Published online Aug 16, 2024. doi: 10.12998/wjcc.v12.i23.5410
Revised: June 12, 2024
Accepted: June 25, 2024
Published online: August 16, 2024
Processing time: 96 Days and 3.5 Hours
Small cell lung cancer (SCLC) exhibits a pronounced tendency for metastasis and relapse, and the acquisition of resistance to chemotherapy and radiotherapy, leading to complexity in treatment outcomes. It is crucial to tackle these chall
We present the first case of a KIF5B-RET fusion in a 65-year-old male patient with SCLC. To date, the patient has received the 4th line chemotherapy with anlotinib for one year and has shown a sustained favorable partial response. According to the results of next generation sequencing, this SCLC patient harbors the KIF5B-RET fusion, suggesting that RET fusion could serve as a promising molecular target for SCLC treatment. Next-generation sequencing (NGS) plays a critical role in comprehensively assessing the genotype and phenotype of cancer.
NGS can provide SCLC patients with personalized and targeted therapy options, thereby improving their likelihood of survival.
Core Tip: This work describes a rare case of a KIF5B-RET fusion in small cell lung cancer (SCLC) and the patient's sustained partial response to the 4th line therapy with anlotinib. The study highlights the potential of RET fusions as a promising molecular target in SCLC treatment and emphasizes the importance of next-generation sequencing for personalized therapy options. The innovative arguments include the identification of a novel fusion in SCLC and the potential for targeted therapy to enhance the survival rates of SCLC patients.
- Citation: Zhang R, He YT, Liu YS, Li H, Zhao F. Small cell lung carcinoma with KIF5B-RET fusion partially responded to the 4th-line therapy with anlotinib: A case report. World J Clin Cases 2024; 12(23): 5410-5415
- URL: https://www.wjgnet.com/2307-8960/full/v12/i23/5410.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i23.5410
Small cell lung cancer (SCLC) is a highly aggressive malignancy often diagnosed with metastases at the time of detection. Approximately 15% of lung cancer cases are classified as SCLC, with a 5-year survival rate less than 7%[1]. SCLC is categorized into limited-stage disease (LS-SCLC) and extensive-stage disease (ES-SCLC). ES-SCLC accounts for around 70% of SCLC patients and carries a poorer prognosis compared to LS-SCLC. Standard therapy comprising cisplatin and etoposide for ES-SCLC has endured as the 1st line chemotherapy for over 30 years. Despite the favorable initial response rates to the 1st line chemotherapy, ES-SCLC patients experience increased relapse rates, leading to an unfavorable overall prognosis primarily attributed to the rapid development of drug resistance. The integration of cancer immunotherapy and gene profiling technology has started to revolutionize the standard treatment of SCLC. The IMpower133 phase III randomized trial assessed the efficacy of atezolizumab in conjunction with carboplatin and etoposide in 403 ES-SCLC patients[2]. The data indicated a noteworthy improvement in median overall survival to 12.3 months when atezolizumab was added, compared to 10.3 months with chemotherapy alone. The Food and Drug Administration (FDA), the European Medicines Agency, and National Medical Products Administration have approved both atezolizumab and durvalumab combined with carboplatin or platinum and etoposide as standards for ES-SCLC 1st line systemic therapy[3]. Furthermore, advancements in gene profiling technologies such as next-generation sequencing (NGS) have significantly accelerated our understanding of SCLC biology. Genomic losses or dysfunctions in retinoblastoma 1 (RB1) and tumor protein P53 (TP53) are prevalent in SCLC. The discovery of RET fusions in 1%-3% of non-SCLCs (NSCLC) presents a promising therapeutic target for oncologic intervention. Despite recent therapeutic advancements, ES-SCLC remains an exceptionally aggressive and challenging disease. RET fusions result from genomic loci rearrangements involving chromosomal inversion or translocation. The most prevalent RET fusion variant in lung cancer is the KIF5B-RET fusion. Previously reported KIF5B-RET fusions in lung cancer have all been associated with NSCLC. An exceptional case reported in 2023, was a 57-year-old female patient with combined SCLC who was found to harbor a KIF5B-RET fusion[4]. Notably, this patient demonstrated a sustained clinical response to the 4th line therapy with selpercatinib, a tyrosine kinase inhibitor. In the present study, we report the case of a patient with ES-SCLC harboring a KIF5B-RET fusion. To the best of our knowledge, this represents the first case of a KIF5B-RET fusion in ES-SCLC. Several RET-specific inhibitors are currently applied in clinical therapy, and have shown promising outcomes in terms of prognosis[5,6]. Thus, RET fusions may serve as promising molecular targets for SCLC therapies, broadening treatment options and improving survival rates. This finding also suggests the potential necessity for RET fusion testing in patients with SCLC.
A 65-year-old male patient was diagnosed with a left lung mass present for over one month and SCLC in the left lung was confirmed more than ten days ago.
The patient fell and subsequently presented to a local hospital seeking medical evaluation. Chest computed tomography (CT) revealed a mass in the upper lobe of the left lung. To pursue a comprehensive diagnosis and treatment plan, the patient sought care at our hospital.
The patient was diagnosed with type 2 diabetes three years previously, with a serum glucose level of 22 mmol/L, and had not received any treatment.
The patient has smoked and consumed alcohol for 45 years. Furthermore, his mother died at the age of 85 years due to cervical carcinoma.
A routine physical examination did not reveal any abnormalities.
Laboratory examinations indicated elevated levels of keratin 19 (2.94 ng/mL), carbohydrate antigen 125 (CA125) (37.00 U/mL), and blood glucose (10.22 mmol/L). In addition, the patient's serum levels of squamous epithelial cell carcinoma antigen, neuron-specific enolase (NSE), carcinoembryonic antigen, and carbohydrate antigen 15-3 were within normal limits. Remarkably, a NGS analysis of the primary lung biopsy specimen revealed a KIF5B-RET fusion, along with mutations in TP53 and RB1.
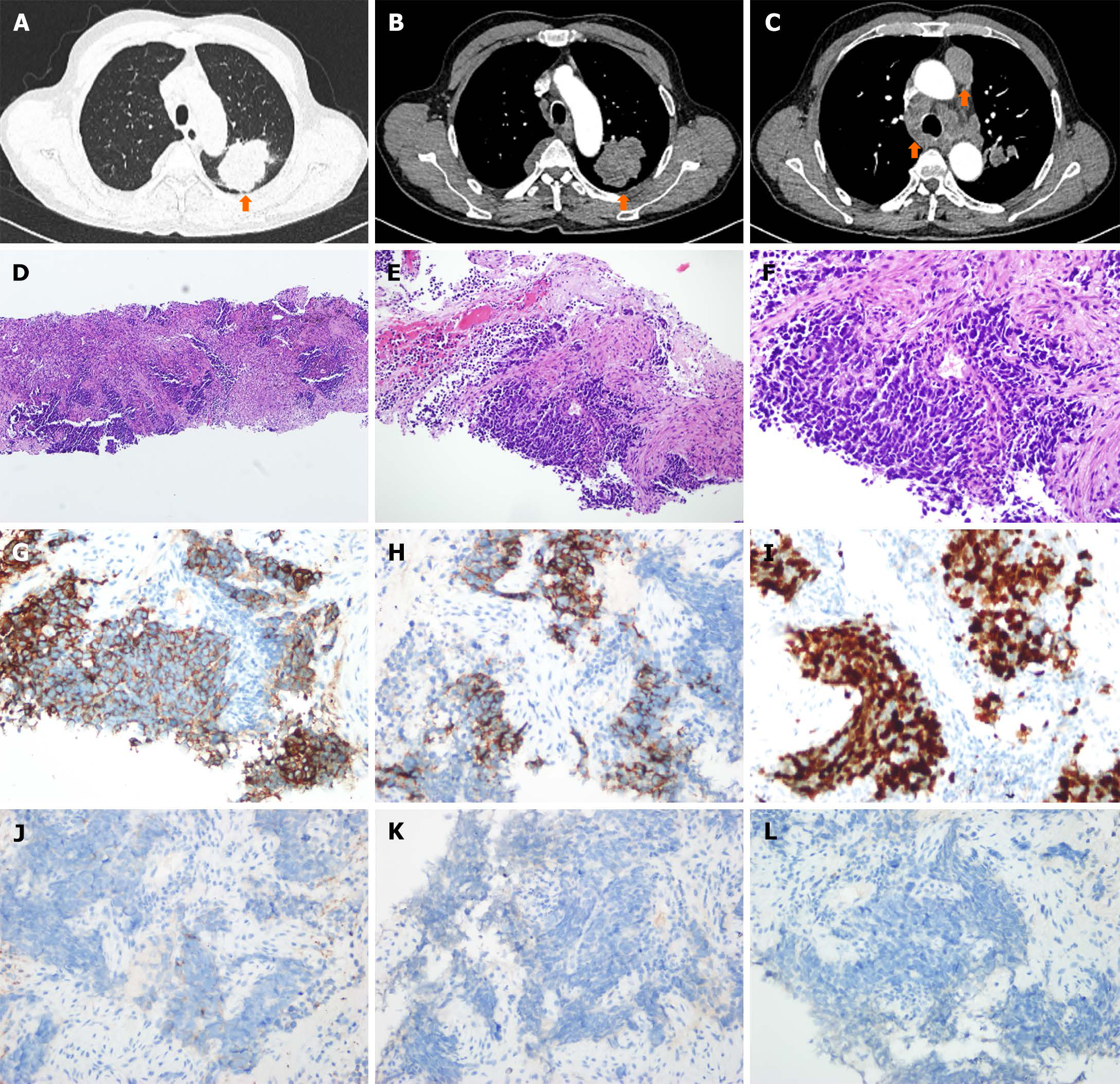
A chest CT scan revealed a lobulated soft tissue mass shadow in the apical posterior segment of the upper lobe of the left lung, measuring approximately 55 mm × 42 mm. The mass invaded the left superior pulmonary artery and the right pleura, and displayed enlargement of mediastinal and hilar lymph nodes (Figure 1A-C), indicating metastasis within the lung and lymph nodes. Histological examination of the primary mass using hematoxylin and eosin staining revealed typical SCLC features with small cell nests (Figure 1D-F). This was further confirmed by immunohistochemistry, which showed positive results for cytokeratin CAM5.2 (Figure 1G) and CD56 (Figure 1H), a Ki67 index of approximately 80% (Figure 1I), focal positivity for synaptophysin (Figure 1J), and negativity for chromogranin A (Figure 1K), and thyroid transcription factor-1 (Figure 1L).
ES-SCLC was diagnosed based on the patient's medical history, and laboratory and imaging examinations.
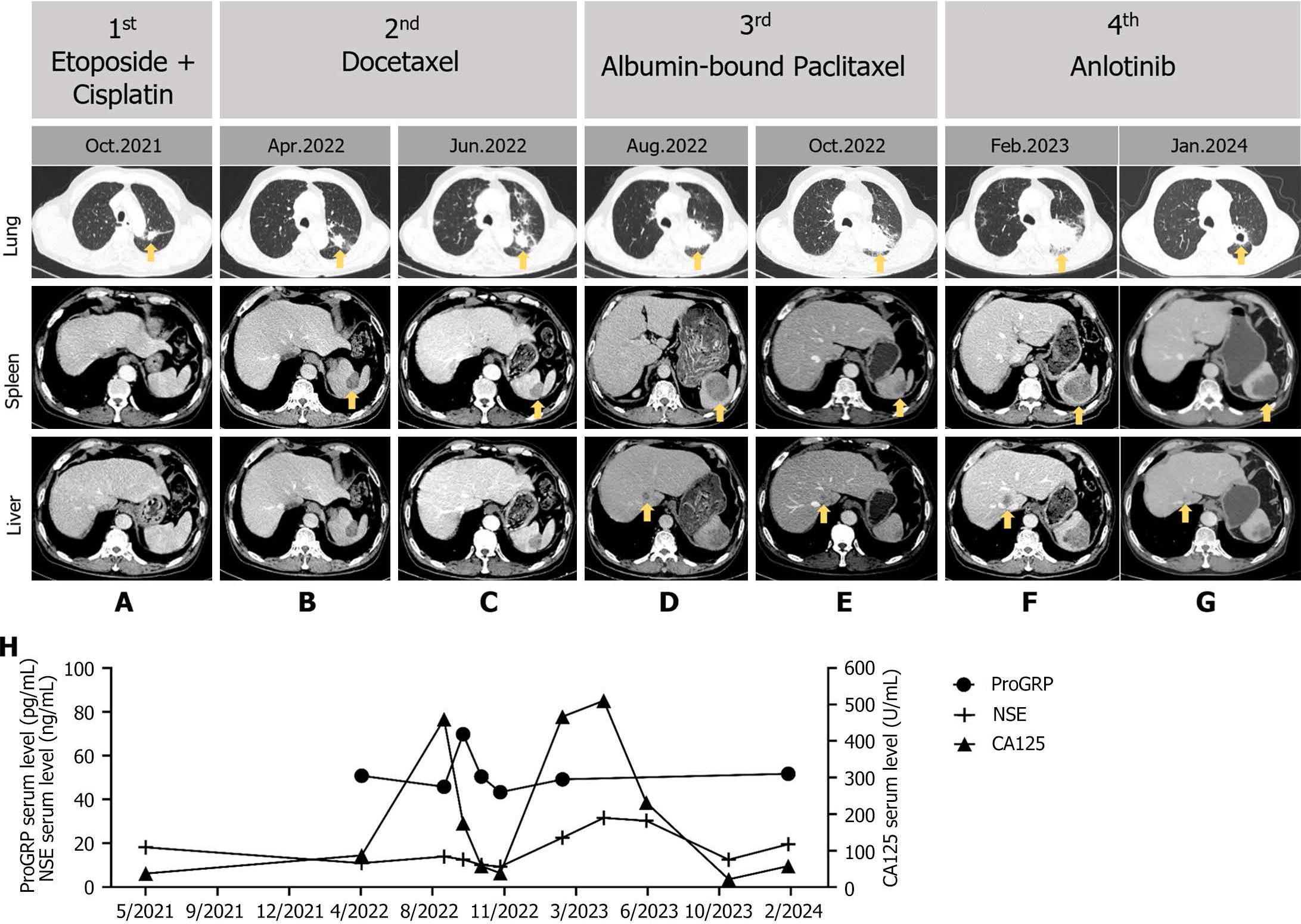
Despite being unable to undergo the preferred regimen of chemotherapy combined with immunotherapy (atezolizumab) due to personal reasons, the patient received six cycles of etoposide/cisplatin as the 1st line chemotherapy until October 2021 (Figure 2A). The patient experienced minimal side effects, primarily slight nausea and poor appetite. Following a partial response (PR) to chemotherapy, the patient underwent thoracic intensity-modulated radiotherapy starting on November 2021, due to residual thoracic lesions. Subsequent evaluation by chest CT scanning showed further reductions in the lung lesions and confirmed a PR. In April 2022, splenic metastatic nodules were confirmed by CT scans (Figure 2B), and the patient received 2nd line chemotherapy with docetaxel, with a PR observed (Figure 2C). However, further treatment was not pursued due to grade IV bone marrow suppression.
In August 2022, recurrence was observed with a size increment in splenic metastatic nodules and newly detected hepatic metastatic nodules (Figure 2D). The patient was treated with albumin-bound paclitaxel as 3rd line chemotherapy, resulting in a PR observed on CT scans (Figure 2E). Subsequent follow-up in February 2023 showed progression of lung, splenic, and hepatic nodules (Figure 2F), leading to the initiation of targeted therapy with anlotinib as the 4th line chemotherapy up to the present, resulting in a sustained PR (Figure 2G). The levels of serum biomarkers, such as progastrin-releasing peptide, NSE and CA125, were measured to monitor the clinical response to chemotherapy and tumor progression (Figure 2H).
Currently, the patient is still alive, taking anlotinib, and undergoing CT scanning every two months.
SCLC accounts for around 15% of all lung cancers and is known for its rapid tumor growth and early spread to multiple organs. Despite progress in cancer treatment, the therapeutic strategy for SCLC has stagnated, with cisplatin and etoposide being the established chemotherapy regimen for several decades. While most patients exhibit a favorable response to initial chemotherapy, recurrent disease is common, leading to a poor prognosis[7]. Current 1st line treatment continues to rely on chemotherapy, yet options remain limited for those with disease progression post-initial therapy[8]. More recent research efforts have concentrated on deciphering the molecular attributes of SCLC to tailor precise treatment approaches aimed at enhancing patient prognoses. Our patient exhibited a genomic loss of both TP53 and RB1 function, a characteristic molecular feature of SCLC. SCLC is known to be highly aggressive, attributed to genomic instability, near-universal inactivation of TP53 and RB1, rapid tumor growth, enhanced vascularity, and pronounced metastatic potential[9,10]. Notably, SCLC patients with RB1 inactivating variants demonstrate sensitivity to platinum-based chemotherapy[11]. RB1 and TP53 inactivating variants often co-occur in SCLC, with combined inactivation of these genes in murine models capable of inducing SCLC formation[12]. RET fusions, a rare oncogene in lung cancer, are detected in 1%-2% of all lung cancers and in approximately 1.6% of Chinese NSCLC cases. The predominant partners involved in RET fusions are KIF5B and CCDC6, contributing to approximately 70%-90% and 10%-25% of cases, respectively[13]. The KIF5B-RET fusion occurs in 1%-2% of lung adenocarcinoma cases, leading to sustained RET activation, a key driver gene in lung adenocarcinoma. RET expression is notably elevated, being 2 to 30 times higher in KIF5B-RET fusion lung adenocarcinoma compared to normal lung tissue[14]. Moreover, RET expression is notably elevated in SCLC compared to lung adenocarcinoma. A subgroup of SCLC patients could potentially derive benefits from tyrosine kinase inhibitors that target RET[15]. Various multikinase inhibitors targeting RET activity, including cabozantinib and vandetanib, have also received FDA approval. Current clinical guidelines suggest selpercatinib and pralsetinib as the preferred treatment for RET-rearranged NSCLC, with cabozantinib as a recommended option. This report marks the first identification of KIF5B-RET fusion (KIF5B exon15-RET exon12) in a patient with ES-SCLC, showcasing the significance of fusion genes in lung cancer pathogenesis and the breakthrough potential of KIF5B-RET fusion discovery for targeted SCLC treatment.
In summary, this report describes a SCLC case featuring a KIF5B-RET fusion. To date, the patient has received the 4th line chemotherapy with anlotinib and shown a favorable sustained PR. Given the rarity of this KIF5B-RET fusion in SCLC, the importance of RET fusion in SCLC patients remains unclear, underscoring the need for additional investigation. Furthermore, RET fusion represents a promising molecular target for SCLC therapies, deserving consideration in future treatment strategies.
| 1. | DiBonaventura MD, Shah-Manek B, Higginbottom K, Penrod JR, Yuan Y. Adherence to recommended clinical guidelines in extensive disease small-cell lung cancer across the US, Europe, and Japan. Ther Clin Risk Manag. 2019;15:355-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, Reck M, Mok T, Lam S, Shames DS, Liu J, Ding B, Lopez-Chavez A, Kabbinavar F, Lin W, Sandler A, Liu SV; IMpower133 Study Group. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379:2220-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 2372] [Article Influence: 338.9] [Reference Citation Analysis (0)] |
| 3. | Gomez-Randulfe I, Leporati R, Gupta B, Liu S, Califano R. Recent advances and future strategies in first-line treatment of ES-SCLC. Eur J Cancer. 2024;200:113581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 4. | Huang Y, Dai S, Yin W, Luo F, Li Y. Sustained Clinical Response to 4th-Line Therapy with Selpercatinib in RET Fusion-Positive Combined Small Cell Lung Cancer. Onco Targets Ther. 2023;16:1015-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Curigliano G, Subbiah V, Gainor J, Lee D, Taylor M, Zhu V, Doebele R, Lopes G, Baik C, Garralda E, Gadgeel S, Kim D, Turner C, Palmer M, Miller S. Treatment with BLU-667, a potent and selective RET inhibitor, provides rapid clearance of ctDNA in patients with RET-altered non-small cell lung cancer (NSCLC) and thyroid cancer. Annals Oncology. 2019;30:v790. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Drilon A, Oxnard G, Wirth L, Besse B, Gautschi O, Tan S, Loong H, Bauer T, Kim Y, Horiike A, Park K, Shah M, Mccoach C, Bazhenova L, Seto T, Brose M, Pennell N, Weiss J, Matos I, Peled N, Cho B, Ohe Y, Reckamp K, Boni V, Satouchi M, Falchook G, Akerley W, Daga H, Sakamoto T, Patel J, Lakhani N, Barlesi F, Burkard M, Zhu V, Moreno Garcia V, Medioni J, Matrana M, Rolfo C, Lee D, Nechushtan H, Johnson M, Velcheti V, Nishio M, Toyozawa R, Ohashi K, Song L, Han J, Spira A, De Braud F, Staal Rohrberg K, Takeuchi S, Sakakibara J, Waqar S, Kenmotsu H, Wilson F, B. nair, Olek E, Kherani J, Ebata K, Zhu E, Nguyen M, Yang L, Huang X, Cruickshank S, Rothenberg S, Solomon B, Goto K, Subbiah V. PL02.08 Registrational Results of LIBRETTO-001: A Phase 1/2 Trial of LOXO-292 in Patients with RET Fusion-Positive Lung Cancers. Journal of Thoracic Oncology. 2019;14:S6-S7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Scott SC, Hann CL. Immunotherapy for small cell lung cancer: established applications and novel approaches. Clin Adv Hematol Oncol. 2021;19:654-663. [PubMed] |
| 8. | Qu Z, Liu J, Luo F, Li L, Zhu L, Zhou Q. [MDT Treatment of Small Cell Lung Cancer Complicated with Adenocarcinoma: A Case Report and Literature Review]. Zhongguo Fei Ai Za Zhi. 2021;24:808-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15305] [Article Influence: 3061.0] [Reference Citation Analysis (4)] |
| 10. | Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17:725-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 524] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 11. | Dowlati A, Lipka MB, McColl K, Dabir S, Behtaj M, Kresak A, Miron A, Yang M, Sharma N, Fu P, Wildey G. Clinical correlation of extensive-stage small-cell lung cancer genomics. Ann Oncol. 2016;27:642-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Semenova EA, Nagel R, Berns A. Origins, genetic landscape, and emerging therapies of small cell lung cancer. Genes Dev. 2015;29:1447-1462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 13. | Sarfaty M, Moore A, Neiman V, Dudnik E, Ilouze M, Gottfried M, Katznelson R, Nechushtan H, Sorotsky HG, Paz K, Katz A, Saute M, Wolner M, Moskovitz M, Miller V, Elvin J, Lipson D, Ali S, Gutman LS, Dvir A, Gordon N, Peled N. RET Fusion Lung Carcinoma: Response to Therapy and Clinical Features in a Case Series of 14 Patients. Clin Lung Cancer. 2017;18:e223-e232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Song M. Progress in Discovery of KIF5B-RET Kinase Inhibitors for the Treatment of Non-Small-Cell Lung Cancer. J Med Chem. 2015;58:3672-3681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Dabir S, Babakoohi S, Kluge A, Morrow JJ, Kresak A, Yang M, MacPherson D, Wildey G, Dowlati A. RET mutation and expression in small-cell lung cancer. J Thorac Oncol. 2014;9:1316-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |