Published online Aug 16, 2024. doi: 10.12998/wjcc.v12.i23.5422
Revised: May 28, 2024
Accepted: June 17, 2024
Published online: August 16, 2024
Processing time: 85 Days and 3 Hours
Multiple acyl-CoA dehydrogenase deficiency (MADD) is a disease of rare auto
We report a severe case of a young man with onset type III MADD induced by drugs and strenuous exercise characterized by rhabdomyolysis and liver dysfunction. Urine analysis indicated 12 out of 70 kinds of organic acids like glutaric acid-2 were detected. Serum analysis in genetic metabolic diseases revealed 24 out of 43 tested items were abnormal, revealing the elevation of several acylcarnitines and the reduction of carnitine in the patient. By next generation sequencing technology for gene sequencing related to fatty acid oxidation and carnitine cycle defects, a rare ETFDH gene variant was identified: NM_004453:4:C.1448C>T(p.Pro483 Leu). The patient was diagnosed with late-onset GAII. He was not responsive to riboflavin and progressively worsened into multiple organ failure that finally led to death.
Type III MADD can also be fatal and not responsive to treatments.
Core Tip: Multiple acyl-CoA dehydrogenase deficiency (MADD) is a disease of rare autosomal recessive disorder of fatty acid, amino acid, and choline metabolism. Here, we report a severe case of a young man with onset type III MADD characterized by rhabdomyolysis and liver dysfunction. His urinary and serum analysis indicated organic acids, the elevation of several acylcarnitines and the reduction of carnitine. Eventually, we identified a rare compound heterozygous variant in the patient. Unfortunately, the patient was not responsive to riboflavin and his condition progressively worsened into multiple organ failure that finally led to death.
- Citation: Li XX, Yang XN, Pan HD, Liu L. Fatal multiple acyl-CoA dehydrogenase deficiency caused by ETFDH gene mutation: A case report. World J Clin Cases 2024; 12(23): 5422-5430
- URL: https://www.wjgnet.com/2307-8960/full/v12/i23/5422.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i23.5422
Multiple acyl-CoA dehydrogenase deficiency (MADD), also called glutaric aciduria type II (GA II) (MIM:231680), is a rare autosomal recessive disorder. A defect in the alpha subunit of fatty acid mitochondrial electron transfer flavoprotein (ETFA) protein, beta subunit of fatty acid mitochondrial electron transfer flavoprotein (ETFB) protein or the electron transfer flavoprotein dehydrogenase (ETFDH) protein could lead to disorder of amino acid and choline metabolism. There are three types of MADD. Type I is a neonatal-onset form with congenital anomalies, Type II is a neonatal-onset form without congenital anomalies, and Type III is considered to a milder form and usually respond to riboflavin. Types I and II are usually considered to be more severe and sometimes fatal. The late-onset form is considered to be milder, and could be mostly responsive to treatments. Here, we report a late-onset MADD that is not responsive to riboflavin and is fatal.
Onset weakness for a month and vomiting for 5 h.
The patient complained of fatigue after strenuous exercise (tug of war) a month ago. He visited a doctor in a clinic and received a febuxostat tablet for hyperuricemia and bicyclol tablet for liver injury. He suffered, vomiting, shortness of breath, chest pain and slurred speech as the disease progressed.
The patient had a history of palpitation and liver dysfunction for 5 years.
The patient denied infectious diseases, genetic diseases, history of surgical trauma and blood transfusion.
On admission, physical examination showed body temperature of 36.5 °C, pulse of 130 times per minute, respiration of 19 times per minute, and blood pressure [p(B)] of 16.226/10.374 kPa. The body check revealed jaundice, neck stiffness, flexor asthenia and weakness of four limbs.
The patient’s blood glucose concentration was 2.7 mmol/L in the emergency room. Blood tests revealed an elevation of creatine kinase, alanine aminotransferase, aspartate aminotransferase on admission. His serum albumin was 37.2 U/L, serum creatinine was 80 μmol/L, and blood urea nitrogen was 6.4 mmol/L on admission, while his blood ammonia was 236 μmol/L, and ceruloplasmin was 13.1 mg/L. His urinalysis indicated occult blood in the urine. Blood clotting functions were abnormal. Blood cell analysis, index of infection, autoantibodies, tumor markers and thyroid function were normal on admission.
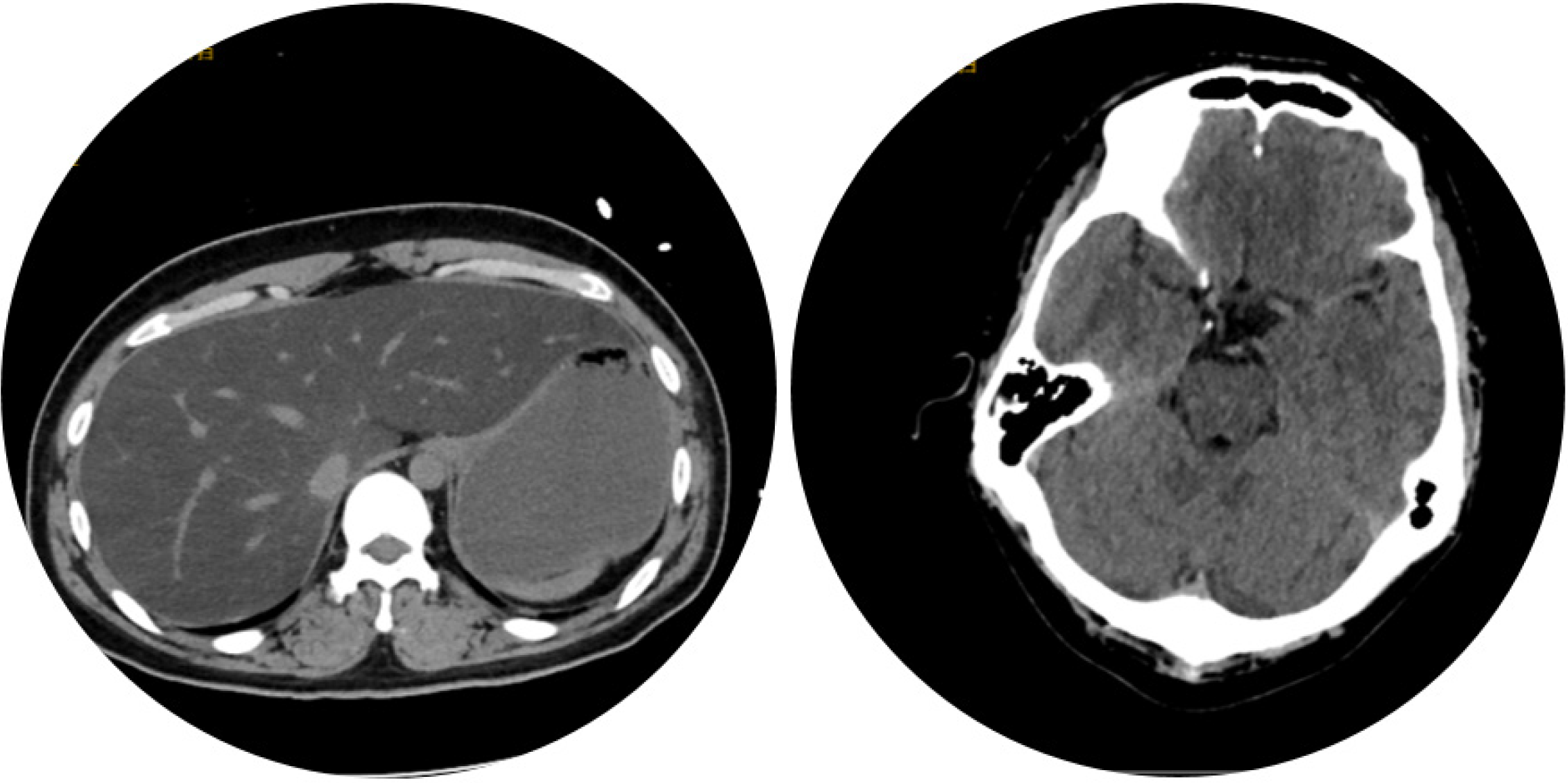
Computer tomography of the patient’s brain and abdomen indicted sallow cerebral sulci and severe fatty liver (Figure 1). Electrocardiograph indicated supraventricular tachycardia (SVT). The heart ultrasound and electromyography were normal.
The patient was diagnosed with rhabdomyolysis, SVT, hepatic failure, acidosis, hypoglycemia and abnormal coagulation function on admission. A high dose of glucose was pumped in until his glucose level was stable. Timely fluid infusion, intravenous glutathione and glycyrrhzin were used for liver protection. Intravenous ornithine aspartate and rice vinegar clysis were applied for reducing blood ammonia level. However, the patient experienced very rapid deterioration in his clinical conditions following the onset of symptoms. His liver function and myocardial enzyme levels continuously increased. On the 9th day after admission, he suffered from onset fatigue, mucocutaneous stained yellow (daily elevated serum bilirubin level over 17 mmol/L), repeated severe hypoglycemia, acidosis, hepatic encephalopathy, SVT, rhab
His coagulation system, respiratory system, and circulatory system were collapsed, accompanied with renal and liver failure. Multiple organ failure could not be explained by the admitting diagnosis. Further, we discovered that his parents were in a consanguineous marriage. Combined with the clinical features of the patient, hereditary disease was con
| Number | Organic acids (normal range) | |
| 1 | Lactic acid -2 (0.0-13.0) | 14.2↑ |
| 2 | Pyruvate-OX-2 (0.0-30.0) | 91.7↑ |
| 3 | 2-hydroxyisovaleric acid-2 (0.0-2.0) | 7.8↑ |
| 4 | 2-ketoisovaleric acid-OX-2 (0.0-0.3) | 1.0↑ |
| 5 | 2-ketoisohexanoic acid-OX-2 (0.0-0.8) | 1.7↑ |
| 6 | Glutaric acid -2 (0.0-8.0) | 516.4↑ |
| 7 | Isoamyl glycine-1 (0.0-1.5) | 11.3↑ |
| 8 | Acetylmalonic acid-2 (0.0-7.0) | 17.6↑ |
| 9 | Methylsuccinic acid-2 (0.0-4.0) | 5.5↑ |
| 10 | Methyl fumaric acid-2 (0.0-2.5) | 3.0↑ |
| 11 | Glutaconic acid-2 (0.0-0.0) | 2.2↑ |
| 12 | Adipic acid-2 (0.0-15.0) | 165↑ |
| Amino acid and acylcarnitine spectrum (normal range) | Amino acid and acylcarnitine spectrum | ||
| Palmitoyl carnitine (C16) (0.20-3.50) | 4.49↑ | C8/C3 (0.01-0.40) | 1.20↑ |
| Palmitoyl carnitine (C16:1) (0.02-0.30) | 2.22↑ | C10/C3 (0.01-0.50) | 2.70↑ |
| Palmolive dienyl carnitine (C16:2) (0.01-0.10) | 0.31↑ | C12/C3 (0.01-0.35) | 3.58↑ |
| Octadecyl carnitine (C18:1) (0.20-2.80) | 3.80↑ | C14/C3 (0.02-0.45) | 9.25↑ |
| C3/Mot (0.02-0.30) | 0.01↓ | C14:1/C8:1 (0.10-6.00) | 12.31↑ |
| C3DC/C4 (0.10-1.50) | 0.07↓ | C16/C2 (0.01-0.20) | 0.82↑ |
| C4/C2 (0.00-0.05) | 0.08↑ | C16/C3 (0.10-3.50) | 22.07↑ |
| C4/C3 (0.00-0.70) | 2.15↑ | C18/C3 (0.05-2.00) | 5.44↑ |
| C5/C2 (0.00-0.05) | 0.12↑ | C16 OH/C3 (0.00-0.15) | 0.22↑ |
| C5/C3 (0.00-0.50) | 3.20↑ | C18 OH/C3 (0.00-0.10) | 0.17↑ |
| C5DC/C3 (0.01-0.40) | 0.65↑ | (C1G+C18:1)/C2 (0.03-0.40) | 1.52↑ |
| C6/C3 (0.01-0.30) | 0.72↑ | C0/(C1G+C18) (6.50-90.00) | 1.12↓ |
The patient was diagnosed with type III (late-onset form) MADD.
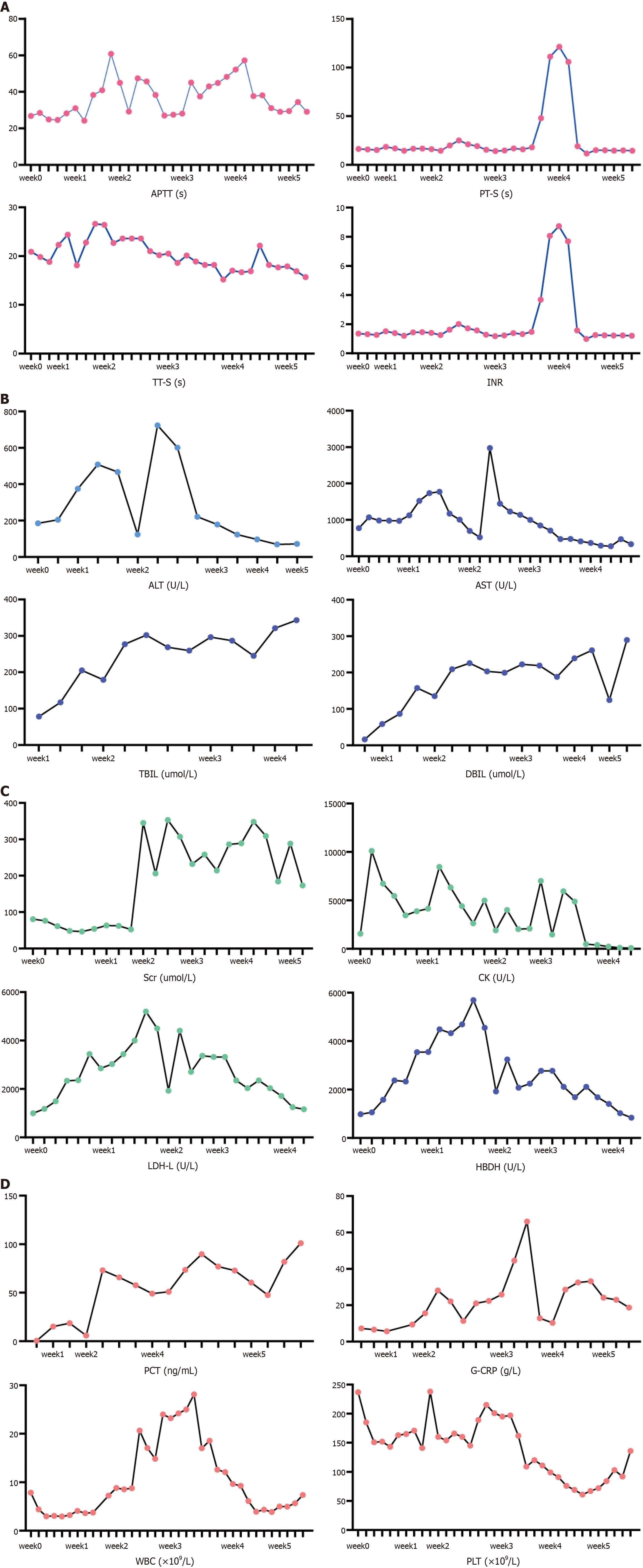
Levocarnitine and riboflavin were intravenously injected at a dose of 60 mg/kg/d and 200 mg/d, respectively. Anti-infective therapy was applied for secondary infection. Unfortunately, the patient fell into a coma after the 9th day of admission. He also suffered malignant arrhythmia with a heart rate over two hundred times per minute. Esmolol and metarbamine were not effective to his heart so synchronized electrical cardioversions were applied seven times on the 11th, 12th and 13th day after his admission. The patients finally fell into a deep coma and depended on mechanical ventilation, continuous renal replacement treatment (CRRT) and artificial liver. Twenty-five days of ventilator support, eleven rounds of CRRT, two rounds of plasma exchange, three rounds of dual plasma molecular adsorption system and nine rounds of alveolar wash were applied within the twenty-five days of his coma. More than 20000 mL of plasma, 12 units of red blood cells, and some blood platelet and cryoprecipitation were used during the treatment. His liver function, kidney function and functions of other systems were improving, as demonstrated by clinical biochemistry (Figure 2).
The young man finally came to after 25 days of treatment, though he was still critically ill. We transferred the patient to Yunnan according to the demand of his parents. Unfortunately, we got the message that he finally passed away in Yun
MADD is also called glutaric aciduria type II (GA II) (MIM:231680). This is a rare autosomal recessive disorder caused by defects in the mitochondrial electron transport chain, which is the electron transport from flavin adenine dinucleotide-containing dehydrogenases to coenzyme Q10. Intra-mitochondrial acyl-CoA dehydrogenation causes MADD.
There are three forms of defects. Defects in the ETFA (OMIM 231680), ETFB protein (OMIM 130410) or ETFDH proteins (OMIM 231675) could lead to disorder of amino acid and choline metabolism. ETFA (15q23-q25) encodes the alpha subunit of ETF, ETFB (19q13.3-q13.4) encodes the beta subunit of ETF, and ETFDH (4q32-q35) encoding ETF-ubiquinone oxidoreductase (ETFQO).
Thus, there are three forms of MADD. Both type I and type II are neonatal-onset forms; type I is associated with congenital anomalies, while type II is not. Types I and II are usually considered to be more severe and sometimes fatal, presenting with hypoketotic hypoglycemia, metabolic acidosis, cardiomyopathy, and hepatomegaly. Type III is a late-onset form which is considered to be milder, characterized by proximal myopathy. Episodic vomiting, encephalopathy, liver and renal impairment, and rhabdomyolysis may occur under catabolic stress[1].
ETFDH variants are the most common cause of the late-onset form[2], which[3] is considered to be more variable. There could be recurrent hypoglycemia, metabolic acidosis, vomiting, and muscle weakness during stress[4]. These atypical symptoms usually make differential diagnosis difficult. Exercise intolerance could be an atypical symptom. In our case, exercise and drugs may have induced rhabdomyolysis and liver dysfunction. Research has also indicated that a prolonged exercise test could be of diagnostic importance[5]. Intolerance for prolonged exercise in patients with anam
Urinary organic acid profiles could indicate MADD, which is important for the diagnosis of GAII. The test is characterized by elevated acids, including isovaleric acid, a-methylbutyrate, ethylmalonic acid, isobutyrate, glutaric acid, aliphatic dicarboxylic acids, etc[7,8]. Urine organic acid analysis could show these acids and their derivatives. During metabolic decompensation, lactate levels and creatine phosphokinase are typically raised. In our case, organic acids tests in the urine indicated 12 of 70 organic acids were abnormal, including lactic acid-2, pyruvate-OX-2, 2-hydroxyisovaleric acid-2, 2-ketoisovaleric acid-OX-2, etc. Among which, the patient’s glutaric acid-2 level was over 64 times higher than the upper limit of the reference level. Adipic acid-2 level was over 11 times higher than the upper limit of the reference level. Detection of urine organic acids are effective in MADD diagnosis that could highly suggest the disease.
Serum characterization of MADD includes acylcarnitine and organic acid profiling. The biochemical test would reveal higher levels of acylglycine conjugates and dicarboxylic acids. The elevated levels of C4-C18 acylcarnitines could be also detected in the blood. All these biochemical findings might normalize during the period of stability in MADD. Acylcarnitine profile could reveal elevations of short-chain, medium-chain and long-chain acylcarnitines[9]. In our case, blood amino acid and acylcarnitine spectrum of genetic metabolic diseases revealed that 24 out of the total 43 tested items were abnormal, including the elevation of several acylcarnitines and the reduction of carnitine.
Defects in the ETFDH protein[10] account for about 90% of MADD cases. ETFDH-c.250G>A is one of the most common mutations, representing a high allelic frequency of about 80% in southern China[11]. ETFDH-c.770A>G and ETFDH-c.1227A>C are more widespread mutations in mainland China[12]. Muscle biopsy could reveal acid and lipid accumulation in skeletal muscles, reducing the activity of mitochondrial respiratory chain complexes[13,14]. In our case, we applied next generation sequencing technology for gene sequencing related to fatty acid oxidation and carnitine cycle defects in the patient’s whole blood. Single nucleotide variation and small fragment insertion deletion variation were detected, which indicated single nucleotide variation of ETFDH gene with Chromosomal location: Chr4:159627503; mutation information: NM.004453.4:c.1448C>T(p.Pro483Leu). The mutation is very rare, and whether it is related to the severity of clinical symptoms could be further explored.
For treatments, type III MADD usually responds to riboflavin, with a reported response rate over 98%[1]. When riboflavin insufficient[15], MADD patients would undergo rapid degradation. Riboflavin supplementation is vital in the treatment of late-onset MADD. Research suggests that ETFDH mutations were responsible for all riboflavin-responsive GA-II patients[16]. More than 80% of patients treated with riboflavin showed a release of the disease. The treatment improved their cardiac and skeletal muscle functions. Patients receiving riboflavin treatment could also respond in urinary organic acids normalization and acylcarnitine species reductions[17]. However, there are controversies sur
In clinic, MADD should be suspected in cases with metabolic abnormalities. Patients with liver damage, kidney failure, hypoglycemia, hyperammonemia, hypoglycemia and rhabdomyolysis, etc would suggest GA-II. Exercise and medicine might be inducements. Early diagnosis depends on urine organic acid analysis, blood amino acid tests, serum acylcarnitine profile analysis, muscle biopsy, and genetic tests. Riboflavin supplementation is the first line medication, though it may be not effective.
In summary, our report describes the clinical, biochemical, and molecular findings of a 21-year-old male with ETFDH-related MADD. Late onset of MADD could be with definite inducement factors like drugs and severe exercise. It could also be fatal. c.1448C>T in ETFDH were found in this young man presented with multiple organ failure, which further expands the list of mutations found in MADD patients that might be riboflavin non-responsive MADD.
The authors are grateful to Dr. Xiao-Yong Zhang, Wan-Fang Tan and Fu-Ren Guan for entering the data.
| 1. | Grünert SC. Clinical and genetical heterogeneity of late-onset multiple acyl-coenzyme A dehydrogenase deficiency. Orphanet J Rare Dis. 2014;9:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 2. | Horvath R. Update on clinical aspects and treatment of selected vitamin-responsive disorders II (riboflavin and CoQ 10). J Inherit Metab Dis. 2012;35:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Olsen RK, Andresen BS, Christensen E, Bross P, Skovby F, Gregersen N. Clear relationship between ETF/ETFDH genotype and phenotype in patients with multiple acyl-CoA dehydrogenation deficiency. Hum Mutat. 2003;22:12-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Ip WC, Hammond JW, Wilcken B. Neonatal multiple acyl-CoA dehydrogenase deficiency: essentially absent fatty acid oxidation activity in proband but normal activity in parental cultured skin fibroblasts. J Inherit Metab Dis. 1996;19:379-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Takken T, Custers J, Visser G, Dorland L, Helders P, de Koning T. Prolonged exercise testing in two children with a mild Multiple Acyl-CoA-Dehydrogenase deficiency. Nutr Metab (Lond). 2005;2:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Loots DT, Wiid IJ, Page BJ, Mienie LJ, van Helden PD. Melatonin prevents the free radical and MADD metabolic profiles induced by antituberculosis drugs in an animal model. J Pineal Res. 2005;38:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Yamada K, Kobayashi H, Bo R, Takahashi T, Purevsuren J, Hasegawa Y, Taketani T, Fukuda S, Ohkubo T, Yokota T, Watanabe M, Tsunemi T, Mizusawa H, Takuma H, Shioya A, Ishii A, Tamaoka A, Shigematsu Y, Sugie H, Yamaguchi S. Clinical, biochemical and molecular investigation of adult-onset glutaric acidemia type II: Characteristics in comparison with pediatric cases. Brain Dev. 2016;38:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Liu J, Wu C, Gao F, Yan Q. A rare condition that mimic myopathy: Late-onset glutaric acidaemia type II. Rheumatol Immunol Res. 2023;4:173-175. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Wen B, Li D, Li W, Zhao Y, Yan C. Multiple acyl-CoA dehydrogenation deficiency as decreased acyl-carnitine profile in serum. Neurol Sci. 2015;36:853-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Goodman SI, Binard RJ, Woontner MR, Frerman FE. Glutaric acidemia type II: gene structure and mutations of the electron transfer flavoprotein:ubiquinone oxidoreductase (ETF:QO) gene. Mol Genet Metab. 2002;77:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Wang ZQ, Chen XJ, Murong SX, Wang N, Wu ZY. Molecular analysis of 51 unrelated pedigrees with late-onset multiple acyl-CoA dehydrogenation deficiency (MADD) in southern China confirmed the most common ETFDH mutation and high carrier frequency of c.250G>A. J Mol Med (Berl). 2011;89:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Zhu M, Zhu X, Qi X, Weijiang D, Yu Y, Wan H, Hong D. Riboflavin-responsive multiple Acyl-CoA dehydrogenation deficiency in 13 cases, and a literature review in mainland Chinese patients. J Hum Genet. 2014;59:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Vergani L, Barile M, Angelini C, Burlina AB, Nijtmans L, Freda MP, Brizio C, Zerbetto E, Dabbeni-Sala F. Riboflavin therapy. Biochemical heterogeneity in two adult lipid storage myopathies. Brain. 1999;122 ( Pt 12):2401-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Olsen RK, Olpin SE, Andresen BS, Miedzybrodzka ZH, Pourfarzam M, Merinero B, Frerman FE, Beresford MW, Dean JC, Cornelius N, Andersen O, Oldfors A, Holme E, Gregersen N, Turnbull DM, Morris AA. ETFDH mutations as a major cause of riboflavin-responsive multiple acyl-CoA dehydrogenation deficiency. Brain. 2007;130:2045-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 227] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 15. | Henriques BJ, Olsen RK, Bross P, Gomes CM. Emerging roles for riboflavin in functional rescue of mitochondrial β-oxidation flavoenzymes. Curr Med Chem. 2010;17:3842-3854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Law LK, Tang NL, Hui J, Fung SL, Ruiter J, Wanders RJ, Fok TF, Lam CW. Novel mutations in ETFDH gene in Chinese patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Clin Chim Acta. 2009;404:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Olsen RKJ, Koňaříková E, Giancaspero TA, Mosegaard S, Boczonadi V, Mataković L, Veauville-Merllié A, Terrile C, Schwarzmayr T, Haack TB, Auranen M, Leone P, Galluccio M, Imbard A, Gutierrez-Rios P, Palmfeldt J, Graf E, Vianey-Saban C, Oppenheim M, Schiff M, Pichard S, Rigal O, Pyle A, Chinnery PF, Konstantopoulou V, Möslinger D, Feichtinger RG, Talim B, Topaloglu H, Coskun T, Gucer S, Botta A, Pegoraro E, Malena A, Vergani L, Mazzà D, Zollino M, Ghezzi D, Acquaviva C, Tyni T, Boneh A, Meitinger T, Strom TM, Gregersen N, Mayr JA, Horvath R, Barile M, Prokisch H. Riboflavin-Responsive and -Non-responsive Mutations in FAD Synthase Cause Multiple Acyl-CoA Dehydrogenase and Combined Respiratory-Chain Deficiency. Am J Hum Genet. 2016;98:1130-1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |