Published online Aug 16, 2024. doi: 10.12998/wjcc.v12.i23.5441
Revised: May 29, 2024
Accepted: June 20, 2024
Published online: August 16, 2024
Processing time: 68 Days and 15.9 Hours
Ulcerative colitis (UC) is an idiopathic, chronic inflammatory bowel disease (IBD) most often located in the rectum, but may involve the entire colon. Extra intestinal manifestations (EIMs) occur with varying frequency depending on the affected organ. The most common ones are musculoskeletal EIMs, affecting up to 33%-40% of IBD patients. These include, among others, inflammatory back pain, tendinitis, plantar fasciitis and arthritis. Only a few case reports in literature discuss Achilles tendinitis.
This report describes a patient with UC and Achilles tendinitis in whom after many unsuccessful attempts of treatment with sulfasalazine, mesalazine, glucoco
This case mentions rare EIMs of UC and suggests that DBT may be an alternative for patient with ulcerative colitis and EIMs.
Core Tip: Achilles tendinitis is one of the rarest extra intestinal manifestations of ulcerative colitis (UC). To our best knowledge only a few case reports in literature discuss this problem. This article is a case report of a patient immobilized by Achilles tendinitis and excluded from social life by a very long-lasting exacerbation of UC. His way to recovery was full of ups and downs. We tried many lines of biological treatment with no permanent success. Only dual biologic therapy with ustekinumab and adalimumab thanks to combining two mechanisms of action brought the desired effect–a steroid free remission of UC and Achilles tendinitis.
- Citation: Filipiuk A, Gonciarz M. Rare extraintestinal manifestations of ulcerative colitis treated with dual biologic therapy: A case report. World J Clin Cases 2024; 12(23): 5441-5447
- URL: https://www.wjgnet.com/2307-8960/full/v12/i23/5441.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i23.5441
Ulcerative colitis (UC) is an autoimmune disease localized in the large intestine. According to the literature, extra intestinal manifestations (EIMs) of UC originating from the musculoskeletal system are in the range of 33% to 40%[1-3]. In recent years significant progress has been made in understanding the pathogenesis of arthritis associated with inflammatory bowel disease (IBD) although the exact pathomechanism linking gut inflammation with arthritis and tendinitis still remains unclear. The coexistence of EIMs including musculoskeletal symptoms worsens the quality of patients' lives and usually requires separate specialized pharmacological treatment and rehabilitation. In 2007, for the first time, a study demonstrating the safety of dual biologic therapy (DBT) was published[4]. Since then, there has been some progress, especially with the introduction of small molecules such as Janus kinase inhibitors and drugs modulating the sphingosine-1-phosphate signaling pathway into the therapy of IBD.
A 47-year-old Caucasian man, tax official with UC was admitted to our department in order to qualify for a biological therapy. On admission, the patient was passing 15 loose, non-bloody stools per day. At that time, the patient denied the presence of EIMs, but in the past had complained of ankle and knee pain. However, during the treatment described here, after glucocorticosteroids (GCs) dose reduction patient complained of severe Achilles tendons pain, leading to patient’s immobility.
The patient was diagnosed with UC 22 years ago and had a long history of treatment with 5-aminosalicylic acid and GCs, but also a short ineffective course of cyclosporine complicated by transient bone marrow aplasia. On admission, the patient was taking 28 mg of methylprednisolone (MP) orally daily.
The patient’s past medical history revealed osteopenia, hyperlipidemia, depression and hypertension.
There was no history of ethanol or drug abuse, herbal use or depot injections. The patient was vaccinated according to the local vaccination schedule. His family history was insignificant.
Physical examination revealed stable vital signs, his body mass index was 29 (height 176 cm, weight 90 kg) and heart rate was 110/min. Also, tenderness in the lower abdomen was found. The negative Otto test 3 cm (n > 3 cm) and a positive Schober test 3 cm (n > 4.5 cm) in physical examination indicated a reduction in mobility of the spine in the lumbar region. The Patrick's test was negative bilaterally.
Abnormalities in laboratory tests included increased calprotectin stool concentration-410.5 mg/kg (n < 50) and C-reactive protein (CRP) serum concentration - 1.1 mg/dL (n: 0-0.8 mg/dL). The liver enzymes and renal parameters were within the normal range. Possible infectious triggers of the exacerbation were excluded: Serological tests for Epstein–Barr virus, cytomegalovirus as well as stool cultures for Salmonella, Shigella, Yersinia, Campylobacter, enterotoxic E. coli, and also Clostridioides were negative. The serum CRP concentration persisted at 1.2 mg/dL (n: 0-0.8) and the peripheral white blood cells remained within normal range. A radiological examination of the chest as well as abdominal ultrasound examination showed no abnormalities. The rheumatoid factor, anti–cyclic citrullinated peptide antibodies and human leukocyte antigen B27 were negative.
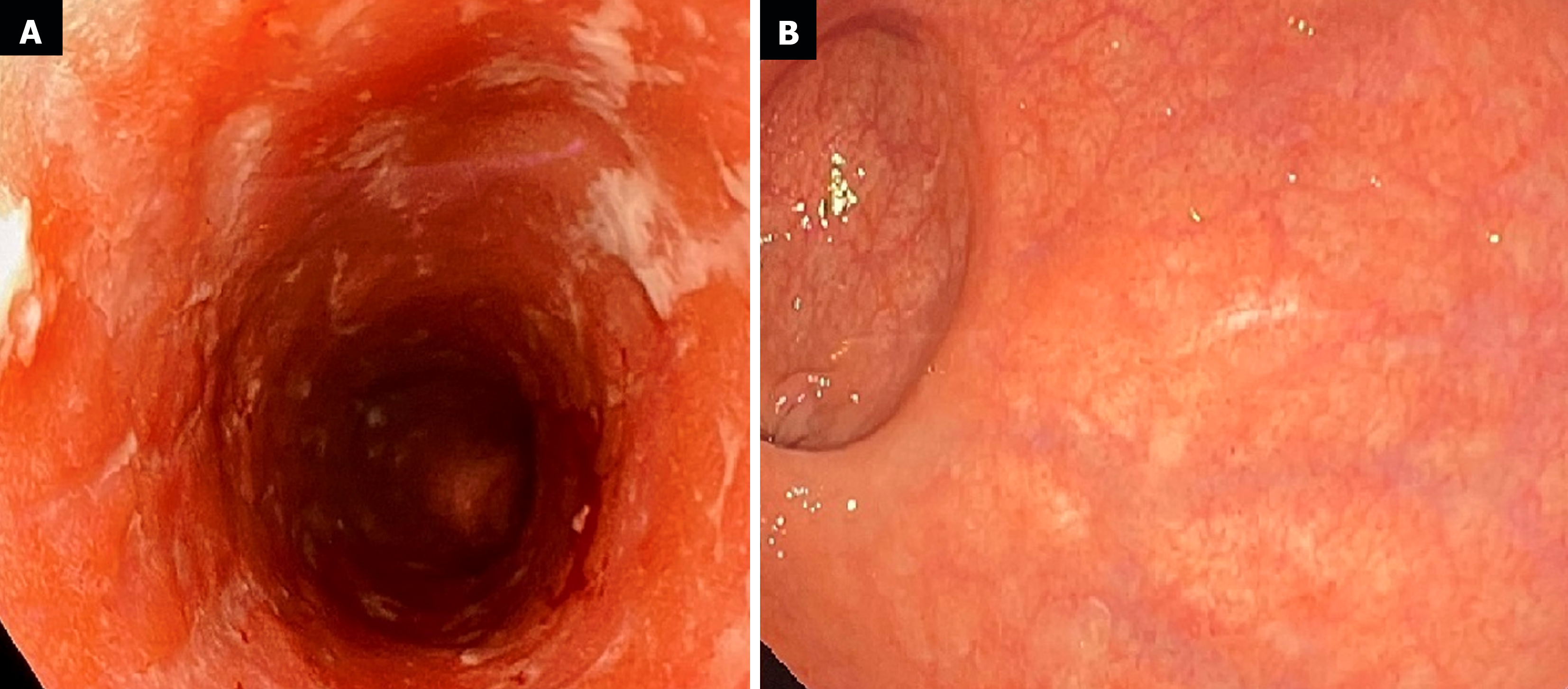
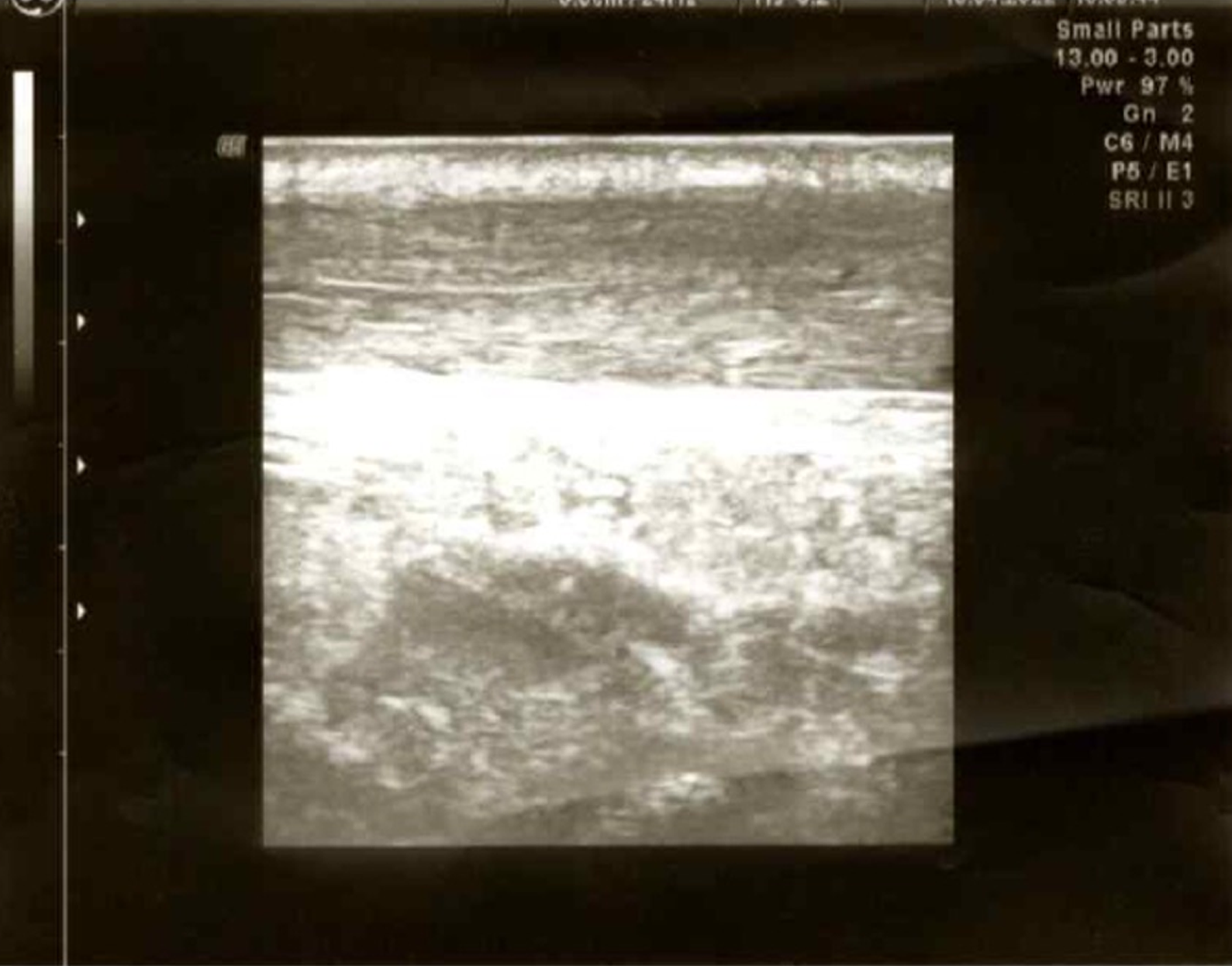
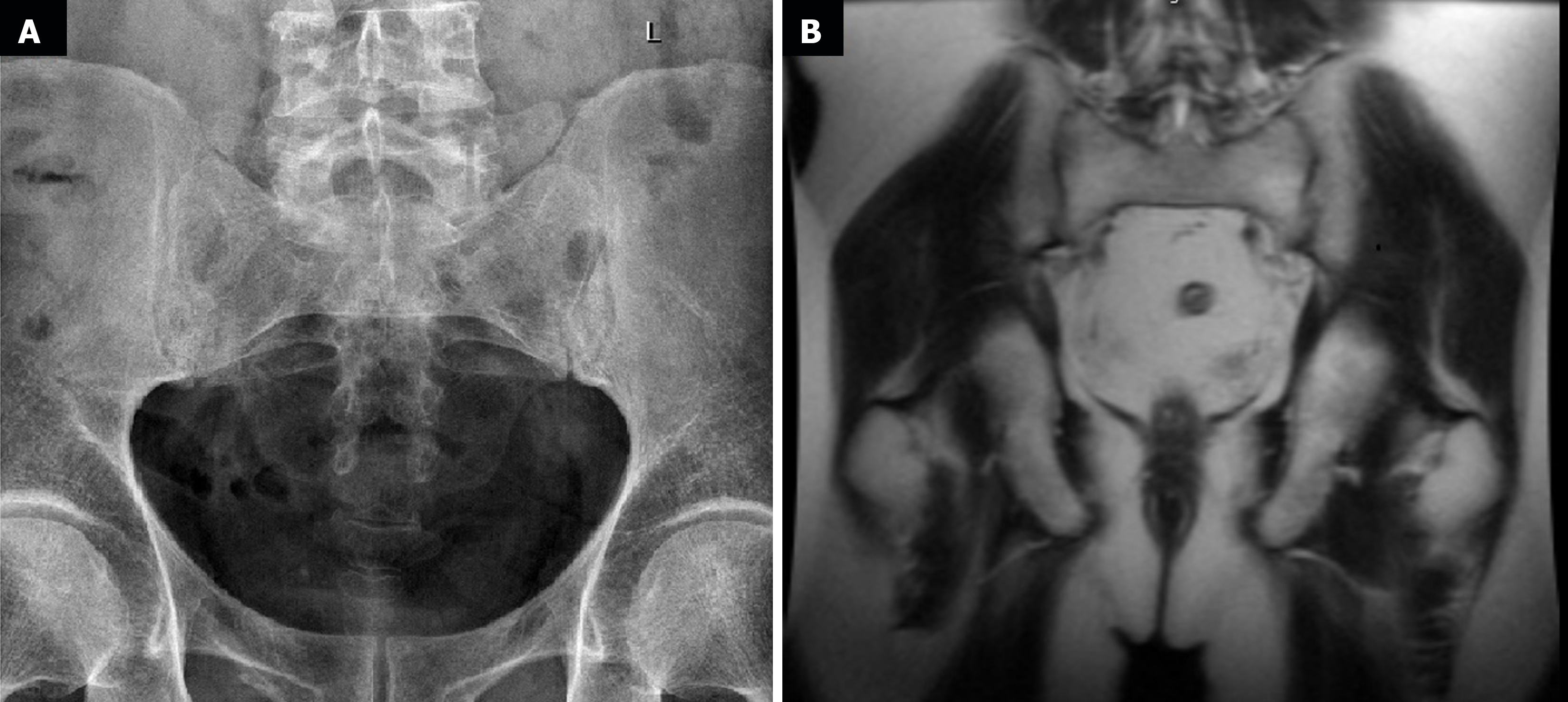
The first endoscopic examination during steroid therapy showed the Mayo endoscopic score of 1 with a full Mayo Score of 6. After a few lines of treatment, another endoscopic examination revealed the disease activity at Mayo 3 (Figure 1A) with a full Mayo Score of 9. Also, an ultrasound of the tendons was performed, confirming their bilateral inflammation (Figure 2). At the time, the patient was also examined for other EIMs of UC, revealing bilateral sacroiliitis and Achilles tendon enthesopathy (Figures 3 and 4). The spine X-ray showed squaring of the vertebral bodies in the area of the thoracic-lumbar junction, right-sided curvature of the spine in the Th/L section and degenerative changes of the intervertebral joints.
In concordance with the Assessment of SpondyloArthritis International Society (ASAS) 2010 criteria, seronegative peripheral SpA was diagnosed as an EIM of UC[5].
Due to the moderate-severe relapse of the UC after many years of ineffective GCs therapy, the patient was qualified for biological therapy with infliximab (INF) administered intravenously at a dose of 5 mg/kg resulting in clinical improvement after induction therapy. Concurrently, MP dose reduction was initiated and when the MP dose was 20 mg per day, the patient complained of Achilles tendon pain for the first time in his life. The treatment with INF was continued, achieving complete steroid-free clinical remission of UC on the 6th week of the treatment. The tendon pain, however, persisted and the patient was additionally on oral non-steroidal anti-inflammatory drugs. On the 12th week of the treatment, the patient experienced an exacerbation of the UC. Physical examination revealed mild abdominal tenderness, as well as swelling and tenderness in the area of the heel attachment of the Achilles tendons bilaterally. Endoscopic examination revealed the disease activity at Mayo 3 with a full Mayo Score of 9 (significant deterioration compared to the beginning of the therapy). Consequently, it was decided to convert biological therapy to tofacitinib (TOFA) at a dose of 10 mg orally twice daily and reintroduce MP at a dose of 16 mg daily. The efficacy of TOFA was evaluated at week 8 of the treatment. The patient remained in clinical remission, and colonoscopy showed Mayo endoscopic score of 1 with a full Mayo score of 3. Unfortunately, it was not possible to completely discontinue MP, as with a dose below 6 mg, recurrence of the tendon pain was observed, yet of a much lower intensity than before. For this reason, it was decided to convert the treatment with mesalazine at a dose of 4 g to sulfasalazine at a dose of 4 g daily. At week 8 of the treatment, after clinical and endoscopic response, the dosage of TOFA was reduced to 5 mg twice a day. At week 15 of the therapy (with MP dose of 4 mg), the patient presented to the outpatient clinic with a significant increase in Achilles tendon pain (6/10 in the numerical rating scale) and also ankle, knee and shoulder joints pain. He remained in clinical remission for intestinal symptoms, sigmoidoscopy showed Mayo endoscopic score of 1 and the dose of TOFA was increased to 10 mg twice daily. At week 26 of therapy, joint symptoms and tendon pain persisted resulting in the patient requiring daily opioids. In addition, an exacerbation of intestinal symptoms has recurred. The sigmoidoscopy revealed an increase in disease activity-Mayo endoscopic score of 2, with the total Mayo score of 8, a decision was made to change treatment to ustekinumab (UST) (520 mg intravenously). Eight weeks after the induction dose, a reduction in the total Mayo score from 8 to 5 was observed, as well as a slight improvement in tendon and joint pain, with no endoscopic improvement. The patient was then placed on DBT, UST dosing was maintained 90 mg subcutaneously every 8 weeks and INF was added at a dose of 5 mg/kg intravenously, but unfortunately with the 2nd dose, the patient experienced anaphylactic shock. Consequently, we decided to combine UST with adalimumab (ADA) at an induction dose of 160 mg subcutaneously, next 80 mg after 2 weeks, and then 40 mg every 2 weeks. After steroid discontinuation the patient suffered from tendons pain again, but of lower intensity. Then, we added methotrexate (MTX) at a dose of 15 mg subcutaneously weekly to DBT.
After 12 weeks of DBT combined with MTX for 2 months, the effectiveness of the treatment was evaluated. The patient has remained in steroid-free clinical remission, joint pains have subsided and tendon pain has remained minimal, requiring only rehabilitation and periodic low doses of analgesics. The colonoscopy showed full endoscopic remission for the first time-Mayo endoscopic score of 0 with total Mayo score of 0 (Figure 1B). The patient continues the treatment with UST (90 mg every 8 weeks), ADA (40 mg every 2 weeks) and MTX (15 mg every week) maintaining a steroid-free remission and no EIMs for the first time in many years. No adverse events have been observed.
The patient achieved clinical remission during anti-TNF treatment (only transiently as it later turned out), but extraintestinal symptoms were still present. The temporary reintroduction of GCs allowed the control of tendon pain and the implementation of appropriate treatment, which proved to be effective in the long term and allowed a steroid-free UC remission after many years. The way to remission was long and difficult, requiring multiple treatment modifications and ultimately the use of DBT.
When analyzing the patient’s disease course and treatment, attention should be paid to three key points. Firstly, the long-term and unjustified use of GCs. Secondly, the long delay in qualifying the patient for biological therapy despite the evident ineffectiveness of standard treatment. Thirdly, the lack of diagnosis of SpA despite the patient's reported symptoms in the form of ankle and knee joints pain in the past. Physicians usually correctly diagnose arthropathies in the course of IBD (although this had not been done for many years in the patient's case), however, they often forget that tendinitis is also an EIM of this group of diseases. In a study to validate a questionnaire for the detection of SpA in IBD patients, it has been shown that 102 (24.4%) patients met ASAS criteria for this diagnosis. Among these patients, enthesitis was present in 26 patients (25.5%)[6]. Despite that, case reports of patients with Achilles tendinitis are extremely rarely reported in the literature. ter Borg et al[7] described a case of a patient with tendinitis accompanying “pouchitis”, ergo this manifestation should not be forgotten even in patients with UC after colectomy. In all described cases, tendinitis was accompanied by the exacerbation of the underlying disease and resolved with the remission of intestinal symptoms[7-10]. However, it was not sufficient in the case discussed here, and required a novel approach.
The benefits of combining two biological drugs with different mechanisms of action are being increasingly discussed in literature[11-14]. For example, Glassner et al[13] described retrospectively 50 patients treated with DBT using different drug combinations (the most often vedolizumab combined with UST). In the described cohort, there were 10 patients who had concomitant rheumatological or dermatological disease, especially psoriasis and psoriatic arthritis. However, so far only one case of UC and concomitant peripheral arthropathy successfully treated with DBT (vedolizumab and UST) have been reported[15]. DBT is most widely referred to in systematic reviews, meta-analysis, retrospective studies and case series, but it is also a promising direction for clinical research. In 2023, randomized double-blind controlled phase 2 trial evaluating guselkumab plus golimumab combination therapy vs guselkumab or golimumab monotherapy in patients with UC was published. Both the efficacy and a good safety profile have been demonstrated for DBT. Despite the growing number of available therapies for some patients, inhibition of only one inflammatory response pathway may be insufficient, especially in challenging IBD patients and those with accompanying EIMs resistant to treatment with a single mechanism of action drugs.
The case of the patient with bilateral Achilles tendinitis discussed in this article represents rarely reported EIMs of UC and suggests that DBT may be an alternative for patient with UC and EIMs who did not respond for standard either first- or second-line medical therapies.
We sincerely appreciate the work of the endoscopists and radiologists.
| 1. | Du L, Ha C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol Clin North Am. 2020;49:643-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 356] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 2. | Malik TF, Aurelio DM. Extraintestinal Manifestations of Inflammatory Bowel Disease. 2023 Mar 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 3. | Salvarani C, Vlachonikolis IG, van der Heijde DM, Fornaciari G, Macchioni P, Beltrami M, Olivieri I, Di Gennaro F, Politi P, Stockbrügger RW, Russel MG; European Collaborative IBD Study Group. Musculoskeletal manifestations in a population-based cohort of inflammatory bowel disease patients. Scand J Gastroenterol. 2001;36:1307-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Sands BE, Kozarek R, Spainhour J, Barish CF, Becker S, Goldberg L, Katz S, Goldblum R, Harrigan R, Hilton D, Hanauer SB. Safety and tolerability of concurrent natalizumab treatment for patients with Crohn's disease not in remission while receiving infliximab. Inflamm Bowel Dis. 2007;13:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 5. | Braun J, van den Berg R, Baraliakos X, Boehm H, Burgos-Vargas R, Collantes-Estevez E, Dagfinrud H, Dijkmans B, Dougados M, Emery P, Geher P, Hammoudeh M, Inman RD, Jongkees M, Khan MA, Kiltz U, Kvien T, Leirisalo-Repo M, Maksymowych WP, Olivieri I, Pavelka K, Sieper J, Stanislawska-Biernat E, Wendling D, Ozgocmen S, van Drogen C, van Royen B, van der Heijde D. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011;70:896-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 734] [Cited by in RCA: 640] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 6. | Benfaremo D, Luchetti MM, Di Carlo M, Laganà B, Picchianti-Diamanti A, Carubbi F, Pica R, Chimenti MS, Lorenzetti R, Scolieri P, Bruzzese V, Benedetti A, Ramonda R, Giacomelli R, Salaffi F, Gabrielli A; GRADES-IBD Study Group. Multicenter Validation of the DETAIL Questionnaire for the Screening of Spondyloarthritis in Patients With Inflammatory Bowel Diseases. J Rheumatol. 2021;48:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | ter Borg EJ, Nadorp JH, Elbers JR. Ileal pouch arthritis: a case report. Eur J Gastroenterol Hepatol. 1996;8:957-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Akbal A, Gökmen F, Döner D, Uysal F. Are inflammatory bowel disease patients aware of Achilles tendonitis? J Crohns Colitis. 2014;8:711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Fremlin GA, Rawlings C, Livingstone JA, Bray AP. An unusual case of bilateral pyoderma gangrenosum with Achilles tendon rupture. Br J Dermatol. 2015;172:522-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Zenda T, Araki I, Nakamiya O, Tokuumi Y, Shimada Y, Komai K, Taniuchi Y. Achilles tendinitis as a rare extraintestinal manifestation of ulcerative colitis. Clin J Gastroenterol. 2016;9:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Buer LCT, Høivik ML, Warren DJ, Medhus AW, Moum BA. Combining Anti-TNF-α and Vedolizumab in the Treatment of Inflammatory Bowel Disease: A Case Series. Inflamm Bowel Dis. 2018;24:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Peter CD, Huang YH, Tian WN. Dual biologic therapy for severe ulcerative colitis, concomitant extraintestinal manifestations and an orbital osteoma: a successful treatment. Rev Esp Enferm Dig. 2022;114:757-758. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Glassner K, Oglat A, Duran A, Koduru P, Perry C, Wilhite A, Abraham BP. The use of combination biological or small molecule therapy in inflammatory bowel disease: A retrospective cohort study. J Dig Dis. 2020;21:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 14. | Yang E, Panaccione N, Whitmire N, Dulai PS, Vande Casteele N, Singh S, Boland BS, Collins A, Sandborn WJ, Panaccione R, Battat R. Efficacy and safety of simultaneous treatment with two biologic medications in refractory Crohn's disease. Aliment Pharmacol Ther. 2020;51:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 15. | Feagan BG, Sands BE, Sandborn WJ, Germinaro M, Vetter M, Shao J, Sheng S, Johanns J, Panés J; VEGA Study Group. Guselkumab plus golimumab combination therapy versus guselkumab or golimumab monotherapy in patients with ulcerative colitis (VEGA): a randomised, double-blind, controlled, phase 2, proof-of-concept trial. Lancet Gastroenterol Hepatol. 2023;8:307-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 59.5] [Reference Citation Analysis (1)] |