stability[34]. There is also support for gait robots to increase the firing rate of motor neurons without altering muscle strength[35]. In addition, the A3 robot's visual feedback and dystonia sensing system will have a beneficial effect on motor control in stroke patients. According to the view of Lam et al[36] scholars, the process of stroke patients restoring walking ability through robot-assisted gait training is an adaptive change. The improvement in the A3 group was the combined effect of multiple interventions including an exoskeleton robot, weight loss training, and visual feedback.
This study also had some limitations. The sample size was small and not representative of the training effect in the majority of stroke patients,. Second, due to the short observation period, we cannot know whether the 2-wk training effect is sustainable. In addition, we did not perform kinetic or electromyographic (EMG) data, nor did we have brain imaging such as electroencephalogram (EEG), near-infrared (NIR) imaging, or MRI, which may help to determine the mechanism of action of the lower limb robot. Third, the balance parameters and gait time parameters we used are probably not comprehensive enough; however, the available assessment tools are not precise enough for certain metrics (e.g., hip, knee, and ankle mobility, which varies partially from patient to patient due to patient size and sensor positioning), and we have chosen the most precise metrics possible.
For prospects, we venture to speculate, based on the results of this study, that patients with subacute phase stroke will require at least 2 wk or more of lower extremity robotic intervention over 6 mo to achieve significant gait and balance improvements, which will need to be verified by further studies. In addition, future research is proposed to combine brain-computer interfaces, EEG, EMG, NIR, and MRI imaging to explore the mechanisms of robotics to improve gait and balance.
The 2-wk A3 robotic intervention combined with conventional lower extremity training significantly improved gait and balance in subacute stroke patients and was more effective than conventional ground walking. In addition, the efficacy of the lower extremity robot may take 2 wk or more to become apparent.
The authors would like to thank the participants for their participation in this trial.
| 1. | Kang HJ, Lee EH, Kim JW, Kim SW, Shin IS, Kim JT, Park MS, Cho KH, Han JS, Lyoo IK, Kim JM. Association of SLC6A4 methylation with long-term outcomes after stroke: focus on the interaction with suicidal ideation. Sci Rep. 2021;11:2710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 2. | Tamburella F, Moreno JC, Herrera Valenzuela DS, Pisotta I, Iosa M, Cincotti F, Mattia D, Pons JL, Molinari M. Influences of the biofeedback content on robotic post-stroke gait rehabilitation: electromyographic vs joint torque biofeedback. J Neuroeng Rehabil. 2019;16:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Arienti C, Lazzarini SG, Pollock A, Negrini S. Rehabilitation interventions for improving balance following stroke: An overview of systematic reviews. PLoS One. 2019;14:e0219781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | Mao YR, Lo WL, Lin Q, Li L, Xiao X, Raghavan P, Huang DF. The Effect of Body Weight Support Treadmill Training on Gait Recovery, Proximal Lower Limb Motor Pattern, and Balance in Patients with Subacute Stroke. Biomed Res Int. 2015;2015:175719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Park SJ. The immediate effects of proprioceptive neuromuscular facilitation with taping on gait parameters in patients with chronic stroke. J Phys Ther Sci. 2017;29:2018-2021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Hsiao H, Knarr BA, Higginson JS, Binder-Macleod SA. The relative contribution of ankle moment and trailing limb angle to propulsive force during gait. Hum Mov Sci. 2015;39:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Washabaugh EP, Krishnan C. A wearable resistive robot facilitates locomotor adaptations during gait. Restor Neurol Neurosci. 2018;36:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Kim CY, Lee JS, Kim HD. Comparison of the Effect of Lateral and Backward Walking Training on Walking Function in Patients with Poststroke Hemiplegia: A Pilot Randomized Controlled Trial. Am J Phys Med Rehabil. 2017;96:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Lee JH, Park JH. Development of an item list to assess bilateral upper extremity function of stroke patients with hemiplegia. NeuroRehabilitation. 2018;42:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Morone G, Bragoni M, Iosa M, De Angelis D, Venturiero V, Coiro P, Pratesi L, Paolucci S. Who may benefit from robotic-assisted gait training? A randomized clinical trial in patients with subacute stroke. Neurorehabil Neural Repair. 2011;25:636-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Peurala SH, Airaksinen O, Huuskonen P, Jäkälä P, Juhakoski M, Sandell K, Tarkka IM, Sivenius J. Effects of intensive therapy using gait trainer or floor walking exercises early after stroke. J Rehabil Med. 2009;41:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Kim J, Kim DY, Chun MH, Kim SW, Jeon HR, Hwang CH, Choi JK, Bae S. Effects of robot-(Morning Walk(®)) assisted gait training for patients after stroke: a randomized controlled trial. Clin Rehabil. 2019;33:516-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Werner C, Von Frankenberg S, Treig T, Konrad M, Hesse S. Treadmill training with partial body weight support and an electromechanical gait trainer for restoration of gait in subacute stroke patients: a randomized crossover study. Stroke. 2002;33:2895-2901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 204] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Hidler J, Nichols D, Pelliccio M, Brady K, Campbell DD, Kahn JH, Hornby TG. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair. 2009;23:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 346] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 15. | Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, Dobkin BH, Rose DK, Tilson JK, Cen S, Hayden SK; LEAPS Investigative Team. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364:2026-2036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 515] [Cited by in RCA: 441] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 16. | Tanikawa H, Ohtsuka K, Mukaino M, Inagaki K, Matsuda F, Teranishi T, Kanada Y, Kagaya H, Saitoh E. Quantitative assessment of retropulsion of the hip, excessive hip external rotation, and excessive lateral shift of the trunk over the unaffected side in hemiplegia using three-dimensional treadmill gait analysis. Top Stroke Rehabil. 2016;23:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Mayr A, Quirbach E, Picelli A, Kofler M, Smania N, Saltuari L. Early robot-assisted gait retraining in non-ambulatory patients with stroke: a single blind randomized controlled trial. Eur J Phys Rehabil Med. 2018;54:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Mehrholz J, Thomas S, Werner C, Kugler J, Pohl M, Elsner B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev. 2017;5:CD006185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 19. | Yu D, Yang Z, Lei L, Chaoming N, Ming W. Robot-Assisted Gait Training Plan for Patients in Poststroke Recovery Period: A Single Blind Randomized Controlled Trial. Biomed Res Int. 2021;2021:5820304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Fabbri M, Martoni M, Esposito MJ, Brighetti G, Natale V. Postural control after a night without sleep. Neuropsychologia. 2006;44:2520-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Zhang M, You H, Zhang H, Zhao W, Han T, Liu J, Jiang S, Feng X. Effects of visual feedback balance training with the Pro-kin system on walking and self-care abilities in stroke patients. Medicine (Baltimore). 2020;99:e22425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Bougard C, Lepelley MC, Davenne D. The influences of time-of-day and sleep deprivation on postural control. Exp Brain Res. 2011;209:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. Int Disabil Stud. 1991;13:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 471] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 24. | Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8741] [Cited by in RCA: 9294] [Article Influence: 273.4] [Reference Citation Analysis (0)] |
| 25. | Qian J, Ren X. Association between comorbid conditions and BADL/IADL disability in hypertension patients over age 45: Based on the China health and retirement longitudinal study (CHARLS). Medicine (Baltimore). 2016;95:e4536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Alotaibi SM, Alotaibi HM, Alolyani AM, Abu Dali FA, Alshammari AK, Alhwiesh AA, Gari DM, Khuda IKMQ, Vallabadoss CA. Assessment of the stroke-specific quality-of-life scale in KFHU, Khobar: A prospective cross-sectional study. Neurosciences (Riyadh). 2021;26:171-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Kim HY, Shin JH, Yang SP, Shin MA, Lee SH. Robot-assisted gait training for balance and lower extremity function in patients with infratentorial stroke: a single-blinded randomized controlled trial. J Neuroeng Rehabil. 2019;16:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Li DX, Zha FB, Long JJ, Liu F, Cao J, Wang YL. Effect of Robot Assisted Gait Training on Motor and Walking Function in Patients with Subacute Stroke: A Random Controlled Study. J Stroke Cerebrovasc Dis. 2021;30:105807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | van Nunen MP, Gerrits KH, Konijnenbelt M, Janssen TW, de Haan A. Recovery of walking ability using a robotic device in subacute stroke patients: a randomized controlled study. Disabil Rehabil Assist Technol. 2015;10:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Bell MB, Bush Z, McGinnis GR, Rowe GC. Adult skeletal muscle deletion of Mitofusin 1 and 2 impedes exercise performance and training capacity. J Appl Physiol (1985). 2019;126:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Banala SK, Kim SH, Agrawal SK, Scholz JP. Robot assisted gait training with active leg exoskeleton (ALEX). IEEE Trans Neural Syst Rehabil Eng. 2009;17:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 32. | Robinovitch SN, Feldman F, Yang Y, Schonnop R, Leung PM, Sarraf T, Sims-Gould J, Loughin M. Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study. Lancet. 2013;381:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 576] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 33. | Finch L, Barbeau H, Arsenault B. Influence of body weight support on normal human gait: development of a gait retraining strategy. Phys Ther. 1991;71:842-55; discussion 855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 178] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Klarner T, Chan HK, Wakeling JM, Lam T. Patterns of muscle coordination vary with stride frequency during weight assisted treadmill walking. Gait Posture. 2010;31:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Chisari C, Bertolucci F, Monaco V, Venturi M, Simonella C, Micera S, Rossi B. Robot-assisted gait training improves motor performances and modifies Motor Unit firing in poststroke patients. Eur J Phys Rehabil Med. 2015;51:59-69. [PubMed] |
| 36. | Lam T, Anderschitz M, Dietz V. Contribution of feedback and feedforward strategies to locomotor adaptations. J Neurophysiol. 2006;95:766-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |