Published online Aug 16, 2024. doi: 10.12998/wjcc.v12.i23.5329
Revised: May 29, 2024
Accepted: June 19, 2024
Published online: August 16, 2024
Processing time: 125 Days and 9.9 Hours
Cervical spine fracture-dislocations in patients with ankylosing spondylitis (AS) are mostly unstable and require surgery. However, osteoporosis, one of the comorbidities for AS, could lead to detrimental prognoses. There are few accurate assessments of bone mineral density in AS patients.
To analyze Hounsfield units (HUs) for assessing bone mineral density in AS patients with cervical fracture-dislocation.
The HUs from C2 to C7 of 51 patients obtained from computed tomography (CT) scans and three-dimensional reconstruction of the cervical spine were inde
The HUs decreased gradually from C2 to C7. The mean values of the left and right levels were significantly higher than those in the middle. Among the 51 patients, 25 patients (49.02%) may be diagnosed with osteoporosis, and 16 patients (31.37%) may be diagnosed with osteopenia.
The HUs obtained by cervical spine CT are feasible for assessing bone mineral density with excellent agreement in AS patients with cervical fracture-dislocation.
Core Tip: In this study, we analyzed Hounsfield units (HU) for assessing bone mineral density in patients with ankylosing spondylitis who developed cervical spine fracture-dislocation. The HU obtained by cervical spine computed tomography are feasible for assessing bone mineral density with excellent agreement. Subsequently, by analyzing the HU, we described the distribution of vertebral HU in ankylosing spondylitis patients with cervical fracture-dislocation. Among the 51 cervical fracture-dislocation patients with AS, 25 patients (49.02%) were diagnosed with osteoporosis, whereas 16 patients (31.37%) were diagnosed with osteopenia.
- Citation: Gao ZY, Peng WL, Li Y, Lu XH. Hounsfield units in assessing bone mineral density in ankylosing spondylitis patients with cervical fracture-dislocation. World J Clin Cases 2024; 12(23): 5329-5337
- URL: https://www.wjgnet.com/2307-8960/full/v12/i23/5329.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i23.5329
Ankylosing spondylitis (AS), characterized by syndesmophytes and ankylosis, is an inflammatory spondyloarthropathy that causes spinal stiffness and reduced mobility[1]. Minor trauma can lead to vertebral fractures due to the increased fragility of the spine, as observed in long bones, with cervical fracture-dislocations as the most severe type[2].
Most cervical fracture-dislocations in patients with AS involve the three columns, resulting in extreme instability and inevitable neurological deterioration[3]. Early surgical intervention can significantly enhance neurological recovery, reduce the incidence of complications, and increase the survival rate[4-6]. However, spinal surgeons face challenges when treating cervical fractures in these patients, including high surgical risks, limited control during surgery, a high risk of internal fixation failure, and postoperative refracture[7,8]. One of the underlying complications contributing to these challenges is osteoporosis, which is frequently mentioned but not adequately addressed[9-12].
Therefore, obtaining reliable bone density measurements is essential for guiding treatment in cervical spine fracture patients with AS. Dual-energy X-ray absorptiometry (DXA) is the international gold standard for the diagnosis of osteoporosis. However, DXA has limitations in measuring bone density in patients with AS[12]. Ligamentous calcification, tissue hyperplasia, and bone redundancy at the measurement site can result in normal or even abnormally high bone density values[13]. Quantitative computed tomography (CT) can accurately measure bone density at specific sites, but its high cost and complex operation limits its availability in most hospitals[14].
CT produces Hounsfield units (HU), offering unique advantages in assessing spinal osteoporosis. In addition, they provide a means of evaluating bone density without the requirement of additional tests and patient costs[15]. Hinze[12] highlighted the feasibility of using HU to assess osteoporosis in AS patients requiring surgical treatment, indicating the value of HU assessment in these patients.
Only a few studies have explored the evaluation of osteoporosis in AS patients with cervical fractures. Consequently, we conducted a retrospective study to investigate the HU patterns in cervical vertebrae of these patients and explored the application of HU in assessing their vertebral bone density.
This retrospective case study was based on data from AS patients who exhibited cervical spine fracture-dislocation and were admitted to The Second Affiliated Hospital of Naval Medical University between June 2018 and June 2023. The inclusion criteria comprised patients with cervical fractures occurring between C1-C7 and those with completely fused cervical segments due to AS. The exclusion criteria included patients with spinal infection, lack of patient consent, and subjects who had undergone spinal surgery previously.
The study cohort comprised 51 patients (44 males, 7 females). Each patient underwent a CT scan and three-dimensional reconstruction of the cervical spine after injury. Subsequently, patients were immobilized using Halo vests and underwent closed reduction immediately after CT or magnetic resonance imaging examinations. After achieving satisfactory reduction outcomes, patients underwent posterior or combined anterior-posterior surgeries under general anesthesia following successful awake nasotracheal intubation.
The ethics review board of our institution granted approval to conduct this study.
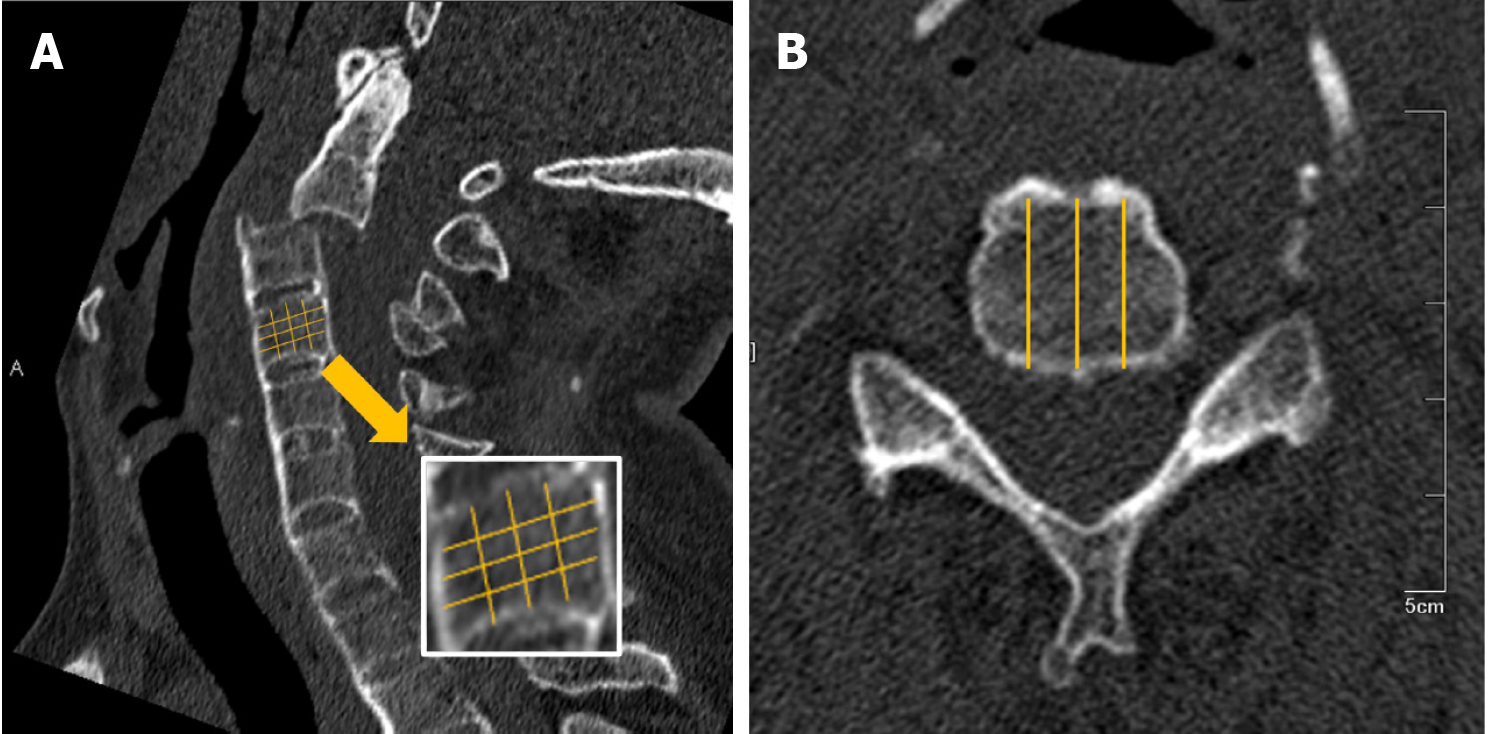
The HUs of C2-C7 vertebrae were measured with Neusoft PACS system by two trained spinal surgeons using the images from the CT scan and three-dimensional reconstruction of the cervical spine. Three sagittal plane reconstruction images, right, middle, and left, were selected for each vertebra. Each vertebrae were divided into approximately 16 regions, and the HUs were measured within each region, excluding areas of bone abnormality such as cortical bone, bone islands, and blood vessels. A detailed description of the measurement process is provided in Figure 1.
The HUs were measured and averaged for every region of each vertebrae. Inter-reader reliability and consistency were evaluated using the interclass correlation coefficient (ICC). An ICC greater than 0.7 indicated consistency between the two datasets. SPSS 22.0 software (IBM Corp., Armonk, NY, United States) was used for statistical analysis.
Among the 51 patients enrolled in this study, 25 were diagnosed with low-energy spinal injuries, whereas 26 patients suffered high-energy injuries. Each patient sustained a single spinal segment injury involving the three columns. The fracture-dislocation levels were distributed as follows: C1/2 in 5 patients, C2/3 in 2 patients, C3/4 in 2 patients, C4/5 in 6 patients, C5/6 in 16 patients, and C6/7 in 20 patients. All patients presented with neurological deficits. Detailed demographics and clinical data for the patients are presented in Table 1.
| Feature | Male, n = 44 | Female, n = 7 | Total, n = 51 |
| Age (years) | 55.14 | 59.57 | 55.4 |
| Injury level | |||
| C1/2 | 5 (11.36) | 0 (0) | 5 (9.80) |
| C2/3 | 2 (4.55) | 0 (0) | 2 (3.92) |
| C3/4 | 2 (4.55) | 0 (0) | 2 (3.92) |
| C4/5 | 5 (11.36) | 1 (14.28) | 6 (11.76) |
| C5/6 | 13 (29.54) | 3 (42.86) | 16 (31.37) |
| C6/7 | 17 (38.64) | 3 (42.86) | 20 (39.23) |
| ASIA grade | |||
| A | 2 (4.55) | 1 (14.28) | 3 (5.88) |
| B | 5 (11.36) | 2 (28.58) | 7 (13.73) |
| C | 14 (31.82) | 3 (42.86) | 17 (33.33) |
| D | 23 (52.27) | 1 (14.28) | 24 (47.06) |
Of the 306 vertebrae examined, 298 (97.39%) were fully assessable for the HUs. Eight vertebrae were excluded from complete assessment due to severe fractures and dislocations affecting all regions. However, the measurable regions of the eight vertebrae were evaluated, and the outcomes were averaged. Statistical analysis of HUs at the right, middle, and left levels of each vertebrae was conducted by the two spinal surgeons. Detailed information on the data is provided in Table 2.
| Segment | Reader 1-Housfield units | Reader 2-Housfield units | ||||
| Right | Middle | Left | Right | Middle | Left | |
| C2 | 337 | 296 | 334 | 336 | 297 | 334 |
| C3 | 266 | 228 | 261 | 266 | 229 | 260 |
| C4 | 250 | 215 | 249 | 253 | 215 | 250 |
| C5 | 233 | 198 | 226 | 235 | 198 | 225 |
| C6 | 218 | 181 | 214 | 217 | 182 | 215 |
| C7 | 201 | 173 | 196 | 202 | 174 | 196 |
| Average | 251 | 215 | 247 | 252 | 216 | 247 |
Both readers observed a cascading decrease in HUs from C2 to C7 (Table 3). At both levels, the highest mean HU values were recorded at C2 (322.83 and 322.10), whereas the lowest values were recorded at C7 (190.20 and 190.41).
| Segment | Readers 1-Housfield units | Readers 2-Housfield units | ICC | ||
| C2 | 322.83 | 322.10 | 0.96 | ||
| C3 | 252.23 | 251.55 | 0.94 | ||
| C4 | 238.95 | 239.57 | 0.96 | ||
| C5 | 219.60 | 218.47 | 0.91 | ||
| C6 | 204.49 | 205.02 | 0.99 | ||
| C7 | 190.20 | 190.41 | 0.98 | ||
| Average | 238.05 | 237.85 | 0.93 | ||
In addition, the mean HU values at different levels of the sagittal plane were analyzed. The results showed that the mean HU values of the left (251 and 252) and right (247 and 247) levels were significantly higher than those at the middle level (215 and 216) level for the two readers (P < 0.5; Table 2).
The inter-reader reliability was excellent, with ICCs ranging from 0.91 to 0.99 across the cervical spine. The C6 vertebrae exhibited the highest concordance with ICC of 0.99, whereas the C5 vertebrae showed the lowest with an ICC of 0.91 (Table 3).
In this study, we evaluated bone mineral density using HUs obtained through CT scan and three-dimensional reconstruction of the cervical spine. The findings demonstrated that HUs are a feasible method for accessing bone mineral density assessment in patients with AS who develop cervical spine fracture-dislocation.
CT examination is associated with additional costs and exposure to increased radiation compared to X-ray examination and DXA. However, using HUs to assess bone mineral density offers several advantages. Firstly, CT scan and three-dimensional reconstructions of the cervical spine are routine procedures for all patients with cervical fracture-dislocations and AS, making them accessible with no additional financial burden on patients. Moreover, the reduced risk of secondary nerve damage resulting from displacements eliminate the need for strict positioning and spatial requirements associated with other examinations such as magnetic resonance imaging and DXA[16]. Secondly, with advancements in CT equipment and supporting software, surgeons can quickly obtain high-definition images and measure the vertebral HUs digitally from images without requiring additional training from radiography specialists. In addition, CT scan is an important diagnostic tool for AS with cervical spine fracture-dislocations, helping prevent missed diagnoses and misdiagnoses often encountered with other examination methods[17]. Moreover, HUs, as a tool for assessing bone mineral density, offer users the ability to set regions of interest. This capability helps to avoid inaccuracies caused by osteochondritis, bone islands, and vascular plexus, significantly enhancing the accuracy of bone mineral density in patients with AS[18]. Furthermore, HUs can be used to predict internal fixation loosening and detect changes in bone mineral density[19].
Marques et al[20] conducted a cross-sectional study and reported that low-dose CT measurement of the HUs is a feasible method to assess vertebral bone density in r-axSpa (AS), demonstrating excellent inter-reader reliability across C3-L5 by two trained readers. This study, comprising 50 patients with r-axSpa, revealed that the HU values decreased from C3 to C7, with ICCs ranging from 0.90 to 1.00 in cervical vertebrae, consistent with the results of our study. The distribution pattern of HU values in cervical vertebrae is presented in Table 2. Notably, the HU values gradually decreased from C2 (322) to C7(190), with the lowest ICC observed at C5 (ICC = 0.91), consistent with the results from Marques et al[20].
In addition, a comparative analysis of the HUs of three levels showed that the HU values of the left and the right sagittal levels were significantly higher than those at the middle sagittal level. This observation may be attributed to the mechanical support provided by extraspinal bone, which can divert gravitational, emotional, and compressive stresses away from the vertebral trabeculae. This phenomenon results in diminished trabecular density, as predicted by Wolf’s law[21].
Swart et al[22] provided insights into the mechanism of osteoporosis in AS/diffuse idiopathic skeletal hyperostosis patients, highlighting that HUs were significantly reduced in the middle of long-segment autofusion, consistent with stress shielding. Alexander also emphasized the variation in bone mineral density between fused and unfused segments, a point that was not addressed by Marques et al[20]. In our study, all patients were diagnosed with AS with completely fused cervical segments. However, it is essential to recognize that the mechanism of cervical spine injuries may differ in patients with incomplete fusion.
Marques et al[20] reported that lower HU values indicated a lower CT attenuation and less dense bones. Despite DXA being the international gold standard for bone mineral density assessment, it has limitations in measuring bone density in patients with AS[12]. Clinal studies have indicated a correlation between the DXA results and the HUs from the same vertebra[23,24]. However, there is no universally accepted standard for diagnosing osteoporosis based on HUs from the cervical spine. Moreover, due to differences in anatomical areas and lack of adequate techniques for cervical bone mineral density, the relationship between DXA results and the HUs from cervical vertebrae remains controversial[25].
Colantonio et al[26] evaluated the relationship between HUs from the C4 vertebra and femur neck bone mineral density, given that the C4 vertebra is considered the least degenerative segment. Han et al[27] conducted a retrospective analysis of cervical vertebral HUs and lumbar T-values obtained by DXA in 439 patients. This analysis demonstrated that the C2 and C3 vertebrae exhibited relatively higher correlation values than other subaxial spinal segments. A strong correlation (r = 0.64-0.70) was observed between cervical spine HUs and spine DXA results, indicating that cervical spine HUs could be a reliable measure for bone mineral density across different measurement planes, age groups, sex, or degrees of degeneration. In addition. Han et al[27] proposed a formula (T-score = 0.01 × HUs - 4.55) and suggested cutoff HU values for osteoporosis and osteopenia as 231.5 and 284.0, respectively.
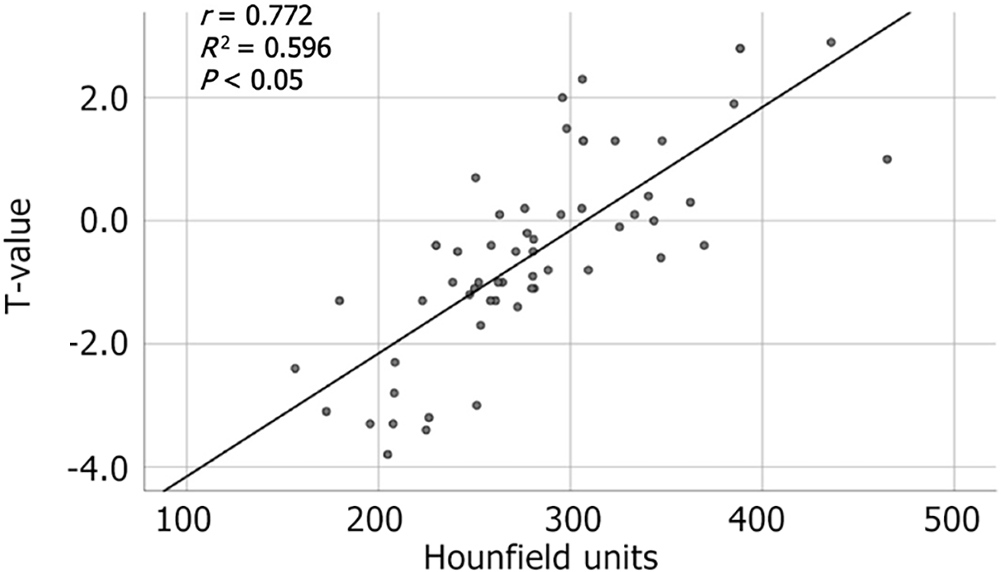
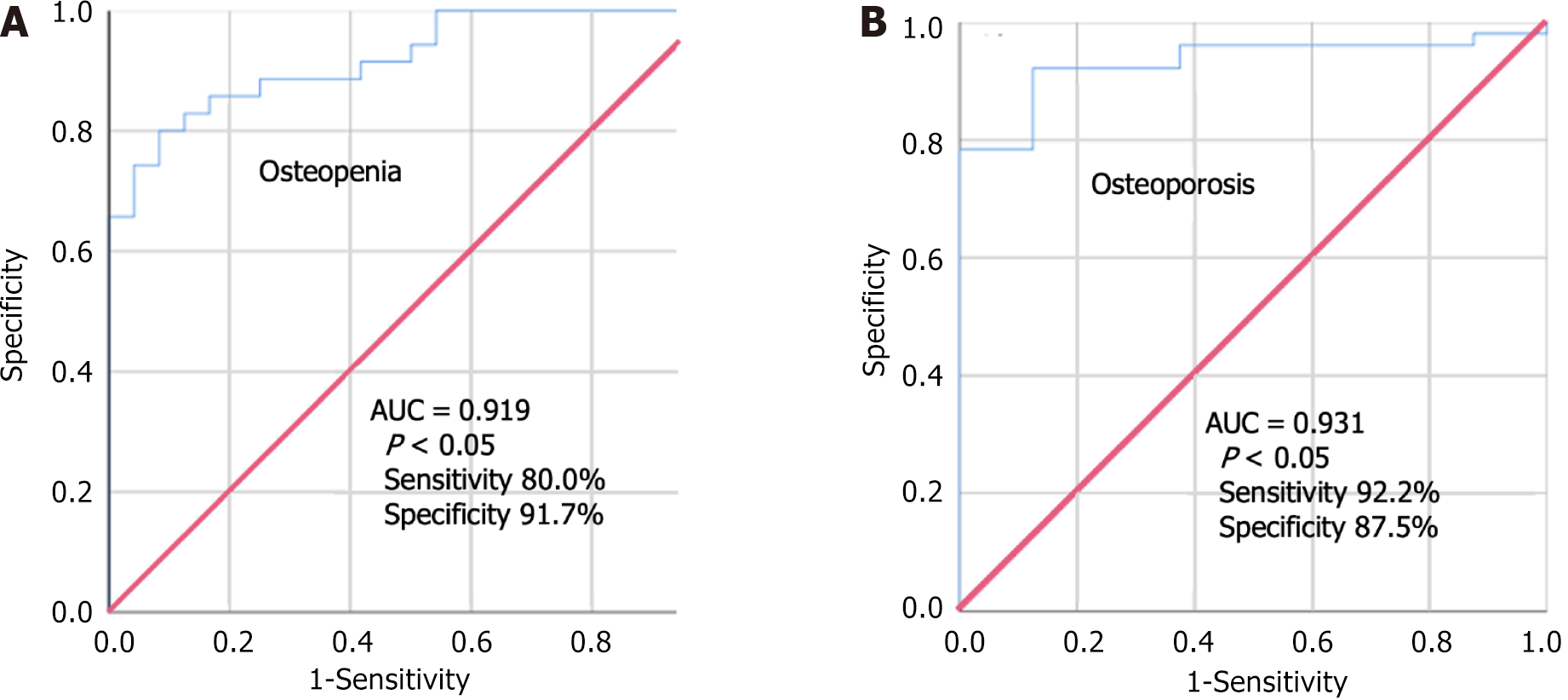
We conducted a retrospective analysis involving 57 patients, excluding patients with AS, diffuse idiopathic skeletal hyperostosis, pathological fractures, and previous spinal surgeries. All the patients underwent a CT scan and three-dimensional reconstruction of the cervical spine within 1 mo of DXA. The method for measuring HUs in C2-C7 vertebrae was consistent with the approach described in the previous studies on AS. Our analysis revealed a strong correlation between HUs in cervical vertebrae and T-scores from DXA (r = 0.772) (Figure 2). We determined HU thresholds for osteopenia and osteoporosis to be 274.33 and 228.08, respectively (Table 4), which were consistent with the data reported by Han et al[27]. Receiver operation characteristic curves (Figure 3) demonstrated that the HU measurement had a sensitivity of 92.2% and specificity of 87.5% with an area under the curve of 0.931 (P < 0.05) in diagnosing osteoporosis and a sensitivity of 80.0% and specificity of 91.7% with an area under the curve of 0.919 (P < 0.05) in diagnosing osteopenia.
| Bone mineral Density | T-value | HU |
| Normal | -1 or greater | ≤ 274.33 |
| Osteopenia | -1 to -2.5 | 228.08-274.33 |
| Osteoporosis | -2.5 or less | ≤ 228.08 |
The findings showed that among the 51 cervical fracture-dislocation patients with AS, 25 patients (49.02%) were diagnosed with osteoporosis, whereas 16 patients (31.37%) were diagnosed with osteopenia. These results indicated that the prevalence of osteoporosis is approximately 49.02% among patients with AS who experience cervical spine fracture-dislocation. This prevalence exceeds the reported osteoporosis rate in normal patients with AS, which is 25% as reported by the World Health Organization[28]. This increased prevalence could be attributed to the higher risk of vertebral fracture among AS patients who develop osteoporosis.
It seems contradictory that AS, characterized by aberrant ossification of extraosseous tissues, often coexists with osteoporosis. However, various studies have shown that AS patients have a significantly higher rate of bone loss compared to healthy people, with osteoporosis occurring even in the early stages of the disease[29]. The cause of osteoporosis in association with AS remains controversial, with the most likely scenario involving a combination of several mechanisms leading to bone loss. These mechanisms include genetic factors, chronic inflammation, medication side effects, asymptomatic bowel disease, and a gradual decrease in spinal mobility due to worsening of ankylosing joints. Notably, persistent inflammation that cannot be effectively alleviated by medications is the main predictor of bone loss in these patients[30].
Most elderly AS patients have reduced bone mass, making them susceptible to vertebral fractures from minor trauma[31]. These fractures are often extremely unstable and frequently associated with neurological dysfunction, complex complications, and high mortality rates[32]. Surgical treatment is typically required for most AS patients who develop cervical spine fracture-dislocations[33]. Stronger internal fixations are required for reduction and stabilization due to the spinal fusion and deformity characteristic of AS. However, osteoporosis in the vertebrae of these patients reduces the grip strength for internal fixation, increasing the surgical difficulty for spinal surgeons[34]. Furthermore, patients with cervical fracture-dislocations often suffer from severe cervical cord injuries resulting in quadriplegia. These patients require prolonged postoperative immobilization, which may exacerbate the progression of osteoporosis.
Therefore, initiating anti-osteoporotic therapy for patients with AS is essential. However, current studies indicate that only a few patients, especially those with compromised self-care abilities, received anti-osteoporotic therapy[35]. Clinical guidelines for treating osteoporosis in AS are not available. Bisphosphonates are currently the first-line drugs for osteoporosis treatment. Previous studies demonstrated that bisphosphonates inhibit osteoclast activity, thereby reducing bone destruction. In addition, they have anti-inflammatory properties, which may alleviate inflammation and bone marrow edema in AS patients[30]. Furthermore, tumor necrosis factor inhibitors are effective in treating osteoporosis by increasing bone mineral density and mitigating the progression of AS[36,37]. A bioinformatic analysis study identified 241 overlapping genes associated with AS and osteoporosis. The study proposed carlumab, bermekimab, rilonacept, rilotumumab, and ficalatuzumab as potential drugs for treating osteoporosis[38]. Although progress has been made in studying drugs for osteoporosis treatment in AS, further research should be conducted to explore the optimal anti-osteoporosis treatment regimen.
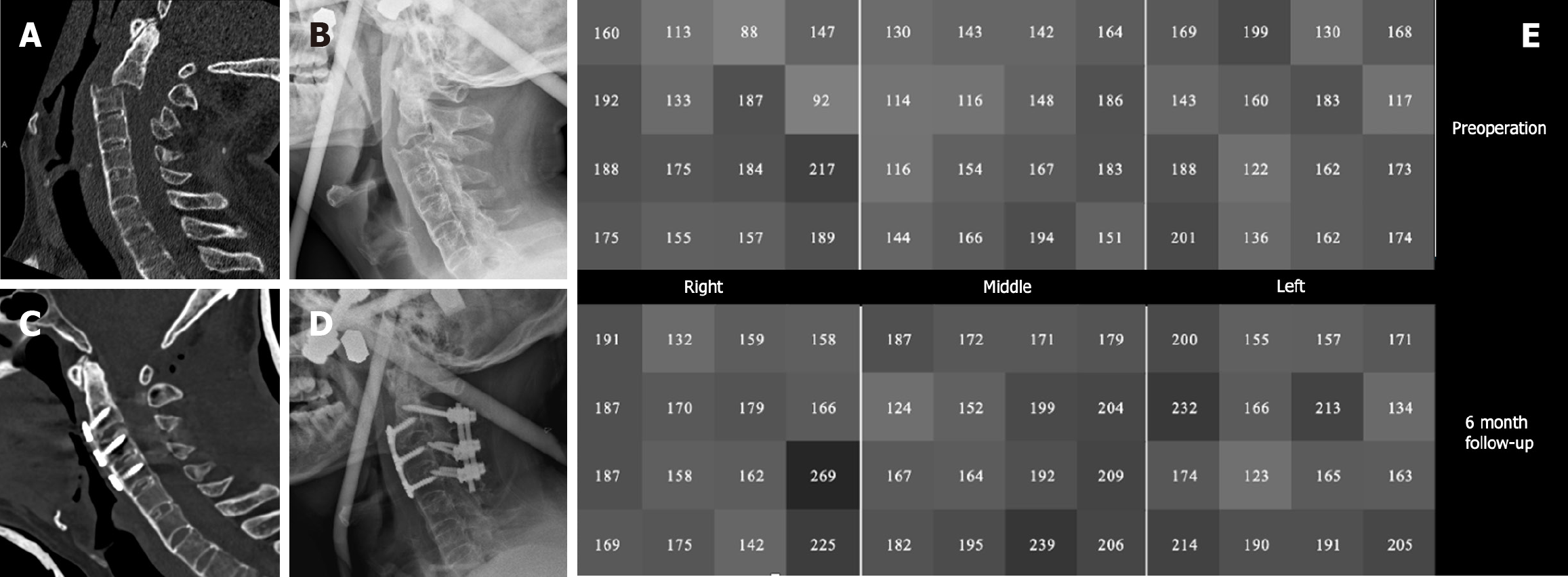
Furthermore, regular follow-up to evaluate bone mineral destiny is crucial alongside anti-osteoporosis treatment, whereby HU measurements are valuable. We measured and analyzed the HU values of cervical vertebrae in non-operated segments to evaluate the effectiveness of postoperative anti-osteoporotic treatment. Figure 4 illustrates a case of a 68-year-old man who developed paralysis after a traffic accident. Preoperative CT scans indicated AS combined with C2/3 fracture-dislocation. We performed an anterior combined posterior surgery after fixation and repositioning with a Halo vest. Bisphosphonates were administered postoperatively for anti-osteoporosis treatment, and cervical CT scans were conducted for 6 mo after the surgery. The HU values of C5 vertebra showed a significant increase in the postoperative HU value (179.67) compared to the preparative value (157.44), as shown in the gray scale diagram.
This study had some shortcomings. Firstly, most studies on the measurement of cervical HU refer to the methodology established by Schreiber et al[24]. In addition, previous research has shown that the average HU of several slices from the same vertebra does not significantly differ from the HU obtained from a single slice[39]. However, in our study, we divided each vertebra into 48 regions to explore the distribution of HUs, which significantly increased the workload. We anticipate leveraging artificial intelligence and upgrading software to streamline this process in future. Secondly, the preliminary diagnostic criteria proposed in this study, based on the relationship between cervical HUs and lumbar T-values obtained from DXA, may not be sufficiently rigorous. Multicenter and large sample clinical studies are required to confirm these findings.
In summary, use of the HUs obtained from cervical spine CT is a feasible approach for assessing bone mineral density in patients with AS who experience cervical spine fracture-dislocation. Although further clinical studies are necessary to refine this approach, it provides a promising and effective method for evaluating bone mineral density of cervical spine.
We thank the Second Affiliated Hospital of Naval Medical University for assistance with planning statistical analyses. Gratitude is also extended to the patients who participated in our study, providing invaluable data. In addition, we appreciate the editorial team of this journal for their insightful review and guidance.
| 1. | Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1266] [Cited by in RCA: 1384] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 2. | Mei J, Hu H, Ding H, Huang Y, Zhang W, Chen X, Fang X. Investigating the causal relationship between ankylosing spondylitis and osteoporosis in the European population: a bidirectional Mendelian randomization study. Front Immunol. 2023;14:1163258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 3. | Graham B, Van Peteghem PK. Fractures of the spine in ankylosing spondylitis. Diagnosis, treatment, and complications. Spine (Phila Pa 1976). 1989;14:803-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 94] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Huang J, Bai H, Tan Q, Hao D, Wu A, Wang Q, Wang B, Wang L, Liu H, Chen X, Jiang Z, Ma X, Liu X, Liu P, Cai W, Lu M, Mao N, Wang Y, Fu S, Zhao S, Zang X, Xie Y, Yu H, Song R, Sun J, Xiang L, Liu X, Li S, Liao B, Wu Z. Instantaneous death risk, conditional survival and optimal surgery timing in cervical fracture patients with ankylosing spondylitis: A national multicentre retrospective study. Front Immunol. 2022;13:971947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Weinstein PR, Karpman RR, Gall EP, Pitt M. Spinal cord injury, spinal fracture, and spinal stenosis in ankylosing spondylitis. J Neurosurg. 1982;57:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 87] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Lukasiewicz AM, Bohl DD, Varthi AG, Basques BA, Webb ML, Samuel AM, Grauer JN. Spinal Fracture in Patients With Ankylosing Spondylitis: Cohort Definition, Distribution of Injuries, and Hospital Outcomes. Spine (Phila Pa 1976). 2016;41:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Lazennec JY, d'Astorg H, Rousseau MA. Cervical spine surgery in ankylosing spondylitis: Review and current concept. Orthop Traumatol Surg Res. 2015;101:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | An SB, Kim KN, Chin DK, Kim KS, Cho YE, Kuh SU. Surgical outcomes after traumatic vertebral fractures in patients with ankylosing spondylitis. J Korean Neurosurg Soc. 2014;56:108-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Liu J, Zhao L, Yang X, Liu C, Kong N, Yu Y, Xuan D, Wan W, Xue Y. Bone mineral density, bone metabolism-related factors, and microRNA-218 are correlated with disease activities in Chinese ankylosing spondylitis patients. J Clin Lab Anal. 2022;36:e24223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Meyer B. Editorial. Is a meta-analysis of a few low-level publications helpful in guiding surgical strategies? Neurosurg Focus. 2021;51:E10. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Davey-Ranasinghe N, Deodhar A. Osteoporosis and vertebral fractures in ankylosing spondylitis. Curr Opin Rheumatol. 2013;25:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Hinze AM, Louie GH. Osteoporosis Management in Ankylosing Spondylitis. Curr Treatm Opt Rheumatol. 2016;2:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Klingberg E, Lorentzon M, Mellström D, Geijer M, Göthlin J, Hilme E, Hedberg M, Carlsten H, Forsblad-d'Elia H. Osteoporosis in ankylosing spondylitis - prevalence, risk factors and methods of assessment. Arthritis Res Ther. 2012;14:R108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Korkosz M, Gąsowski J, Grzanka P, Gorczowski J, Pluskiewicz W, Jeka S, Grodzicki T. Baseline new bone formation does not predict bone loss in ankylosing spondylitis as assessed by quantitative computed tomography (QCT): 10-year follow-up. BMC Musculoskelet Disord. 2011;12:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158:588-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 640] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 16. | Schiefer TK, Milligan BD, Bracken CD, Jacob JT, Krauss WE, Pichelmann MA, Clarke MJ. In-hospital neurologic deterioration following fractures of the ankylosed spine: a single-institution experience. World Neurosurg. 2015;83:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Zhang Z, Liu C, Hu F, You Y, Hu W, Zhang X. Are Both Preoperative Full-Spine 3Dimensional Computed Tomography Scans and X-Ray Films Necessary for Patients with Ankylosing Spondylitis Kyphosis? Orthop Surg. 2022;14:2618-2624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Pu M, Zhang B, Zhu Y, Zhong W, Shen Y, Zhang P. Hounsfield Unit for Evaluating Bone Mineral Density and Strength: Variations in Measurement Methods. World Neurosurg. 2023;180:e56-e68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Pickhardt PJ, Lauder T, Pooler BD, Muñoz Del Rio A, Rosas H, Bruce RJ, Binkley N. Effect of IV contrast on lumbar trabecular attenuation at routine abdominal CT: correlation with DXA and implications for opportunistic osteoporosis screening. Osteoporos Int. 2016;27:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Marques ML, Pereira da Silva N, van der Heijde D, Reijnierse M, Baraliakos X, Braun J, van Gaalen FA, Ramiro S. Low-dose CT hounsfield units: a reliable methodology for assessing vertebral bone density in radiographic axial spondyloarthritis. RMD Open. 2022;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Frost HM. Wolff's Law and bone's structural adaptations to mechanical usage: an overview for clinicians. Angle Orthod. 1994;64:175-188. [PubMed] [DOI] [Full Text] |
| 22. | Swart A, Hamouda A, Pennington Z, Lakomkin N, Mikula AL, Martini ML, Shafi M, Subramaniam T, Sebastian AS, Freedman BA, Nassr AN, Fogelson JL, Elder BD. Significant Reduction in Bone Density as Measured by Hounsfield Units in Patients with Ankylosing Spondylitis or Diffuse Idiopathic Skeletal Hyperostosis. J Clin Med. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Choi MK, Kim SM, Lim JK. Diagnostic efficacy of Hounsfield units in spine CT for the assessment of real bone mineral density of degenerative spine: correlation study between T-scores determined by DEXA scan and Hounsfield units from CT. Acta Neurochir (Wien). 2016;158:1421-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 167] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 24. | Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am. 2011;93:1057-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 620] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 25. | Amin MFM, Zakaria WMW, Yahya N. Correlation between Hounsfield unit derived from head, thorax, abdomen, spine and pelvis CT and t-scores from DXA. Skeletal Radiol. 2021;50:2525-2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Colantonio DF, Saxena SK, Vanier A, Rodkey D, Tintle S, Wagner SC. Cervical Spine Computed Tomography Hounsfield Units Accurately Predict Low Bone Mineral Density of the Femoral Neck. Clin Spine Surg. 2020;33:E58-E62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Han K, You ST, Lee HJ, Kim IS, Hong JT, Sung JH. Hounsfield unit measurement method and related factors that most appropriately reflect bone mineral density on cervical spine computed tomography. Skeletal Radiol. 2022;51:1987-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Dubrovsky AM, Lim MJ, Lane NE. Osteoporosis in Rheumatic Diseases: Anti-rheumatic Drugs and the Skeleton. Calcif Tissue Int. 2018;102:607-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | El Maghraoui A. Extra-articular manifestations of ankylosing spondylitis: prevalence, characteristics and therapeutic implications. Eur J Intern Med. 2011;22:554-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | El Maghraoui A. Osteoporosis and ankylosing spondylitis. Joint Bone Spine. 2004;71:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Montala N, Juanola X, Collantes E, Muñoz-Gomariz E, Gonzalez C, Gratacos J, Zarco P, Fernandez Sueiro JL, Mulero J, Torre-Alonso JC, Batlle E, Carmona L. Prevalence of vertebral fractures by semiautomated morphometry in patients with ankylosing spondylitis. J Rheumatol. 2011;38:893-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Thumbikat P, Hariharan RP, Ravichandran G, McClelland MR, Mathew KM. Spinal cord injury in patients with ankylosing spondylitis: a 10-year review. Spine (Phila Pa 1976). 2007;32:2989-2995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Charles YP, Buy X, Gangi A, Steib JP. Fracture in ankylosing spondylitis after minor trauma: radiological pitfalls and treatment by percutaneous instrumentation. A case report. Orthop Traumatol Surg Res. 2013;99:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Sari I, Haroon N. Radiographic Progression in Ankylosing Spondylitis: From Prognostication to Disease Modification. Curr Rheumatol Rep. 2018;20:82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5:898-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 682] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 36. | Haroon NN, Sriganthan J, Al Ghanim N, Inman RD, Cheung AM. Effect of TNF-alpha inhibitor treatment on bone mineral density in patients with ankylosing spondylitis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Molnar C, Scherer A, Baraliakos X, de Hooge M, Micheroli R, Exer P, Kissling RO, Tamborrini G, Wildi LM, Nissen MJ, Zufferey P, Bernhard J, Weber U, Landewé RBM, van der Heijde D, Ciurea A; Rheumatologists of the Swiss Clinical Quality Management Program. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management cohort. Ann Rheum Dis. 2018;77:63-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 195] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 38. | Wang C, Wang L, Li Q, Wu W, Yuan J, Wang H, Lu X. Computational Drug Discovery in Ankylosing Spondylitis-Induced Osteoporosis Based on Data Mining and Bioinformatics Analysis. World Neurosurg. 2023;174:e8-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Schreiber JJ, Anderson PA, Hsu WK. Use of computed tomography for assessing bone mineral density. Neurosurg Focus. 2014;37:E4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |