Published online Aug 26, 2024. doi: 10.12998/wjcc.v12.i24.5468
Revised: April 20, 2024
Accepted: May 17, 2024
Published online: August 26, 2024
Processing time: 115 Days and 8.4 Hours
The management of refractory ulcerative colitis (UC) and acute severe UC (ASUC) is challenging due to the lack of standardized approaches in cases resistant to multiple treatments. In this editorial, I investigate the efficacy and safety of Janus kinase inhibitors, particularly upadacitinib and tofacitinib, in controlling severe and refractory disease. I highlight a notable case report by Xu et al, which explores the case of a patient with primary nonresponse to two classes of biologics and two fecal microbiota transplants who exhibited a remarkable response to upadacitinib. Furthermore, I discuss the use of tofacitinib in refractory UC and ASUC, either as monotherapy or in combination with biologics, which has shown promising res
Core Tip: This editorial explores the efficacy and safety of Janus kinase inhibitors, specifically upadacitinib and tofacitinib, in refractory ulcerative colitis (UC) and acute severe UC (ASUC). Highlighting a compelling case report, it underscores the potential of these small-molecule therapies, either alone or in combination with biologics, to achieve and maintain disease remission. Furthermore, it emphasizes the importance of considering overlapping infections in ASUC and the need for pro
- Citation: Soldera J. Navigating treatment resistance: Janus kinase inhibitors for ulcerative colitis. World J Clin Cases 2024; 12(24): 5468-5472
- URL: https://www.wjgnet.com/2307-8960/full/v12/i24/5468.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i24.5468
Managing ulcerative colitis (UC), especially cases of refractory acute severe UC (ASUC), poses significant challenges due to poor patient response or intolerance to conventional and biological treatments. There is no standardized approach for cases resistant to multiple treatments. However, recent research has shed light on the efficacy and safety of small-mo
A report by Xu et al[3] describes the case of a patient who showed primary nonresponse to two biologics and two fecal microbiota transplants (FMT) but exhibited a remarkable response to upadacitinib[3]. It should be noted that FMT for ASUC is considered an off-label indication. The available evidence supporting its use remains primarily anecdotal, and its application should be restricted to the treatment of Clostridioides difficile (C. difficile) infection, or to clinical trials, according to the American Gastroenterology Association[4,5].
Despite limited evidence, both upadacitinib and tofacitinib have shown promise in the management of refractory UC, either as monotherapy or in combination with biologics as rescue therapy. At our center, we have extensive experience in employing off-label combined therapy for ASUC. I am particularly inclined to share a specific case that I believe would enrich the ongoing discussion initiated by Xu et al[3], presenting an alternative approach.
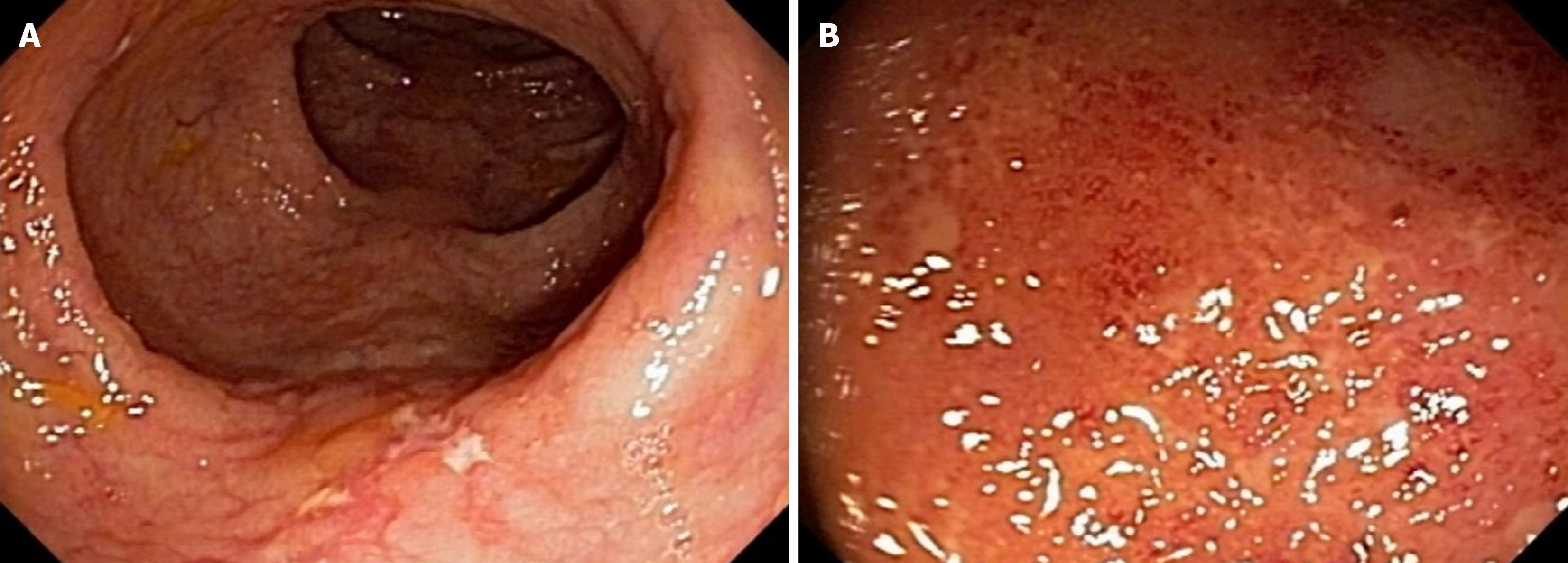
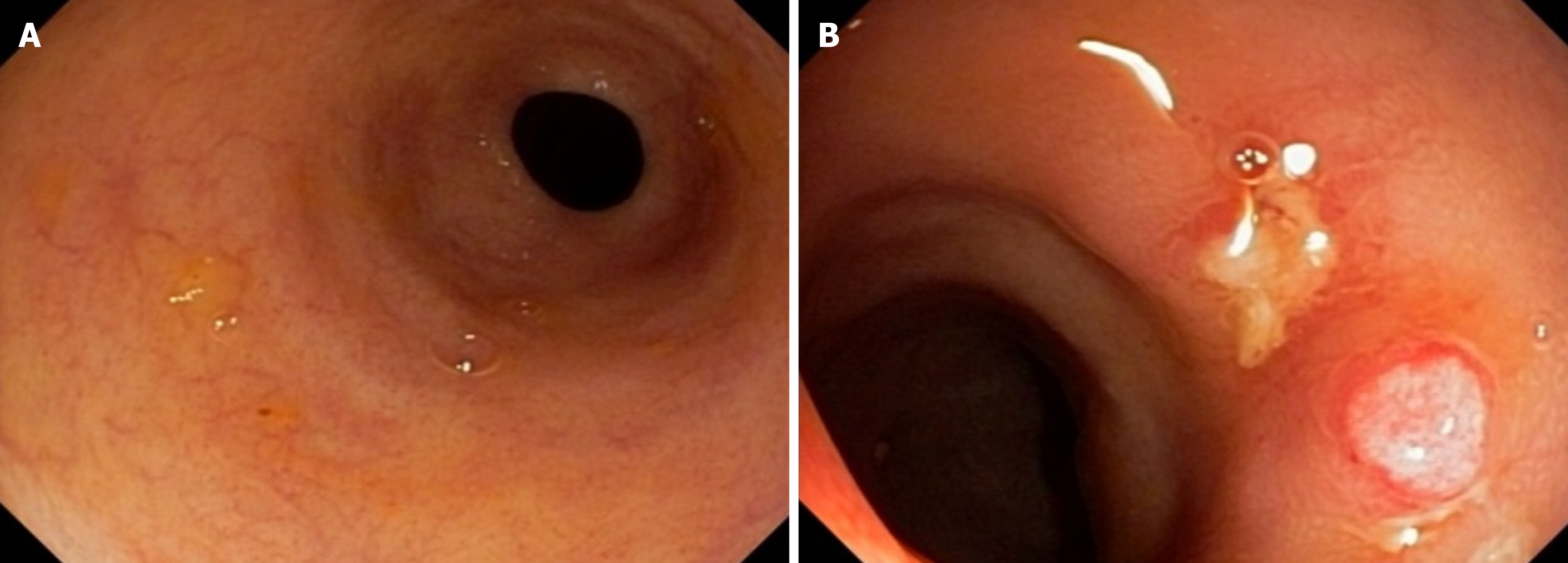
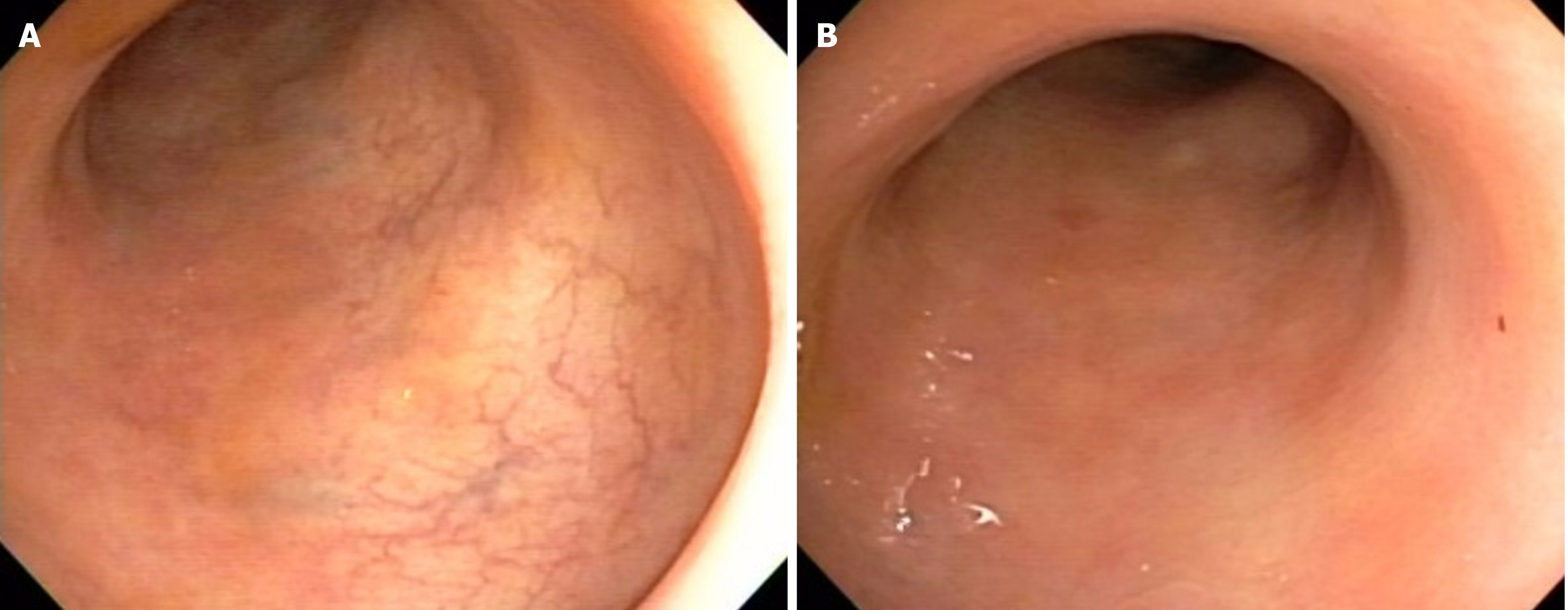
A 57-year-old male patient with a decade-long history of UC presented with recurrent symptomatic episodes despite several treatments. Initially responsive to mesalazine 4 g daily and a course of prednisone, the patient relapsed after a few years. Subsequent treatment with azathioprine 150 mg daily and mesalazine 4 g daily controlled the disease for another few years. However, his symptoms recurred, requiring the use of oral enteric budesonide 9 mg daily. Despite these in
Tests for C. difficile toxins A and B and immunohistochemistry for cytomegalovirus were negative. Colonoscopy re
Tofacitinib 5 mg twice daily was introduced as adjunctive therapy alongside infliximab, replacing azathioprine, with the aim of achieving disease remission, as suggested in the case series by Gilmore et al[6]. Over the subsequent 3 months, the patient’s symptoms gradually improved, with a reduction in stool frequency and resolution of fecal incontinence and bleeding. Treatment with tofacitinib was then interrupted, and the patient was maintained on infliximab 5 mg/kg/dose infusions every 6 wk, adjusted to infliximab serum levels, and azathioprine 100 mg daily. He has remained in deep re
There are two crucial points I believe warrant discussion. First, the possibility of overlapping infections in ASUC (i.e., C. difficile and cytomegalovirus) was ruled out before escalation of therapy[7-9]. Second, colorectal surgeons must be aware that the clinical condition of patients with refractory UC or ASUC can rapidly deteriorate, requiring urgent co
There is increasing evidence supporting the utilization of tofacitinib in cases of infliximab-refractory ASUC[12]. A previous randomized controlled trial reported a response rate as high as 83% in ASUC patients refractory to corticosteroids after 7 d of tofacitinib treatment[13]. Similarly, a retrospective study demonstrated a response rate of 87.5%[14]. Moreover, a noteworthy case series administered a higher dose of tofacitinib to eleven ASUC patients, achieving disease control in nine of them. Subsequently, these patients were transitioned to infliximab for maintenance therapy[15].
Tofacitinib has been investigated in combination with other biologics for the management of ASUC[16], including its use as replacement therapy for azathioprine alongside infliximab, demonstrating both efficacy and safety[6]. Of note, a systematic review revealed that the use of tofacitinib in ASUC led to a pooled 90-day and 6-month colectomy-free rate of 79.9%[17].
Although the use of tofacitinib as monotherapy in this setting has been extensively researched, some reports have also described the efficacy of upadacitinib in ASUC. For instance, a case report documented a positive response to upada
In conclusion, both cases underscore the complexity of managing refractory ASUC and highlight the potential synergistic effect of employing small molecule therapies either in combination or sequentially with biologics to achieve and sustain disease remission. Further studies are warranted to elucidate the optimal treatment strategies for patients who have com
| 1. | Danese S, Vermeire S, Zhou W, Pangan AL, Siffledeen J, Greenbloom S, Hébuterne X, D'Haens G, Nakase H, Panés J, Higgins PDR, Juillerat P, Lindsay JO, Loftus EV Jr, Sandborn WJ, Reinisch W, Chen MH, Sanchez Gonzalez Y, Huang B, Xie W, Liu J, Weinreich MA, Panaccione R. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399:2113-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 331] [Article Influence: 110.3] [Reference Citation Analysis (0)] |
| 2. | Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, Danese S, Feagan BG, Reinisch W, Niezychowski W, Friedman G, Lawendy N, Yu D, Woodworth D, Mukherjee A, Zhang H, Healey P, Panés J; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2017;376:1723-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1189] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 3. | Xu X, Jiang JW, Lu BY, Li XX. Upadacitinib for refractory ulcerative colitis with primary nonresponse to infliximab and vedolizumab: A case report. World J Clin Cases. 2024;12:1685-1690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Costello SP, Day A, Yao CK, Bryant RV. Faecal microbiota transplantation (FMT) with dietary therapy for acute severe ulcerative colitis. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Peery AF, Kelly CR, Kao D, Vaughn BP, Lebwohl B, Singh S, Imdad A, Altayar O; AGA Clinical Guidelines Committee. Electronic address: clinicalpractice@gastro.org. AGA Clinical Practice Guideline on Fecal Microbiota-Based Therapies for Select Gastrointestinal Diseases. Gastroenterology. 2024;166:409-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 76] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 6. | Gilmore R, Hilley P, Srinivasan A, Choy MC, Grace JA, de Cruz P. Tofacitinib Is Safe and Effective When Used in Combination With Infliximab for the Management of Refractory Ulcerative Colitis. Clin Gastroenterol Hepatol. 2021;19:1302-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Furlan RG, Soldera J, Balbinot RS, Terres AZ, Balbinot SS, Balbinot RA. Ulcerative Proctosigmoiditis Exacerbated by Recurrent Clostridium colitis: Case Report. J Gastroenterol Hepatol Endosc. 2017;2:1025. |
| 8. | Kanika A, Soldera J. Pulmonary cytomegalovirus infection: A case report and systematic review. World J Meta-Anal. 2023;11:151-166. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Reference Citation Analysis (5)] |
| 9. | da Cruz ER, Forno AD, Pacheco SA, Bigarella LG, Ballotin VR, Salgado K, Freisbelen D, Michelin L, Soldera J. Intestinal Paracoccidioidomycosis: Case report and systematic review. Braz J Infect Dis. 2021;25:101605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 10. | Mowlah RK, Soldera J. Risk and management of post-operative infectious complications in inflammatory bowel disease: A systematic review. World J Gastrointest Surg. 2023;15:2579-2595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 11. | Dall'Oglio VM, Balbinot RS, Muscope ALF, Castel MD, Souza TR, Macedo RS, Oliveira TB, Balbinot RA, Balbinot SS, Brambilla E, Soldera J. Epidemiological profile of inflammatory bowel disease in Caxias do Sul, Brazil: a cross-sectional study. Sao Paulo Med J. 2020;138:530-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 12. | Fortuny Bauzá M, Cañete Pizarro F, Calm Salvans A, Calafat Sard M, Domènech Morral E. Tofacitinib for the treatment of acute severe ulcerative colitis refractory to infliximab. Gastroenterol Hepatol. 2022;45 Suppl 1:11-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Singh A, Goyal MK, Midha V, Mahajan R, Kaur K, Gupta YK, Singh D, Bansal N, Kaur R, Kalra S, Goyal O, Mehta V, Sood A. Tofacitinib in Acute Severe Ulcerative Colitis (TACOS): A Randomized Controlled Trial. Am J Gastroenterol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 14. | Malakar S, Kothalkar S, Shamsul Hoda U, Ghoshal UC. Tofacitinib in Steroid-Refractory Acute Severe Ulcerative Colitis: A Retrospective Analysis. Cureus. 2023;15:e45416. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Eqbal A, Hilley P, Choy M, Srinivasan A, de Cruz P. Outcomes out to 12 months after sequential use of high-dose tofacitinib following infliximab in acute severe ulcerative colitis. Intern Med J. 2023;53:1497-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 16. | Hilley P, Gilmore R, Srinivasan A, Choy M, De Cruz P. Combined Targeted Treatment Using Biologic-Tofacitinib Co-Therapy in Chronic Active Ulcerative Colitis. Inflamm Bowel Dis. 2021;27:e105-e106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Mpakogiannis K, Fousekis FS, Christodoulou DK, Katsanos KH, Narula N. The current role of Tofacitinib in acute severe ulcerative colitis in adult patients: A systematic review. Dig Liver Dis. 2023;55:1311-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Ali NM, Shehab MA. Upadacitinib as a Rescue Therapy in Acute Severe Ulcerative Colitis: A Case Report and Review of the Literature. Am J Case Rep. 2023;24:e940966. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Gilmore R, Tan WL, Fernandes R, An YK, Begun J. Upadacitinib Salvage Therapy for Infliximab-Experienced Patients with Acute Severe Ulcerative Colitis. J Crohns Colitis. 2023;17:2033-2036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 20. | Dalal RS, Winter RW, Gupta S, Sasson GF, Hamilton MJ, Allegretti JR. Clinical Outcomes at 8-16 Weeks After Upadacitinib Initiation for Acute Severe Ulcerative Colitis: A Case Series in the United States. Inflamm Bowel Dis. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Berinstein JA, Karl T, Patel A, Dolinger M, Barrett TA, Ahmed W, Click B, Steiner CA, Dulaney D, Levine J, Hassan SA, Perry C, Flomenhoft D, Ungaro RC, Berinstein EM, Sheehan J, Cohen-Mekelburg S, Regal RE, Stidham RW, Bishu S, Colombel JF, Higgins PDR. Effectiveness of Upadacitinib for Patients With Acute Severe Ulcerative Colitis: A Multicenter Experience. Am J Gastroenterol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (0)] |