Published online Aug 16, 2024. doi: 10.12998/wjcc.v12.i23.5346
Revised: May 24, 2024
Accepted: June 11, 2024
Published online: August 16, 2024
Processing time: 67 Days and 6.5 Hours
Mycoplasma pneumoniae (MP) frequently causes respiratory infections in children, whereas Epstein-Barr virus (EBV) typically presents subclinical manifestations in immunocompetent pediatric populations. The incidence of MP and EBV co-infections is often overlooked clinically, with the contributory role of EBV in pulmonary infections alongside MP remaining unclear.
To evaluate the serum concentrations of interleukin-2 (IL-2) and interleukin-12 (IL-12) in pediatric patients with MP pneumonia co-infected with EBV and assess their prognostic implications.
We retrospectively analyzed clinical data from patients diagnosed with MP and EBV co-infection, isolated MP infection, and a control group of healthy children, spanning from January 1, 2018 to December 31, 2021. Serum IL-2 and IL-12 levels were quantified using enzyme-linked immunosorbent assay. Logistic regression was employed to identify factors influencing poor prognosis, while receiver operating characteristic (ROC) curves evaluated the prognostic utility of serum IL-2 and IL-12 levels in co-infected patients.
The co-infection group exhibited elevated serum IL-2 and C-reactive protein (CRP) levels compared to both the MP-only and control groups, with a reverse trend observed for IL-12 (P < 0.05). In the poor prognosis cohort, elevated CRP and IL-2 levels, alongside prolonged fever duration, contrasted with reduced IL-12 levels (P < 0.05). Logistic regression identified elevated IL-2 as an independent risk factor and high IL-12 as a protective factor for adverse outcomes (P < 0.05). ROC analysis indicated that the area under the curves for IL-2, IL-12, and their combination in predicting poor prognosis were 0.815, 0.895, and 0.915, respectively.
Elevated serum IL-2 and diminished IL-12 levels in pediatric patients with MP and EBV co-infection correlate with poorer prognosis, with combined IL-2 and IL-12 levels offering enhanced predictive accuracy.
Core Tip: This study presents a novel exploration of the interaction between immune response markers and co-infection outcomes in pediatric respiratory infections, focusing on Mycoplasma pneumoniae and Epstein-Barr virus. Our research addresses a critical gap in understanding the immunological dynamics in co-infected pediatric patients, offering insights into the prognostic values of interleukins interleukin-2 and interleukin-12. Through a comprehensive analysis of clinical data and a robust methodological approach, we provide evidence that serum levels of these cytokines are significantly associated with disease prognosis in co-infected individuals. The findings suggest a nuanced role of the immune system in managing co-infections, with potential implications for therapeutic strategies.
- Citation: Hao YP. Evaluating the role of interleukin-2 and interleukin-12 in pediatric patients with concurrent Mycoplasma pneumoniae and Epstein-Barr virus infections. World J Clin Cases 2024; 12(23): 5346-5353
- URL: https://www.wjgnet.com/2307-8960/full/v12/i23/5346.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i23.5346
Mycoplasma pneumoniae (MP), a common respiratory infection, is the cause of over 40% of community-acquired pneumonia cases in children. It often presents as mycoplasma pneumonia, which is characterized by fever and a persistent, dry cough[1,2]. Simultaneously, Epstein-Barr virus (EBV), a DNA virus, is recognized for its high infectivity, specifically targeting the respiratory system and other organs, and has the potential to remain in a dormant state within the host indefinitely[3,4]. Recent studies have shown an increasing tendency in co-infections involving MP and EBV. This clinical scenario is sometimes difficult to diagnose because it lacks unique symptoms, which worsens lung damage, prolongs episodes of fever, and complicates treatment interventions[5,6]. It is worth mentioning that there have been reports of children with MP and EBV co-infection experiencing more severe clinical symptoms, often affecting several organ systems[7,8].
MP, a primary causative agent of respiratory infections, triggers inflammation and increased sensitivity in the airways when transmitted through respiratory droplets[1,9]. Due to their developing immune systems, children are especially vulnerable to experiencing severe symptoms when they have both MP and EBV infections. This may be because these pathogens trigger a combined immunological response that worsens the symptoms. Interleukin-2 (IL-2) and interleukin-12 (IL-12) are important cytokines primarily produced by activated Th1 cells that have significant functions in the immune system[10]. IL-2 stimulates the growth and development of T and B lymphocytes by binding to their receptors on target cells, coordinating a cellular immune response[11]. On the other hand, IL-12, which plays a crucial role in the body's natural defense system, is essential for cellular immunological functions[12]. The participation of IL-2 in the development of MP and the significance of IL-12 as a crucial Th1 cytokine in regulating innate immunity highlight the intricate nature of the immune response to MP and EBV infections. While the exact pathogenic mechanisms behind co-infections of MP and EBV are not yet fully understood, existing literature highlights their connection with immune-mediated processes. The impaired immune response in pediatric MP pneumonia is commonly recognized, however there are differing views on the prevalence of Th subtypes (Th1/Th2)[10,13].
This study seeks to clarify the functions of IL-2 and IL-12 in the cellular immune response to co-infection of MP and EBV. It will achieve this by analyzing the levels of IL-2 and IL-12 in the blood serum of children who have MP pneumonia and EBV co-infection. The study aims to provide insights into the prognostic significance of these cytokines.
This study conducted a retrospective analysis of clinical data from patients who were diagnosed with MP co-infected with EBV, patients with isolated MP infection, and a control group consisting of healthy youngsters. The participants were enlisted from Maternal and Child Health Hospital between January 1, 2018 and December 31, 2021.
Inclusion criteria encompassed: (1) Clinical presentation of pneumonia at admission characterized by symptoms such as fever, cough, abnormal pulmonary sounds upon auscultation, and novel infiltrative patterns observable on chest radiographs; and (2) Confirmation of MP infection through positive MP polymerase chain reaction (PCR) assays[14], and EBV infection validated by EBV PCR positive results.
The exclusion criteria were established to exclude instances with inadequate data, poor immune function, concurrent bacterial or viral infections, and other respiratory diseases such as pulmonary tuberculosis and bronchial asthma.
Before the patients' guardians participated, they were given informed consent. The study's approach strictly followed the ethical principles specified in the Declaration of Helsinki and obtained clearance from the Hospital's Medical Ethics Committee.
Data encompassing demographic details, clinical manifestations, laboratory findings, and radiological assessments were collated retrospectively. Specimens for laboratory analysis, including blood, nasopharyngeal aspirate (NPA), and bronchoalveolar lavage fluid were collected. Initial blood samples were analyzed to determine white blood cell counts, neutrophil percentages, platelet counts, levels of C-reactive protein (CRP), and cytokines such as IL-2 and IL-12. To rigorously exclude the presence of other common viral (e.g., respiratory syncytial virus, influenza, metapneumovirus, adenovirus, and parainfluenza virus) and bacterial co-infections, NPA samples were collected within 24 hours of admission for comprehensive viral antigen detection and bacterial culture. Additionally, to mitigate the low sensitivity of blood cultures and limited viral detection, we employed a multi-panel PCR assay capable of identifying a broader range of bacterial and viral pathogens. This assay complements our standard procedures by enhancing the specificity of our infectious agent detection, thereby reducing the potential for undetected co-infections that could influence cytokine levels.
The prognosis of pediatric patients, including cases involving negative outcomes such as death or long-term damage to lung function caused by co-infection of MP and EBV, were thoroughly recorded. The exclusion criterion for unfavorable outcomes involved mortality or functional impairments resulting from dysfunctions in other organs, as established through autopsy.
After collecting 3 mL of fasting venous blood samples, the samples were subjected to centrifugation at a speed of 2500 rpm for a duration of 10 minutes. This process was done to separate the serum, which was thereafter stored at a temperature of -80°C. CRP levels were measured using an automated biochemistry analyzer (AU5800; Beckman Coulter, Brea, CA, United States). IL-2 and IL-12 serum levels were measured using enzyme-linked immunosorbent assays (ELISA) following the manufacturer’s protocol (Shanghai Jianglai Biotechnology Co., Ltd., Shanghai, China) The duration of the fever was also documented.
The statistical analysis was performed using SPSS version 20.0 (IBM Corp., Armonk, NY, United States). The categorical variables were represented as frequencies and percentages. Chi-square tests were used to compare the different groups. The continuous variables were reported as the mean ± standard deviation. Pairwise comparisons were conducted using t-tests, while multigroup comparisons were performed using one-way ANOVA. Post-hoc analysis was conducted using SNK-q tests. Utilizing multivariate logistic regression, we analyzed the characteristics that contribute to a negative outcome in pediatric patients with MP and EBV co-infection. The diagnostic efficacy of serum IL-2 and IL-12 levels in predicting negative outcomes was assessed using receiver operating characteristic (ROC) curves. A P value less than 0.05 was considered statistically significant.
Upon comparing the general characteristics across the MP group, the co-infection group (MP and EBV), and the control group, no significant disparities were observed in age or sex distribution among these groups (P > 0.05). Notably, serum CRP levels were markedly elevated in the co-infection group compared to both the MP group and the control group, with the MP group also displaying higher CRP levels than the control group (P < 0.05). Furthermore, the duration of fever in the co-infection group significantly exceeded that in the MP group (P < 0.05, Table 1).
In the comparison between the poor prognosis and the non-poor prognosis groups, age and sex did not significantly differ. However, the CRP levels and fever durations were significantly higher in the poor prognosis group compared to the non-poor prognosis group (P < 0.05, Table 2).
Analysis of serum IL-2 and IL-12 levels among the MP group, the co-infection group, and the control group revealed that IL-2 levels in the co-infection group were significantly elevated compared to both the MP group and the control group, with the MP group also having higher IL-2 levels than the control group. Conversely, IL-12 levels in the co-infection group were significantly lower than those in both the MP group and the control group, with the MP group exhibiting lower IL-12 levels than the control group (P < 0.05, Table 3).
When comparing the poor prognosis group with the non-poor prognosis group, the poor prognosis group showed significantly higher serum IL-2 levels and significantly lower IL-12 levels (P < 0.05, Table 4).
A logistic regression analysis incorporating serum IL-2, IL-12, CRP levels, and fever duration as independent variables, and the poor prognosis of children with EBV co-infection in MP pneumonia as the dependent variable, identified high serum IL-2 as an independent risk factor for poor prognosis. Conversely, high serum IL-12 emerged as a protective factor against poor prognosis in these patients (P < 0.05, Table 5).
| Variate | β | SE | Wald χ2 | P value | OR | 95%CI |
| IL-2 | 1.186 | 0.496 | 5.847 | 0.013 | 3.865 | 1.235-7.475 |
| IL-12 | 1.074 | 0.426 | 6.194 | 0.012 | 0.384 | 0.156-0.836 |
| CRP | 0.516 | 0.319 | 2.496 | 0.136 | 1.858 | 0.843-3.285 |
| Fever | 0.173 | 0.264 | 0.395 | 0.586 | 1.124 | 0.656-2.184 |
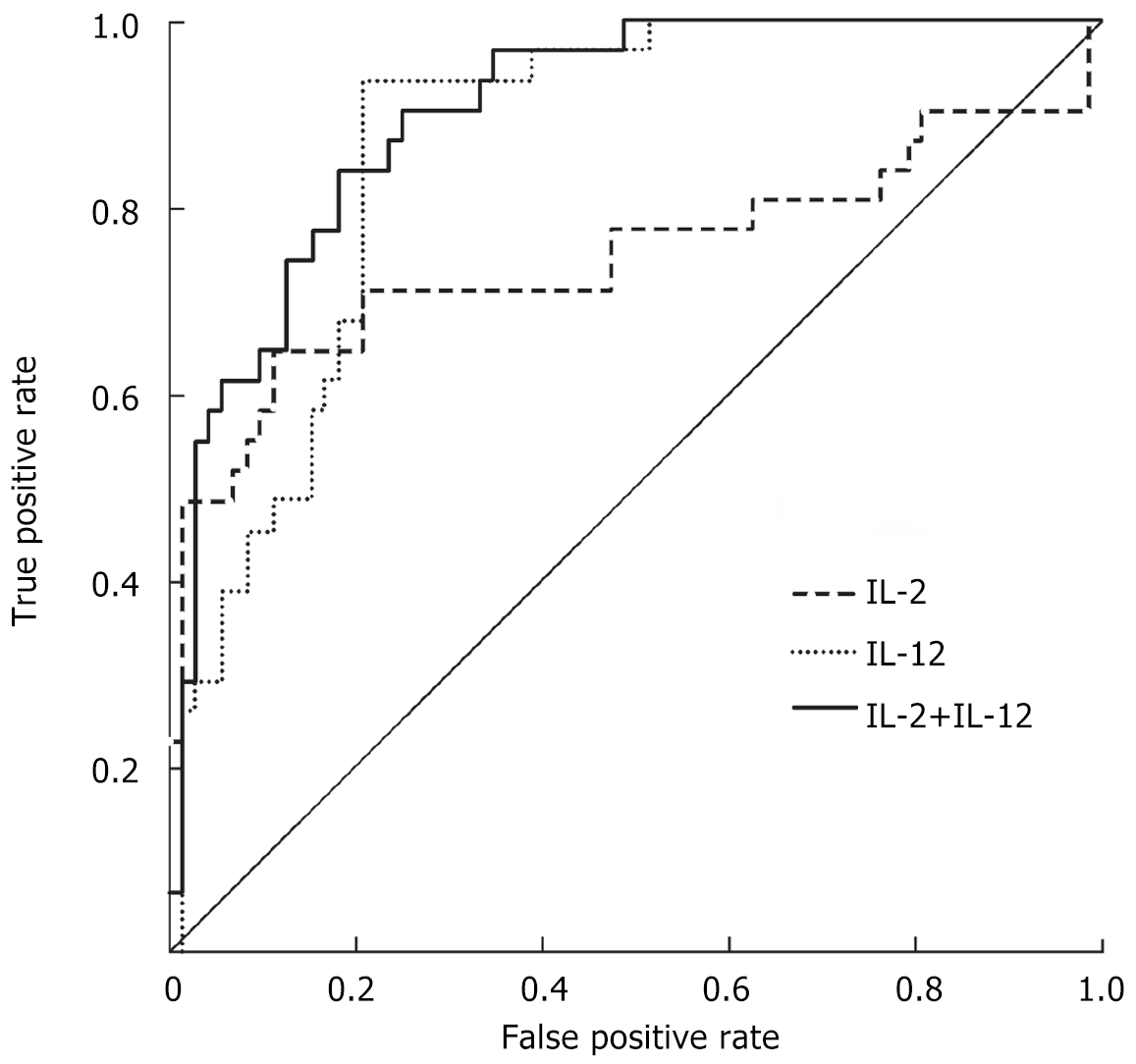
ROC curve analysis illustrated that the area under the curve (AUC) for serum IL-2 in predicting poor prognosis in children with MP and EBV co-infection was 0.815 (95%CI 0.653-0.903, P < 0.001), with a cutoff value of 3.265 ng/L, a sensitivity of 73.3%, and a specificity of 90.2%. The AUC for serum IL-12 in this context was 0.895 (95%CI 0.836-0.953, P < 0.001), with a cutoff value of 13.895 ng/L, a sensitivity of 94.2%, and a specificity of 82.5%. The combined AUC for serum IL-2 and IL-12 was 0.915 (95%CI 0.858-0.971, P < 0.001), yielding a sensitivity of 85.1% and a specificity of 84.3% (Figure 1).
The medical community has rarely reported or acknowledged cases of co-infections involving both MP and EBV in juvenile respiratory infections among patients with normal immune function[1,9,15]. Our analysis found that the clinical and radiological characteristics of children with simultaneous MP and EBV infections closely resemble those of MP infection alone. Nevertheless, the group of patients with co-infections displayed extended periods of fever and increased CRP levels, indicating more severe clinical symptoms in these individuals. This finding is consistent with similar case studies in scientific literature, such as the report by Li et al[7] on a patient with co-infection who experienced splenic infarction, and the description by Yenson et al[16] of a 7-year-old with MP and EBV co-infection who had agglutination of white and red blood cells. These reports further support the idea that co-infection can result in more severe manifestations of the disease.
IL-2 is essential for the immune response and is mostly produced by activated Th1 cells. It helps regulate immunological responses by activating regulatory T lymphocytes[17]. The findings of our study revealed a notable increase in serum IL-2 levels in the co-infection group compared to both the MP group and the control group. This suggests that co-infection might stimulate a heightened release of IL-2, which in turn could result in an intensified inflammatory response. The atypical manifestation of IL-2, linked to immunological dysfunction, could be a crucial element in the development of MP and EBV co-infection. Significantly, the study found that higher IL-2 levels were strongly linked to a negative prognosis in the group being studied. This suggests that IL-2 levels may have the capacity to predict unfavorable outcomes. Logistic regression study revealed that elevated blood IL-2 is an autonomous risk factor for worse prognosis in children with MP and EBV co-infection. Additionally, ROC curve analysis confirmed the predictive significance of serum IL-2 levels for undesirable outcomes.
IL-12, mostly released by mononuclear macrophages, plays a crucial role in stimulating Th1 cellular immune responses and controlling the equilibrium between Th1 and Th2 cells[18,19]. Extensive studies on IL-12 have revealed its ability to boost T cell function, promote Th1 responses, and regulate the balance between Th1 and Th2 cellular immune responses. This plays a vital role in cellular immunity during viral infections[10,20]. The results of this work suggest that individuals with co-infection have a dominant Th1 response, which is associated with an enhanced cellular immunological reactivity resulting in systemic immune damage and more severe clinical symptoms. The co-infection group exhibited a significant decrease in blood IL-12 levels compared to both the MP-only and control groups. Similarly, within the MP group, serum IL-12 levels were lower compared to the control group. These findings imply that serum IL-12 concentrations could potentially be used as indications of the severity of infection[21]. Moreover, the reduced levels of IL-12 in the group with bad prognosis, compared to the group with non-poor prognosis, suggest an imbalance in the Th1/Th2 cellular immune response in co-infected children. This highlights the importance of IL-12 as a crucial regulatory component in determining their prognosis.
The investigation demonstrated that elevated blood IL-12 levels serve as a safeguard against unfavorable prognosis in children with MP and EBV co-infection, confirming the strong correlation between IL-12 levels and patient outcomes. There was a correlation between the decrease in serum IL-12 levels and a higher probability of experiencing a negative prognosis. The ROC curve study revealed that serum IL-12 is a strong predictor of bad prognosis in co-infected children, with an AUC value of 0.895. The sensitivity of the test was 94.2%, meaning it correctly identified 94.2% of the children with poor prognosis. The specificity of the test was 82.5%, indicating that it correctly identified 82.5% of the children without poor prognosis. An association was found between a serum IL-12 threshold of ≤ 13.895 ng/L and an increased likelihood of experiencing negative outcomes. In addition, when serum IL-2 and IL-12 levels were analyzed together, the AUC was found to be 0.915. This analysis showed a sensitivity of 85.1% and a specificity of 84.3%. These results suggest that the combined assessment of these cytokines provides a more accurate prediction of outcomes for children with co-infections and could be an important prognostic marker.
Due to the notable increase in CRP levels observed in our study, it is essential to understand the complex interplay between EBV and MP co-infection. While EBV infections are typically subclinical in immunocompetent children, the scenario may differ substantially when co-infection with another pathogen such as MP is present. Studies have shown that EBV can enhance the host's inflammatory response, especially when co-infected with other pathogens[22]. Furthermore, MP is known to provoke a significant inflammatory response, potentially leading to elevated CRP levels[23]. In the context of co-infection, as in our study, the synergistic effects of EBV and MP may intensify the inflammatory response, thereby causing an increase in CRP levels beyond what is typically observed with viral infections alone. Moreover, CRP, as an acute-phase protein, is a sensitive marker of inflammation and is elevated in various infectious contexts. Our findings of elevated CRP levels are corroborated by the existing literature suggesting that the presence of additional pro-inflammatory stimuli, such as a bacterial infection with MP, can significantly enhance the CRP response even in the setting of a primary viral infection[24]. Therefore, the apparent anomaly in CRP elevation in our study is consistent with the expected biological response to EBV and MP co-infection, rather than an indication of data error. This response underscores the importance of considering co-infections and their cumulative impact on inflammatory markers in clinical assessments and research.
This study had significant limitations, particularly its retrospective approach, which prevented the determination of an ideal sample size and may have led to selection bias and reduced testing effectiveness due to the very small number of participants. Additionally, due to the study being conducted at a single center, there is a possibility of biases that may restrict the applicability of the results to a broader population. Future research should focus on establishing cooperation between other centers to validate these findings and expand our understanding of the role of IL-12 in predicting the outcome of young patients with MP and EBV co-infections.
Our study reveals that elevated serum IL-2 and reduced IL-12 levels in pediatric MP pneumonia with EBV co-infection are indicative of poor prognosis, with their combined assessment offering superior predictive accuracy. This underscores the necessity for further investigation into the underlying mechanisms and interactions of MP/EBV co-infection to enhance clinical management and outcomes.
Thank you to the patients who participated in this study.
| 1. | Kashyap S, Sarkar M. Mycoplasma pneumonia: Clinical features and management. Lung India. 2010;27:75-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Jiang W, Wu M, Zhou J, Wang Y, Hao C, Ji W, Zhang X, Gu W, Shao X. Etiologic spectrum and occurrence of coinfections in children hospitalized with community-acquired pneumonia. BMC Infect Dis. 2017;17:787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343:481-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1170] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 4. | Nowalk A, Green M. Epstein-Barr Virus. Microbiol Spectr. 2016;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Zhang X, Chen Z, Gu W, Ji W, Wang Y, Hao C, He Y, Huang L, Wang M, Shao X, Yan Y. Viral and bacterial co-infection in hospitalised children with refractory Mycoplasma pneumoniae pneumonia. Epidemiol Infect. 2018;146:1384-1388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Xu Y, Li S, Liu J, Zhou J, Jin F, Chen X, Wang Y, Jiang Y, Chen Z. Impact of Epstein-Barr virus coinfection in Mycoplasma pneumoniae pneumonia. Medicine (Baltimore). 2020;99:e19792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Li Y, Pattan V, Syed B, Islam M, Yousif A. Splenic infarction caused by a rare coinfection of Epstein-Barr virus, cytomegalovirus, and Mycoplasma pneumoniae. Pediatr Emerg Care. 2014;30:636-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Martínez Roig A, Busquets Monge RM, López Segura N, Herrero Pérez S, Esteban Torné E. [Epstein-Barr virus and Mycoplasma pneumoniae coinfection in two girls with community-acquired pneumonia]. An Esp Pediatr. 2002;56:69-70. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Ferwerda A, Moll HA, de Groot R. Respiratory tract infections by Mycoplasma pneumoniae in children: a review of diagnostic and therapeutic measures. Eur J Pediatr. 2001;160:483-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 987] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 11. | Tanaka H, Honma S, Abe S, Tamura H. Effects of interleukin-2 and cyclosporin A on pathologic features in Mycoplasma pneumonia. Am J Respir Crit Care Med. 1996;154:1908-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Salvatore CM, Fonseca-Aten M, Katz-Gaynor K, Gomez AM, Mejias A, Somers C, Chavez-Bueno S, McCracken GH, Hardy RD. Respiratory tract infection with Mycoplasma pneumoniae in interleukin-12 knockout mice results in improved bacterial clearance and reduced pulmonary inflammation. Infect Immun. 2007;75:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Li W, Liu YJ, Zhao XL, Shang SQ, Wu L, Ye Q, Xu H. Th1/Th2 Cytokine Profile and Its Diagnostic Value in Mycoplasma pneumoniae Pneumonia. Iran J Pediatr. 2016;26:e3807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Xu D, Li S, Chen Z, Du L. Detection of Mycoplasma pneumoniae in different respiratory specimens. Eur J Pediatr. 2011;170:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Oumei H, Xuefeng W, Jianping L, Kunling S, Rong M, Zhenze C, Li D, Huimin Y, Lining W, Zhaolan L, Xinmin L, Hua X, Zhiyan J, Yanning L, Yan H, Baoqing Z, Xiaochun F, Chunhui H, Yonghong J, Xue Z, Wei W, Zi W. Etiology of community-acquired pneumonia in 1500 hospitalized children. J Med Virol. 2018;90:421-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 16. | Yenson PR, Fleming A, Kaikov Y, Wadsworth LD. Combined neutrophil and erythrocyte agglutination in a 7-year-old boy. J Pediatr Hematol Oncol. 2007;29:664-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Mitra S, Leonard WJ. Biology of IL-2 and its therapeutic modulation: Mechanisms and strategies. J Leukoc Biol. 2018;103:643-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 18. | Dittrich A, Hessenkemper W, Schaper F. Systems biology of IL-6, IL-12 family cytokines. Cytokine Growth Factor Rev. 2015;26:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Sutterwala FS, Mosser DM. The taming of IL-12: suppressing the production of proinflammatory cytokines. J Leukoc Biol. 1999;65:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Komastu T, Ireland DD, Reiss CS. IL-12 and viral infections. Cytokine Growth Factor Rev. 1998;9:277-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Li H, Li S, Zheng J, Cai C, Ye B, Yang J, Chen Z. Cerebrospinal fluid Th1/Th2 cytokine profiles in children with enterovirus 71-associated meningoencephalitis. Microbiol Immunol. 2015;59:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Jangra S, Yuen KS, Botelho MG, Jin DY. Epstein-Barr Virus and Innate Immunity: Friends or Foes? Microorganisms. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Shi S, Zhang X, Zhou Y, Tang H, Zhao D, Liu F. Immunosuppression Reduces Lung Injury Caused by Mycoplasma pneumoniae Infection. Sci Rep. 2019;9:7147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018;9:754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 899] [Cited by in RCA: 1712] [Article Influence: 244.6] [Reference Citation Analysis (0)] |